Abstract
Ischemic stroke is caused primarily by an interruption in cerebral blood flow, which induces severe neural injuries, and is one of the leading causes of death and disability worldwide. Thus, it is of great necessity to further detailly elucidate the mechanisms of ischemic stroke and find out new therapies against the disease. In recent years, efforts have been made to understand the pathophysiology of ischemic stroke, including cellular excitotoxicity, oxidative stress, cell death processes, and neuroinflammation. In the meantime, a plethora of signaling pathways, either detrimental or neuroprotective, are also highly involved in the forementioned pathophysiology. These pathways are closely intertwined and form a complex signaling network. Also, these signaling pathways reveal therapeutic potential, as targeting these signaling pathways could possibly serve as therapeutic approaches against ischemic stroke. In this review, we describe the signaling pathways involved in ischemic stroke and categorize them based on the pathophysiological processes they participate in. Therapeutic approaches targeting these signaling pathways, which are associated with the pathophysiology mentioned above, are also discussed. Meanwhile, clinical trials regarding ischemic stroke, which potentially target the pathophysiology and the signaling pathways involved, are summarized in details. Conclusively, this review elucidated potential molecular mechanisms and related signaling pathways underlying ischemic stroke, and summarize the therapeutic approaches targeted various pathophysiology, with particular reference to clinical trials and future prospects for treating ischemic stroke.
Similar content being viewed by others
Introduction
Epidemiology, diagnosis, and treatment for ischemic stroke
Ischemic stroke is caused by an interruption in cerebral blood flow, induced by thrombosis or embolism. It represents the second leading cause of deaths worldwide, with 5.9 million deaths and 102 million disability-adjusted life years lost.1,2 Several risk factors have been implicated in the pathogenesis of ischemic stroke, including diabetes, cigarette smoking, hyperlipidemia, and hypertension.3 Based on the etiology, the cause of ischemic stroke can be traced to embolism from the heart, artery-to-artery embolism, and in situ small vessel disease.2,4 Typically, stroke symptoms include sudden unilateral weakness, numbness, diplopia, slurred speech, ataxia, and non-orthostatic vertigo.5 Various efforts have been made to improve outcome after stroke onset. Immediate clinical interventions, such as intravenous thrombolytic treatment and mechanical thrombectomy, contribute to the recanalization of cerebral blood vessels.5 While antithrombotic therapies, including antiplatelet or anticoagulant agents, are recommended for nearly all patients with no contraindication,3 pharmacological approaches against ischemic stroke remain limited, suggesting the need for new treatments.
Morphological changes in ischemic stroke
In the pathogenesis of ischemic stroke, various types of cells in the central nervous system experience different morphological alterations facing ischemic damages. In the ischemic core, neurons undergo morphological changes where the cell bodies and axons disappear.6,7 Swelling of the cytoplasm and nucleolus disappearance are often seen in neurons as well as glial cells. While in the penumbra, neurons, which are referred to as ‘ischemic neurons’ and relatively viable, usually experience several changes such as endoplasmic ribosomes and Nissl bodies disintegration.8 Besides neurons, glial cells, including microglia and astrocytes, also experience morphological changes after ischemia. Ramified microglia can transform into an “activated state”, characterized by swollen ameboid-like cells, accompanied by the production of pro-inflammatory substances, including cytokines, chemokines, and reactive oxygen species (ROS),9 while astrocytes usually undergo gradual alterations both in molecular expression profiles and morphologies, which serves to protect neurons in the ischemic penumbra.10,11 After ischemia, increased blood-brain barrier (BBB) permeability contributes to the infiltration of several immune cells including leukocytes, monocytes, and macrophages, into the ischemic lesions, which release a variety of neurotoxic or neurotrophic factors to exert either neuroprotective or detrimental effects on ischemic brain tissues.12,13,14,15,16,17
The temporal and spatial alterations in ischemic stroke are illustrated in Fig. 1.
Experimental models of ischemic stroke
Efforts have been made to elucidate the pathophysiological mechanisms and screen potential therapeutical targets of ischemic stroke, and models both in vivo and in vitro are utilized to mimic ischemic circumstances. The most frequently used experimental ischemic stroke model is middle cerebral artery occlusion model (MCAO), in which a filament is utilized to block cerebral blood flow from the middle cerebral artery to induce a transient occlusion.18,19 This model was mostly used for studying blood-brain barrier disruption and inflammatory response in cerebral ischemia.20,21 Besides MCAO model, photothrombosis model is also utilized to induce cerebral ischemia in both mice and rats. In this model, Rose Bengal, a photosensitive dye is injected systematically into the animal, while a 532 nm wavelength laser is directly illuminated onto the skull and react with the photosensitive dye.22 Advantage of this model include the possibility to select a specific cortical brain region for ischemia and the high reproducibility with very low mortality.18 Correspondingly, the most frequently used in vitro model to mimic ischemic stroke is the oxygen and glucose deprivation (OGD) model, in which oxygen is replaced by N2 and glucose in the medium is omitted. Often this model is combined with cell co-cultures to study cellular interactions under ischemic circumstances.23 However, a limitation still remains that the in vitro model should be combined with in vivo studies to better comprehensively understand ischemic stroke.18
Pathophysiological mechanisms involved in ischemic stroke
As a hallmark of ischemic stroke, interrupted cerebral blood flow depletes the brain of oxygen and glucose, which leads to disrupted ATP synthesis and energy deficiency, as well as impaired ion homeostasis and acid-base imbalance.24,25 All these dysfunctions result in cerebral neuropathological changes, such as brain edema, neuroinflammation, and neural cell death, eventually underpinning severe neurological deficits.26 Progress has been made in unveiling the pathogenesis and mechanisms of stroke, including cellular excitotoxicity,27 mitochondrial dysfunctions,28 neuroinflammation,29 BBB impairment,30 and cell death processes.31 Various signaling pathways become activated in these pathological transitions, and their targeted regulation could serve as a potential therapeutic strategy. Given the complex pathophysiology of ischemic stroke, the accompanying injury and signaling mechanisms should be first identified and then further elucidated to develop targeted interventions.
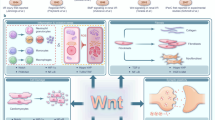
The present review describes various signaling pathways associated with ischemic stroke pathophysiology (Fig. 2) and categorizes the corresponding therapeutic approaches (Table 1). Additionally, we summarize evidence from national clinical trials assessing therapies targeting ischemic stroke (Table 2).
A brief summary for the pathophysiology involved in ischemic stroke. a Excitotoxicity in ischemic stroke, in which excessive glutamate are released and both synaptic and extra-synaptic NMDARs are involved; b Cell death signaling pathways, which mainly involves autophagy, apoptosis and necroptosis in ischemic stroke; c Neuroinflammation and BBB breakdown in ischemic stroke. Here we've presented the participation of various immune cells and chemokines and cytokines released, which thus contribute to blood-brain barrier breakdown; d Oxidative stress, which is mainly characterized by ROS production and mitochondrial dysfunction that involves Ca2+ influx into mitochondria and MPTP in ischemic stroke
Pathophysiology and signaling pathways involved in ischemic stroke
Energy deficiency due to a lack of glucose and oxygen
Immediately after ischemic stroke, cerebral blood flow is significantly reduced, which limits the availability of glucose and oxygen, especially in neurons. Energy disruption leads to mitochondrial dysfunction and oxidative stress-induced damage, triggered by the production of ROS.32 Concurrently, energy deficiency contributes to an ionic imbalance that affects Na+, K+, and Ca2+ levels, leading to cell depolarization and prompting glutamate release.33 The excessive glutamate activates N-methyl-D-aspartate receptors (NMDARs), inducing toxicity, cell death, and finally severe damage of the central nervous system.34,35,36 Taken together, deficiency in glucose and oxygen may eventually lead to cellular excitotoxicity and mitochondrial dysfunctions, which serve as the initiating session of ischemia-induced damage and subsequently cause other cascade of injuries. In this section, the review focuses on the various signaling pathways involved in glutamate and NMDAR-induced cell toxicity, namely, excitotoxicity, as well as oxidative stress and mitochondrial dysfunction in ischemic stroke.
Excitotoxicity and related signaling pathways
Glucose and oxygen deficiency during cerebral ischemia induces neuronal cell depolarization and glutamate release. The latter then stimulates Na+/Ca2+ channels coupled with NMDARs.37 Enhanced Ca2+ influx perturbs ionic homeostasis, resulting in Ca2+ overload in both the mitochondria38,39,40 and cytosol. These changes stimulate a variety of proteases, lipases, kinases, phosphatases, endonucleases, and free radicals,41,42 as well as biological processes causing cell death, such as calpain activation,43 oxidative stress, and mitochondrial impairment.44,45 Overall, these cellular dysfunctions are termed excitotoxicity and involve NMDARs, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, and kainite receptors.1,46
Despite their involvement in ischemic stroke-related excitotoxicity, NMDARs act as a double-edged sword. Functional and structural studies have revealed that activation of NMDARs containing the GluN2B subunit triggers excitotoxicity during ischemic stroke and subsequent neuronal apoptosis, whereas activation of NMDARs containing the GluN2A subunit exerts a neuroprotective effect.33,47 Similarly, it has been hypothesized that synaptic NMDARs promote neuronal survival, whereas extra-synaptic NMDARs play detrimental roles in neuronal activity.48 The analogy between synaptic vs. extra-synaptic NMDARs and GluN2A-containing NMDARs vs. GluN2B-containing NMDARs demonstrates the dual effect of NMDARs and their regulation of signaling pathways with neuroprotective or detrimental effects on ischemic stroke (Fig. 3).
Excitotoxicity and signaling pathways involved in ischemic stroke. NMDAR N-methyl-D-aspartate receptors, PI3K Phosphatidylinositol 3 kinase, BDNF Brain-derived neurotrophic factor, CREB cAMP-response element-binding protein PTEN Phosphate and tension homology deleted on chromosome ten, PIP3 plasma membrane intrinsic protein 3, DAPK1 Death-associated protein kinase 1, PSD95 Postsynaptic density protein 95, nNOS Neuronal nitric oxide synthase
Phosphatidylinositol 3-kinase (PI3K)-Akt signaling pathway
Stimulation of synaptic NMDARs activates the pro-survival PI3K/Akt signaling pathway, thereby exerting a neuroprotective effect. PI3K is an intracellular kinase classified into three categories (I, II, and III) based on structure and substrate specificity. In neurons, activation of the PI3K/Akt signaling pathway by NMDAR occurs via Ca2+ and calmodulin, which recruit phosphoinositide-dependent protein kinase 1. At the same time, Ca2+ triggers tyrosine phosphorylation of insulin receptor substrate 1, reinforcing NMDAR-induced Akt activation.49,50,51 The protective effect of the PI3K/Akt signaling pathway on ischemic stroke has been reported both in in vitro neurons during hypoxia52,53,54 and in vivo against ischemic neuronal death,52,55,56,57, and PI3K/Akt signaling inhibition aggravates ischemia-induced neuronal death in experimental stroke animals.55,56,58,59 Mechanistically, the neuroprotective effect of Akt is related to the phosphorylation and inactivation of various downstream targets, including glycogen synthase kinase 3 beta (GSK3β), pro-apoptotic B-cell lymphoma 2 (Bcl2)-associated BAD,60 c-Jun N-terminal kinase (JNK)/p38 activator ASK1,61 and apoptotic p53.54 These effects not only exist in neurons, but also in other neural cell types, which are possibly related to the inhibition of synaptic excitotoxicity and thus exert neuroprotective effects in ischemic stroke.
Brain-derived neurotrophic factor (BDNF) and cAMP-response element-binding protein (CREB)-related gene products
Synaptic NMDAR activation and Ca2+ influx activate the Ras/extracellular signal-regulated kinase (ERK) signaling pathway and nuclear Ca2+/calmodulin-dependent protein kinases, which in turn phosphorylate and activate CREB.62,63 Together with NMDAR and BDNF, CREB promotes the expression of numerous pro-neuronal survival genes.64,65,66,67 BDNF production in the brain relies on Ca2+ influx through NMDAR.64,68,69 Synaptic NMDARs promote BDNF gene expression,70 whereas extra-synaptic NMDARs block CREB-mediated BDNF expression.71 In experimental ischemic stroke models, BDNF is secreted into the brain and protects against ischemia-induced injury via neuronal GluN2A-NMDAR activation.72,73 Together, these results show that BDNF and, to some extent, the upstream CREB signaling pathway contribute to the neuroprotective effect associated with synaptic excitotoxicity in cerebral ischemia.
Phosphatase and tensin homolog (PTEN) signaling pathway
Extra-synaptic NMDARs are closely linked to signaling pathways associated with cell death and often contradict the effects triggered by synaptic NMDARs. Upon activation by Ca2+ influx through NMDARs, PTEN is recruited to GluN2B-NMDARs. The direct interaction between PTEN and the GluN1 subunit of GluN2B-NMDARs enhances current flow through the channel, tightening the junctions between PTEN and the neuronal death signaling complex. Concurrently, the excitotoxic stimulation of NMDARs initiates PTEN nuclear translocation, thus significantly lowering the phosphorylation of phosphatidylinositol-trisphosphate and Akt and consequently blocking PI3K/Akt signaling.74,75 Thus, contrary to the protective effect of PI3K/Akt, PTEN signaling may decrease cell survival and induce neuronal death.76 In agreement with this hypothesis, downregulating PTEN expression reportedly inhibits extra-synaptic NMDAR currents and protects neurons from experimental ischemic injury.74 The above evidence hints at the detrimental role of PTEN in ischemic stroke, which is largely mediated by regulation of extra-synaptic NMDAR activities.
Death-associated protein kinase 1 (DAPK1) signaling pathway
DAPK1 is a Ca2+/calmodulin-dependent serine/threonine-protein kinase, whose phosphorylation contributes to apoptotic cell death.77,78 DAPK1 participates in excitotoxicity in ischemic stroke. During ischemia, NMDAR overactivation promotes Ca2+ influx, activates Ca2+/calmodulin, and stimulates calcineurin phosphatase, which subsequently dephosphorylates and activates DAPK1.79 The latter is then transferred to the GluN2B subunit of NMDARs, aggravating ischemic injury.80 Preventing the interaction between GluN2B and DAPK1 attenuated neuronal excitotoxicity in mouse ischemic stroke models and downregulated the NMDAR current in vitro.80 In addition, NMDAR-regulated calcineurin activation contributes to DAPK1 activation, whereas NMDAR or calcineurin inhibition prevents DAPK1 dephosphorylation. DAPK1 inhibition protects against ischemic injury both in cultured neurons and in vivo, suggesting that potential treatments for ischemic stroke could be based on inhibiting DAPK1.81 It is interesting to note that the pro-survival signaling factor ERK serves as a downstream effector of DAPK1, and the DAPK1-ERK interaction could block the neuroprotective effect of ERK on experimental ischemic stroke, possibly by retaining ERK in neuronal cytoplasm.82
Postsynaptic density protein-95 (PSD95)/neuronal nitric oxide synthase (nNOS) signaling pathways and excitotoxicity-induced cell death
Neuronal NMDARs contribute to nitric oxide production, which is associated with calcium/calmodulin and is regulated by nNOS.83 NMDAR subunits bind directly to PSD95, which is composed of three PDZ domains.84,85,86 The binding of PSD95 to NMDAR and nNOS enhances Ca2+ influx, a hallmark of excitotoxicity.87,88 PSD95/nNOS signaling may play a pivotal role in ischemic stroke, as evidenced by the amelioration of neurological deficits in animals suffering from cerebral ischemia and whose nNOS activity was inhibited by either pharmacological or genetic means.89 Cerebral ischemia has been shown to enhance NMDAR/PSD95/nNOS interactions in neurons, thus further aggravating brain injuries after experimental ischemic stroke.90 All these results show that signaling through the PSD95/nNOS complex is crucial for excitotoxicity in ischemic stroke and contributes to the neurotoxic effects of extra-synaptic NMDARs.
Mitochondrial dysfunction, oxidative stress, and related signaling pathways
Mitochondria are essential for maintaining energy homeostasis. When ATP synthesis and energy balance are disrupted by a lack of glucose and oxygen, the status and function of mitochondria become substantially altered. Ca2+ influx leads to mitochondrial permeability transition pore (MPTP) opening and cytochrome c release.91,92 At the same time, insufficient ATP supply triggers mitochondrial membrane depolarization, which is characterized by the influx of Na+ and efflux of K+.93,94,95 Besides mitochondrial dysfunction, energy deficiency in cerebral ischemia leads to oxidative stress, which severely damages cells and brain tissues.96 Oxidative stress accompanies several pathological processes and results from increased ROS production,97 mostly via oxidative phosphorylation in the mitochondria.98 Considering the intimate link between ROS and mitochondrial metabolism, mitochondrial dysfunction is often related to oxidative stress pathologies. During ischemia, oxidative damage and excessive Ca2+ levels contribute to MPTP induction, which further promotes succinate release and mitochondrial damage-associated molecular patterns including the activation of downstream inflammatory responses.99,100,101,102 Consequently, all these damaging factors lead to neurotoxic and cell death processes, in which a plethora of signaling pathways are involved (Fig. 4).
Oxidative stress and mitochondrial dysfunctions and signaling pathways involved in ischemic stroke. MPTP mitochondrial permeability transition pore, ROS Reactive oxygen species, ATP Adenosine triphosphate, HIF-1 Hypoxia-induced factor, Nrf2 Nuclear factor E2-related factor 2, ARE Antioxidant response element, CK2 Casein kinase 2, PARP-1 Poly ADP-ribose polymerase 1, AIF Apoptosis-inducing factor, PINK1 PTEN induced putative kinase 1, NF-kB Necrosis factor-kB
Hypoxia-inducible factor (HIF) signaling pathway
HIF-1, a key transcription factor activated during cerebral ischemia and hypoxia, comprises two subunits: HIF-1α and HIF-1β.103,104,105 HIF-1 enhances the expression of several glycolysis-associated genes under hypoxic conditions, thus helping cells and tissues become accustomed to hypoxia.106 Also, HIF-1α expression strongly correlates with ROS levels, with HIF-1α chains stabilized by the large quantities of ROS generated under hypoxia.28,107 In a positive feedback loop, lack of oxygen and glucose due to ischemia may enhance HIF-1 expression, thereby causing oxidative stress and further stimulating HIF-1 activity.
Conversely, it has been reported by other studies that HIF-1α may also play protective roles in the regulation of energy metabolism, especially in neurons. Consequently, HIF-1α depletion in mouse embryo fibroblasts results in excessive ROS, reduced glycolytic metabolism, and cell death.108 Besides controlling ROS production, the activation of HIF-1α may benefit cellular homeostasis by maintaining the redox equilibrium.109 Knockout of HIF-1α has been shown to disrupt redox homeostasis and glucose metabolism, such as pentose phosphate pathway and glucose transportation in SHSY5Y cell lines cultured under oxygen-glucose deprivation.110 In summary, HIF signaling may be closely associated with oxidative stress. Although there is still debate whether HIF-1α signaling enhances oxidative stress or not, activation of HIF-1α may be closely associated with production of ROS and oxidative stress, which consequently affects cellular redox equilibrium and biological activities.
Nuclear factor E2-related factor 2 (Nrf2) signaling pathway
Nrf2 regulates cellular redox homeostasis and counteracts oxidative stress. Nrf2 activation protects individuals against cerebral ischemic damage. In the resting state, Nrf2 is coupled to Keap1, its specific cytoplasmic receptor. The structure of Keap1 changes upon electrophilic or oxidative stress. As Nrf2 is phosphorylated through the protein kinase C pathway, it becomes uncoupled from Keap1, leading to enhanced expression of various anti-inflammatory proteins, antioxidant enzymes, and growth factors.111,112 In ischemic stroke, oxidative stress caused by elevated ROS levels induces Nrf2 accumulation in the nucleus, where it binds to antioxidant response elements (ARE) and maintains normal mitochondrial function.113 In contrast, insufficient Nrf2 contributes to neuronal mitochondrial depolarization, ATP depletion, and respiratory function impairment. suggested the beneficial role of Nrf2 in mitochondria.114
A variety of downstream signaling pathways, including PI3K/Akt, ERK/mitogen-activated protein kinase (MAPK), and nuclear factor kappa beta (NF-κB), potentially mediate the antioxidant effect of Nrf2 during ischemia. The neuroprotective PI3K/Akt pathway induces the nuclear translocation of Nrf2, which in turn stimulates the production of various antioxidants.115,116 Likewise, ERK/MAPK signaling pathway during ischemia is associated with a variety of neuroprotective biological processes, such as preventing apoptosis or enhancing Nrf2 phosphorylation and translocation.117,118 Also, NRF2 and NF-κB signaling pathways closely interact with each other under a variety of circumstances. On the one hand, deletion of NRF2 results in increased inflammation, as well as high levels of NF-κB; on the other hand, the elevated expression of NRF2 inhibits NF-κB-regulated pro-inflammatory and immune responses.119 This show the neuroprotective effects of NRF2 against NF-kB-induced inflammatory responses in cerebral ischemia.
In summary, Nrf2 is a crucial player against oxidative stress and mitochondrial dysfunction in ischemic brain injuries, possibly via the regulation of various downstream signaling pathways.
Casein kinase 2 (CK2) signaling pathway
CK2, an important oncogenic kinase, is crucial for counteracting ROS accumulation.120 First, it exerts a protective effect by inhibiting NADPH oxidase via regulating Rac1, a GTPase which significantly activate NADPH oxidase, possibly through interactions with other subunits and link the cytosolic subunits with the cell membrane.121,122,123 Second, CK2 reportedly phosphorylates Janus kinase and signal transducer and activator of transcription 3 (STAT3), enabling ROS detoxification by superoxide dismutase 2 (SOD2).124,125 Third, CK2 activates HIF-1α and phosphorylates NF-κB to promote the release of vascular endothelial growth factor (VEGF) and angiogenic proteins under in vitro hypoxic conditions.126,127 Conversely, CK2 inhibition in the ischemic region contributes to poly (ADP-ribose) polymerase 1 accumulation, which leads to the release of mitochondrial cytochrome c and apoptosis-inducing factor (AIF), with subsequent activation of downstream apoptotic events.120 These findings reveal the protective effect of CK2 against oxidative stress and inflammation, while promoting the release of angiogenic factors under hypoxia.
Notably, CK2 was shown to activate ROS-generating NADPH oxidase isoform 2 in an experimental ischemic stroke model, which induced AIF release into the mitochondria and subsequent DNA damage and apoptosis.128 Moreover, studies have shown that cyclin dependent kinase 5 and AKT/GSK3β are activated by CK2 in ischemia/reperfusion injuries.129 Given that inhibition of cyclin dependent kinase 5 reportedly alleviates cerebral ischemic stroke-induced damage, CK2 may do more harm than good.130,131 Taken together, the CK2 signaling pathway and related molecules play either protective or detrimental roles in ischemic stroke, especially in relation to oxidative stress. Importantly, downstream effectors of CK2 may function as potential targets against ischemic stroke.
Mitophagy and related signaling pathways
Mitophagy describes the process whereby mitochondrial content is taken up by mitochondria-derived vesicles and then transferred to lysosomes or peroxisomes for degradation. Mitophagy is essential for maintaining cellular homeostasis and serves as a protective strategy in various central nervous system diseases.132 Signaling pathways, such as PTEN induced kinase 1 (PINK1)/Parkin, Bcl2-interacting protein 3 (Bnip3), BNIP3-like, and FUN14 domain containing 1 pathways, are reportedly involved in mitophagy during ischemia–reperfusion. In the reperfusion stage, the levels of the free radical ONOO− are increased, which leads to dynamin related protein 1 recruitment to the mitochondria and PINK1/Parkin-associated mitophagy.133 Meanwhile, elevated ROS levels upregulate the levels of Parkin RBR E3 ubiquitin protein ligase, which is recruited by PINK1, further exacerbating mitophagy.134 Interestingly, PINK1-regulated mitophagy is mechanistically associated with MPTP opening, whereas Bnip3-induced mitophagy is independent of MPTP.135,136
The activated mitophagy pathway may alleviate oxidative stress-induced cell injuries by promoting the degradation of damaged mitochondria.137 Enhanced mitophagy has been shown to possibly ameliorate ROS accumulation in cerebral ischemic stroke.138 In conclusion, mitophagy is significantly involved in the pathophysiology of ischemic stroke, along with the activation of various signaling pathways. Targeting these signals could potentially ameliorate the pathological changes and symptoms of ischemic stroke; however, the mechanisms remain to be elucidated.
Cell death signaling pathways in ischemic stroke
Damage caused by excitotoxicity, oxidative stress, and mitochondrial dysfunctions in ischemic stroke may induce a variety of cellular signaling cascades, which lead neural cells to undergo either programmed or unprogrammed death.139 Usually, programmed cell death includes apoptosis and autophagy, which are normal cellular functions,140 whereas unprogrammed cell death involves necrosis and is likely caused by external stimuli.141 Lack of oxygen and glucose in the ischemic core often leads to irreversible necrosis; in contrast, relatively minor damage in the penumbra is responsible for reversible death processes, such as apoptosis and autophagy.40 A variety of signaling pathways are highly involved in cell death, and they could either enhance or inhibit the process (Fig. 5).
Cell death signaling pathways involved in ischemic stroke. GSK3β Glycogen synthase kinase-3β; Bcl-2 B-cell lymphoma-2; ERK Ras/extracellular signal-regulated kinase; CAMKs Ca2+/calmodulin-dependent protein kinases; MAPK Mitogen-activated protein kinase; TNF Tumor necrosis factor; mTOR mammalian target of rapamycin; AMPK 5′-AMP-activated protein kinase; FADD Fas-associating protein with a novel death domain; TRADD TNFRSF1A Associated Via Death Domain; RIPK Receptor-interacting protein kinase; MLKL Mixed lineage kinase domain-like protein; RIP1 Receptor interaction protein 1; RIP3 Receptor interaction protein 3; PGAM5 Phosphoglycerate Mutase Family Member 5; MLKL mixed lineage kinase domain like pseudokinase; Atg5 Autophagy related 5; Atg12 Autophagy related 12; TFEB Transcription factor EB; ULK1 Unc-51 Like Autophagy Activating Kinase 1; AMPK 5′-AMP-activated protein kinase; mTOR mammalian target of rapamycin; Apaf-1 Apoptotic peptidase activating factor 1
Signaling pathways related to autophagy in stroke
Autophagy is a self-protective pathway that maintains cell homeostasis and promotes cell survival by degrading circulating long-lived proteins, misfolded and aggregated proteins, and damaged organelles to obtain energy or in response to cellular stress.142 Subsequently, autolysosomes are newly formed to cleave the cargo for subsequent recycling.143 Emerging evidence indicates that autophagy is activated in various cell types following ischemic stroke, including neurons, glial cells, and endothelial cells. Autophagy can exert either beneficial or detrimental effects on cerebral ischemic injuries, as moderate autophagy may help degrade aggregated proteins,144,145,146 whereas inadequate or excessive autophagy may eventually lead to cell death.147 The dual role of autophagy in ischemic stroke may be explained by the involvement of multiple signaling pathways, such as mammalian target of rapamycin (mTOR), 5′-AMP-activated protein kinase (AMPK), MAPK, NF-κB, p53, HIF-1, and Bcl2 pathways.148
mTOR-related signaling pathways
mTOR is a serine/threonine protein kinase that comes in two major forms: mTORC1 (rapamycin-sensitive) and mTORC2 (rapamycin-insensitive). The former is responsible for cell growth and cell cycle progression, whereas the latter contributes to cellular skeleton formation. mTOR is a key regulator of the initial phase of autophagy, as it senses changes in signaling within the cell. Usually, mTOR limits autophagy by inhibiting phosphorylation of the Atg1/ULK1 protease complex.149 During ischemic stroke, mTOR interacts with multiple signaling pathway components that regulate autophagy,150 including PI3K/Akt, AMPK, and MAPK.
Akt, which is involved in various biological processes, can affect cellular autophagy through multiple signaling pathways, of which PI3K/Akt/mTOR is the most important one.151,152,153 The PI3K/Akt signaling pathway was suggested to exert a neuroprotective effect on ischemic stroke, possibly by regulating mTORC and, hence, autophagy in both mice MCAO models and OGD-treated primary neurons in vitro.154 Another study found that inhibition of mTOR by rapamycin activated the PI3K/Akt signaling pathway and, in turn, autophagy, thus protecting neonatal rats against hypoxia.155 Interestingly, homocysteine exerts a neurotoxic effect, possibly owing to excessive autophagy following downregulation of PI3K/Akt/mTOR signaling in neural stem cells, suggesting the bi-faceted role of autophagy in ischemic stroke.156
AMPK is a member of the serine/threonine kinase family and serves as an important endogenous defense factor against cerebral ischemia.151 During cerebral ischemia or hypoxia, energy deficiency and the consequent elevated AMP/ATP ratio contribute to AMPK phosphorylation, which activates autophagy to enhance energy production.157 Several studies on animal experimental ischemic stroke models have found that protective autophagy can be induced by regulating the AMPK/mTOR signaling pathway, thereby alleviating cerebral ischemic injury.105,158 A variety of downstream and upstream factors contribute to AMPK activity in both in vivo experimental ischemic stroke models and in vitro. Mechanistically, AMPK inhibits mTORC1 activity by phosphorylating and stimulating the TSC1/TSC2 complex during ischemia, thereby promoting autophagy.24 Furthermore, during ischemic stroke, Ca2+ overload can activate AMPK via calcium/calmodulin-dependent protein kinase beta and thus activate autophagy via the AMPK/mTOR pathway.159 Meanwhile, cytosolic p53 has been shown to directly inhibit autophagosome formation, whereas activated p53 functions to promote AMPKβ expression and inhibits mTOR expression to promote autophagy.160 These molecules contribute to the function of AMPK in autophagy in ischemic stroke.
MAPK is another important regulator of autophagy associated with ischemic stroke.161 MAPKs act as upstream regulators of mTORC1 and modulate autophagy through the MAPK/mTOR signaling pathway in ischemic stroke.162 Wang et al. found that autophagy protected against animal experimental cerebral ischemic injury through induction of an Akt-independent MAPK/mTOR signaling pathway, wherein ERK negatively regulated mTORC1.163 In contrast, Zhang et al. found that ERK negatively controlled autophagy by activating mTOR, which contributed to neuronal survival after experimental ischemic stroke injuries.164 Furthermore, an in vitro OGD/R study revealed that ERK could modulate autophagy by regulating mTOR in oxygen-glucose deprivation models.165 Therefore, the MAPK/ERK signaling pathway family could exert either a positive or negative regulation over mTOR in ischemic stroke; however, the exact mechanisms will require further investigation.
Beclin1/Bcl2 signaling pathway
Beclin1 plays a significant role in the early stage of autophagy. Local cerebral ischemia can upregulate Beclin1 expression and induce autophagy-like cell death, suggesting the involvement of Beclin1/Bcl2 signaling in the regulation of autophagy in ischemic stroke.166 Qi et al. found that Bcl2 phosphorylation after cerebral ischemia in rats perturbed the Beclin1-Bcl2 complex and triggered distal ischemic conditional autophagy, thereby alleviating mitochondrial damage.167 Moreover, peroxisome proliferator-activated receptor γ (PPAR-γ) expression increases during experimental cerebral ischemic injury. Activated PPAR-γ inhibits Beclin1-mediated autophagy, possibly by upregulating the expression of Bcl2/BclXL.168 Thus, either detrimental or neuroprotective factors impact on Beclin1-Bcl2 signal activities, subsequently affecting autophagy in ischemic stroke.
Other autophagy-related pathways
Several other signaling pathways are also involved in autophagy during ischemic stroke. Under ischemic conditions, the accumulation of misfolded proteins and disruption of Ca2+ homeostasis lead to self-protective events in the unfolded protein response (UPR) pathway.151 The UPR can promote autophagy by stimulating the PERK/eIF2 and Ire1/TRAF2/JNK pathways.169 The UPR signaling pathway mediator, activating transcription factor 6, can also affect autophagy in stroke.105 Rab7, a lysosome-associated small Rab GTPase, regulates autophagy during cerebral ischemia and provides neuroprotection against ischemic brain injury.169 Specifically, Rab7 enables the fusion of autophagosomes with lysosomes, thus affecting autophagosome maturation, lysosome formation, and maintenance of lysosomal function.164 However, the actual mechanisms of UPR signaling and that of Rab7 in ischemic stroke require further investigation.
Signaling pathways related to apoptosis in stroke
Apoptosis is a highly regulated, energy-dependent form of cell death characterized by distinct morphological changes, such as cell shrinkage, cytoplasmic condensation, nuclear membrane breakdown, and apoptotic body formation.170 Apoptosis, especially neuronal apoptosis, is involved in the pathology of post-ischemic stroke. Cerebral ischemia leads to a decrease in ATP, which causes cellular apoptosis in the ischemic penumbra. Anti-apoptotic signals enable the potential recovery of dysfunctional neurons, while pro-apoptotic signals contribute to neuronal death, thus modulating the balance between pro-apoptotic and anti-apoptotic signals serve as potential therapeutic targets.171 Stroke triggers two principal apoptotic pathways: the extrinsic (or death receptor) pathway and the intrinsic (or mitochondrial) pathway. Initiated by a variety of both external and internal damaging stimuli, apoptosis eventually triggers a caspase cascade, which leads to the cellular injuries experienced during ischemic stroke.
Apoptosis by the extrinsic/death receptor pathway
The extrinsic apoptotic pathway is triggered by the combination of ligands, including TNF-α, FasL, and TRAIL, and the corresponding death receptors (TNF-α receptor 1, Fas/CD95/APO1, and TRAIL-R, respectively) on the cell surface.172 In the event of an ischemic stroke, the receptor recruits the death domain adaptor proteins FADD and TRADD, which form a complex by binding to procaspase-8.173 This complex induces a variety of downstream damaging processes and eventually leads to activation of caspase-8.174 Once activated, caspase-8 triggers downstream effector caspases, either directly via proteolytic cleavage or indirectly by cleaving BH3-interacting domain (BID) to its truncated form, which mediates apoptotic cell death via the mitochondria-dependent pathway.175,176 Furthermore, during ischemic injury, neurons and glial cells release TNF-α, increasing Fas mRNA and protein levels. These could function as stimuli for the extrinsic apoptotic pathway and ultimately lead to neuronal death.31
Apoptosis by the intrinsic/mitochondrial pathway
The intrinsic pathway, also called the mitochondrial pathway, is a receptor-independent signaling cascade that affects mitochondrial energy metabolism. Apoptotic stimuli, such as excessive Ca2+ accumulation and oxidative stress, mediate mitochondrial cell death.177,178 Lack of ATP due to oxygen and glucose deficiency results in cellular depolarization and excessive glutamate release, both of which further enhance Ca2+ influx.179,180,181,182,183 Ca2+ overload triggers calpain activation, which mediates the cleavage of Bcl2-interacting BID into its truncated active form, together with caspase-8 in the death receptor pathway.177,184 Truncated BID interacts with pro-apoptotic Bcl2 family members, forming a dimer and causing MPTP opening.185 These changes trigger the release of various pro-apoptotic factors, including cytochrome c, endonuclease G, and AIF,186 which ultimately lead to apoptosome formation by binding to apoptotic protease activating factor-1.187 Upon apoptosome formation, procaspase-9 becomes activated into caspase-9, which triggers downstream effector caspases (caspase-3, caspase-6, and caspase-7) that promote neuronal cell apoptosis.31
p53-mediated apoptotic pathway
Besides the extrinsic and intrinsic apoptotic pathways, another programmed cell death process activated by ischemic stroke depends primarily on p53. The tumor suppressor p53 becomes activated in ischemic areas of the brain, whereby it contributes to neuronal apoptosis. By translocating to the nucleus and binding to its specific DNA site, p53 induces apoptosis in ischemic brain cells.188 A plethora of detrimental signals could stimulate p53. One is DNA damage, which can activate the apoptotic pathway via p53 phosphorylation.189 Another is represented by hypoxia and oxidative stress, which can also upregulate p53 protein levels.190 Concurrently and mechanistically, some upstream cascade proteins, including JNKs, p38, DAPK, ASK1, and Notch may also lead to p53 activation.31 All these factors stimulate p53 activity and lead to cellular apoptosis in ischemic stroke.
p53-induced apoptosis involves a variety of downtream genes and molecules, such as the pro-apoptotic genes Bax, Noxa, p53AIP1, and PUMA, all of which act directly on mitochondria to induce apoptosis.190 Subsequently, p53 leads to the intrinsic apoptotic pathway, releasing pro-apoptotic factors, forming an apoptosome, activating effector caspases, and inducing neuronal apoptosis.191 In addition, p53 mediates apoptosis by inducing the expression of paternally expressed 3 and blocking cell survival signaling.190 All these processes contribute to the onset and progress of p53-mediated apoptosis.
Notch signaling pathways in apoptosis
Notch signaling pathways, the most important component of which is Notch1, plays pivotal roles in a variety of biological processes in the central nervous system. Activation of Notch1, as well as other signaling pathways, including NF-κB, p53, contributes to neuronal death processes. It has been reported that p53 and Pin1 are highly associated with Notch and NICD in ischemic stroke. As an important mediator of apoptosis, p53 is activated by damages such as hypoxia.192 The combination of Notch with p53 is crucial for neuronal apoptosis during ischemic stroke, which majorly involves stabilization of p53 and transcriptional regulation of p53 and NICD target genes.193 Besides, Pin1, an isomerase that regulates p53 transactivation, is deemed to be involved in the pathogenesis of ischemic stroke, which is also related with Notch signaling and is responsible for ischemic stroke-induced neuronal death and neurological deficits.194
In the meantime, studies have shown that Notch plays significant roles in modulating NF-κB-related cell death pathways. For instance, γ-secretase inhibitors down-regulate levels of NICD and protect against ischemic stroke damages. This protection effect is possibly via regulating NF-κB-related signals.195 Meanwhile, γ-secretase inhibitors block Notch signals and alleviates microglial activation.196 All these reveal the interactions between Notch and NF-kB pathways in both neurons and microglia in cerebral ischemia.
Besides, it has also been reported that ischemic stroke increases HIF-1α expression levels, which could directly bind with NICD and NF-κB.197,198 Inhibition of both γ-secretase/Notch and HIF-1α significantly reduced cell apoptosis, while enhanced expression of NICD and HIF-1α increased NF-kB levels. All these show the close interactions among NICD, p53, HIF-1α and NF-kB, which are highly associated with neuronal death processes, especially neuronal apoptosis in ischemic stroke.
Necrosis or necroptosis in cerebral ischemia
Following the onset of stroke, cerebral blood flow in the infarct area becomes significantly reduced, which induces necrotic death of resident neurons.199 Necrosis is an unprogrammed cell death process caused mainly by decreased ATP in ischemia.31 Recent studies have reported necrosis to be a highly regulated process involving various signaling pathways.200 The major downstream signaling pathways controlled by TNF-α include receptor-interacting protein kinase (RIPK1 and RIPK3) and mixed lineage kinase domain-like pathways.201
Facing cerebral ischemic damage, a complex containing TRADD, RIPK1, and ubiquitin 3 ligases is recruited by the combination of TNF-α and its TNFR1120 receptor. Complex IIb is subsequently activated in both ischemia and hypoxia, contributing to the phosphorylation and association of RIPK1 and RIPK3.202,203,204 Within the complex formed by this association, mixed lineage kinase domain-like is further activated by RIPK3, which eventually leads to cell death.205 Concurrently, a cascade of inflammatory reactions, including secretion of pro-inflammatory cytokines, favors necrosis damage and exacerbates ischemic brain injuries.206
Pyroptosis and ferroptosis in ischemic stroke
Majorly observed in ischemic penumbra, pyroptosis potentially induces pro-inflammatory pathways in ischemic stroke.207 During the process of pyroptosis, cells get swollen and cellular organelles are released to induce inflammation, in which caspase-1 is activated and form inflammasomes.208 All these contribute to pyroptotic cell death and secretion of inflammatory factors, such as IL-1β and IL-18.208,209
Another less mentioned but important cell death pathway is ferroptosis. Ferroptosis is regulated by peroxidation, which requires sufficient accessible iron.210 In ischemic brain regions, enhanced cellular excitotoxicity leads to the decrease in activity of GPX4 and reduction in GSH production,211 which accumulates excessive ferric ion and subsequently induces ferroptotic cell death. Also, damaged blood-brain barrier induces the iron to be transferred into neuronal cells, which further enhances ferroptosis.212 From another perspective, ferroptosis is also closely associated with oxidative stress, in which signaling pathways such as calcium-related signals, ATF4 and Keap1-Nrf2 signaling pathways play a role.213 Despite being less frequently discussed, ferroptosis may also be greatly involved in the pathogenesis of ischemic stroke,with a variety of signaling pathways potentially participating in.
Neuroinflammation, BBB disintegration, and related signaling pathways in ischemic stroke
Inflammation is a key component of ischemic stroke pathologies. Existing in nearly all stages of ischemic stroke, neuroinflammation is initiated by the release of DAMPs from injured or dead cells. These DAMPs, including adenosine, heat shock proteins, high mobility group box 1, and interleukin-33, are subsequently recognized by corresponding immune cells, and then trigger a variety of downstream signaling pathways.214,215 During the whole process of inflammation, various immune cells including microglia, macrophages, and T lymphocytes are activated.216,217 Also, the production of inflammation-related cytokines are stimulated, as well as interferons or chemokines including monocyte chemoattractant protein-1 (MCP-1).218 Upregulation of levels of several adhesion molecules assists leukocytes in adhering to vascular surfaces,219 which facilitates the infiltration of immune cells. An abundance of pro-inflammatory cytokines leads to BBB disintegration via activation of endothelial cells and pericytes,220,221 along with the release of specific markers, such as von Willebrand factor and nerve growth factor.222,223 BBB leakage results in cerebral edema, as well as astrocytic aquaporin 4 expression.224,225 All these factors, including MCP-1, von Willebrand factor, nerve growth factor, and aquaporin4, could induce immune cell adhesion to the vascular wall and then infiltrate into the central nervous system, consequently contribute to BBB disintegration and cellular edema.
Several signaling pathways are involved in neuroinflammatory processes and BBB breakdown in ischemic stroke; they are strongly associated with each other and determine the pathophysiology of cerebral ischemia (Fig. 6).
Neuroinflammation, BBB breakdown and related signaling pathways involved in ischemic stroke. DAMPs Damage-associated molecular patterns; AQP4 Aquaporin 4; HMGB1 High-mobility group box protein 1, TLR2 Toll-like receptor 2; TLR4 Toll-like receptor 4; MAPK Mitogen-activated protein kinase; NF-kB Necrosis factor-kB; NLRP3 Nod-like receptor protein-3; MCP-1 monocyte chemoattractant protein-1; MIP Macrophage inflammatory protein; CCL2 Chemokine-chemokine ligand 2; IL-1β Interleukin-1β; IL-6 Interleukin-6; TNF Tumor necrosis factor; BBB Blood-brain barrier; S1PRs Sphingosine-1-phosphate receptor; VCAM Vascular cell adhesion molecule; LFA Lymphocyte Function-associated Antigen; ICAM Intercellular cell adhesion molecule; DC Dendritic cells; MMP Matrix metalloproteinase
Cytokine- and chemokine-induced signaling pathways in neuroinflammation
During ischemic stroke, microglia, which represent the main resident immune cells in the brain, are the first cells recruited to infarct lesions. They secrete both pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor alpha (TNF-α), and anti-inflammatory cytokines, including IL-1R antagonist (IL-1Ra) and IL-10.15,226,227,228 Together, these cytokines form a complex signaling network in ischemic stroke-induced neuroinflammation.
Cytokines
TNF is the most studied cytokine in ischemic stroke; it comprises a secreted form (solTNF) and a transmembrane form (tmTNF).229 The signal from both types of TNF is transferred via two different receptors, TNFR1 and TNFR2, respectively.230 The solTNF-TNFR1 signal is deemed responsible for pro-inflammatory effects of TNF, which trigger cell death signaling pathways. Instead, TNFR2 promotes cell growth and regeneration.204,230,231 Given the important regulatory role of TNF signals in inflammation and other neurological processes, TNFs are likely involved in the pathophysiology of ischemic stroke. Genome-wide association studies have identified a polymorphism in the TNF gene, which enhances stroke susceptibility, suggesting a pivotal role of TNF/TNFR1 in the etiopathogenesis of stroke.232 Moreover, TNF levels are significantly upregulated upon cerebral ischemia, whereby they mediate neuronal plasticity.233 As previously mentioned, TNF is secreted mainly by microglia, which protect against cerebral ischemia. Specific myeloid cell-TNF-knockout mice have been found to have larger infarct volumes and more severe neurological deficits than control mice.227,234 Removal of solTNF in mice reportedly alleviates the symptoms and pathology of cerebral ischemia, suggesting that elimination of solTNF and retention of tmTNF ameliorate cerebral ischemic injuries.235 Thus, different forms of TNF impact ischemic stroke, corroborating the important role of TNF in this disease.
The IL-1 family constitutes a huge and complex network of pleiotropic pro-inflammatory cytokines closely involved in regulating immune cells and inflammatory processes.236 Among IL-1 family members, IL-1α, IL-1β, and IL-1Rα have been studied in detail in relation to ischemic stroke. A polymorphism in the IL-1A gene has been associated with increased susceptibility to stroke;237 conversely, a polymorphism in the IL-1B gene lowers stroke risk.238 IL-1α expression is significantly increased in cerebral ischemia.228 Platelet-derived IL-1α contributes to neurovascular inflammation and causes the infiltration of neutrophils to ischemic lesions.239 Primarily secreted by microglia and macrophages,15,240 IL-1β affects neurons, glial cells, and the vasculature.241 IL-1β levels are significantly increased in the cerebrospinal fluid at days 2 and 3 post-stroke, suggesting a predictive value in stroke pathophysiology.233,242 The IL-1 family has been shown to exacerbate stroke pathology, as revealed by reduced infarct volumes in experimental ischemic stroke models of IL-1α/β knockout mice.243 Conversely, IL-1β administration worsens the outcomes of mice subjected to ischemic stroke.244 Overall, the IL-1 family plays a detrimental role in the pathophysiology of cerebral ischemic stroke and could serve as a potential therapeutic target.
Another vital member among pro-inflammatory interleukins is IL-6, which is secreted by a variety of cells, including monocytes, neurons, and glial cells.245,246 The IL-6 signaling pathways can be classified into classic signaling, which requires IL-6R and gp130, and trans-signaling, whereby IL-6 is linked to sIL-6R.247 Reportedly, the former is deemed to be neuroprotective and helps maintain neuronal homeostasis,248 whereas the latter contributes to IL-6-induced pro-inflammatory outcomes.249,250 IL-6 levels are upregulated during cerebral ischemia, which correlates with infarct volumes and survival rates.251,252 Interestingly, IL-6 levels are seemingly upregulated by IL-1β.253 The fact that brain-derived IL-6 promotes neurogenesis after stroke, and thus contributes to long-term functional recovery, points to its potential neuroprotective effect following cerebral ischemia.254 Even though only a few studies have focused on the role of IL-6 in ischemic stroke, its pleiotropic effects are worth further investigation.
Contrary to the aforementioned pro-inflammatory cytokines, IL-10 is released primarily by type-2 helper T cells and serves as an anti-inflammatory cytokine, reducing inflammation and limiting cellular apoptosis.255 IL-10 gene polymorphism is associated with the risk of stroke subtypes.256 In experimental ischemic stroke models, transgenic mice with enhanced IL-10 expression showed reduced infarct volumes and cellular apoptosis.257 Likewise, clinical studies have shown that low IL-10 levels correlate with poor stroke outcomes, worse neurological deficits, and extravagated inflammatory reactions.258,259,260 These results indicate that the anti-inflammatory properties of IL-10 serve as a potential clue for the diagnosis and prognosis of ischemic stroke.
Chemokines
In addition to cytokines, chemokines represent another group of small signaling proteins that contributes to the inflammatory processes in ischemic stroke. Immediately after cerebral ischemia, pro-inflammatory cytokines, such as TNF-α and IL-1β, induce the secretion of chemokines, such as MCP-1, fractalkine, macrophage inflammatory protein 1, microglial response factor-1, and cytokine-induced neutrophil chemoattractant.261 Chemokine-chemokine ligand 2 (CCL2) and its corresponding receptor, CCR2, are involved in regulating the inflammatory response in ischemia, possibly via immune cell recruitment and adhesion to cerebral endothelial cells.151,262 CCL2 expression becomes enhanced in the ischemic penumbra, cerebrospinal fluid, and serum after ischemia or ischemia-reperfusion.153,263 Moreover, CCL2/CCR2 expression correlates positively with infarct area and lesion enlargement,151,262 and enhanced CCL2 expression further aggravates ischemic injury in mice.153 Ischemic damage significantly increased MCP-1 mRNA (CCL2) expression, which further exacerbated ischemic brain injury, together with abundant infiltration of inflammatory cells in an experimental ischemic stroke model.264 All these findings suggest the detrimental role that CCL2/CCR2 signaling pathways play in ischemic stroke.
Besides the most frequently discussed CCL2, other chemokines are also involved in the pathogenesis of ischemic stroke. For instance, CCL3 has been reported to be upregulated in experimental ischemic stroke models.265 Consistently, external administration of CCL3 to brain ventricles exacerbated ischemia-induced injuries.266 Meanwhile, another chemokine CCL5 has been found to regulate ischemia/reperfusion (I/R) injuries in experimental ischemic stroke models.267 Clinical studies have also shown that plasma CCL5 levels were increased in symptomatic patients in comparison with asymptomatic ones.268 Besides the CC chemokine family, the CXC chemokines, also plays crucial roles in ischemic stroke pathogenesis. Among them, those ELR+ CXC chemokines, including CXCL1, CXCL2, and CXCL8, directly function to neutrophils toward ischemic brain regions; however, those ELR− CXC chemokines, including CXCL10, CXCL12, and CXCL16, mainly induce Th1-cell infiltration in postischemic inflammation.269
High-mobility group box protein 1 (HMGB1)/Toll-like receptor (TLR) and NF-κB signaling pathways in neuroinflammation
Various immune cells, as well as the corresponding cellular products, are associated with oxidative stress and necrosis activate the innate immune system, probably via the TLR signaling pathway. TLRs, which are expressed on both the cell surface and in the intracellular space, regulate the status and function of numerous immune cells.270,271,272,273,274,275 TLR signaling can be categorized based on two major downstream adaptor proteins: myeloid differentiation primary response 88 (MyD88)-dependent and adapter-inducing interferon-β-dependent pathways.276 Both TLR signaling pathways activate NF-κB, which subsequently triggers the release of pro-inflammatory cytokines.277,278,279
Interestingly, TLRs may act as another double-edged sword in ischemic stroke. In case of relatively moderate ischemic injury, TLR2 and TLR4/NF-κB signaling pathways are inhibited, whereas interferon regulatory factor 3 signaling is enhanced. Both of these processes exert neuroprotective effects on ischemia.280 Pretreatment with TLR2, TLR3, TLR4, TLR7, or TLR9 agonists alleviates the symptoms and pathological damage in various ischemic stroke models.281,282 Administration of lipopolysaccharide prior to ischemic insult protects against cerebral ischemia, possibly by modulating the TLR4 signaling pathway and inhibiting NF-κB after ischemic stroke attack.283 In contrast, elevated levels of plasma lipopolysaccharide appear to promote the expression of TLR4, causing the release of inflammatory cytokines, larger infarct volumes, and more severe functional deficits in rat cerebral ischemia models.284 These seemingly contradictory results suggest that LPS modulation of TLR4 response possibly depends on whether activation occurs before or after ischemic insult.
One key component in the TLR-related signaling pathway is HMGB1, which triggers downstream neuroinflammatory responses during stroke.285 HMGB1 levels are significantly elevated in the brain, specifically in microglia, astrocytes, and blood vessel cells, which are closely associated with neuroinflammation and cellular stress such as stroke.286,287,288,289 As one of the major ligands for TLRs, extracellular HMGB1 interacts with TLR2 or TLR4 and in turn NF-κB to elicit pro-inflammatory reactions.280,290,291 Moreover, the release of HMGB1 activates TLR4 and enhances IL-1β production through Nod-like receptor protein 3 (NLRP3) inflammasome activation.292 Furthermore, HMGB1 enhances the secretion of several pro-inflammatory cytokines, including inducible NOS, cytochrome c oxidase subunit 2, IL-1β, and TNF-α, promoting neuronal cell death during ischemia.293 These results suggest that both inflammatory reactions and cell death signaling pathways are induced by HMGB1/TLR signals in ischemic stroke, possibly aggravating ischemic injury.
MAPK signaling pathway in inflammation and BBB dysfunction
MAPK comprises three main effectors: ERK1/2, JNK, and p38.294 Stress-activated protein kinases, JNK, p38 MAPK, and ERK exert detrimental effects during cerebral ischemia.295 Specifically, the MAPK signaling pathway is activated soon after the onset of ischemic injury, and p38 MAPK regulates the expression of various pro-inflammatory cytokines.296 Activation of the p38/MAPK/AR-related signaling pathway has been shown to promote the microglial pro-inflammatory phenotype in cerebral ischemia.297 Activation of MAPK/ERK signaling and consequent stimulation of metalloproteinase (MMP) expression could exacerbate BBB damage in ischemic stroke, further enhancing the expression of pro-inflammatory factors.298 Similarly, BBB damage in cerebral ischemia induced by a high-salt diet, has been associated with the p38/MAPK/SGK1 signaling pathway.299 These results suggest that MAPK-related signaling pathways exacerbate ischemic brain injury, possibly by enhancing neuroinflammatory processes and BBB dysfunction.
MMPs and BBB dysfunction in ischemic stroke
MMPs are crucial for the function and structure of the BBB in both human and animal stroke models.300,301 The elevated production of MMPs and myeloperoxidase in ischemic stroke favors BBB breakdown.302 In particular, MMP9 induces proteolysis of the BBB basal lamina.300,301 Clinical studies have shown that baseline MMP9 serves as an important indicator of BBB disruption in ischemic stroke and is related to the hyperintense acute reperfusion injury marker used in magnetic resonance imaging.303 Hypothermia followed by rapid rewarming enhances the permeability of the BBB in ischemic stroke, along with elevated MMP9 expression levels and damage to tight junctions.304 MMP12 levels have been found to be elevated in rat cerebral ischemic stroke models, whereas suppressing MMP12 alleviates the symptoms induced by ischemia.305 Concurrently, MMP2 may participate in the pathophysiology of ischemic stroke, together with VEGF signaling. The latter is likely involved in the initial stages of ischemic stroke, whereby hypoxic preconditioning exacerbates BBB injury and brain edema.306 Furthermore, it has been shown that recovery from BBB damage is associated with both the MMP2 and VEGF pathways in acute cerebral ischemia, suggesting a close link between MMP2 and VEGF.307
Sphingosine-1-phosphate receptor (S1PR)-related signaling pathways during neuroinflammation in ischemic stroke
S1PRs form a group of G protein-coupled receptors abundant in microglia and are thought to regulate inflammatory responses in ischemic stroke.308 In vitro studies have shown that the addition of S1P to microglia subjected to oxygen-glucose deprivation/reperfusion exacerbates hypoxia-induced neuronal apoptosis.309 In experimental ischemic stroke models, sphingosine kinase 1 phosphorylates sphingosine to S1P, which binds to S1PR3 and confers microglia a pro-inflammatory phenotype. Sphingosine kinase 1 enlarges the brain infarct volume and exacerbates neurological symptoms by upregulating the expression of pro-inflammatory cytokines.310 Intriguingly, the S1PR agonist fingolimod has been recently reported to switch microglia from a pro-inflammatory to an alternatively activated phenotype in a chronic hypo-perfused ischemic stroke model in mice.311 Thus, the pro-inflammatory mechanism of S1PRs in ischemic stroke requires further exploration.
Inflammasome activation in ischemic stroke
Inflammasomes are large multiprotein complexes,312,313 which can mediate neuroinflammation and contribute to neural cell death in ischemic stroke.314 Both in vivo and in vitro model studies suggest that the NLRP3 inflammasome plays a pivotal role in microglia-associated neuroinflammation in ischemic stroke, possibly through alterations to the microglial phenotype.315 These effects may be linked to activation of the NF-κB signaling pathway.316 Additionally, NLRP1 is related to cerebral ischemic injuries, and its inhibition alleviates neuroinflammation in ischemia.317 Thus, inflammasome activation, either via NLRP1 or NLRP3, contributes to the pathogenesis of ischemic stroke and could provide a therapeutic target against cerebral ischemia.
Microglial phagocytosis and complement activation
Microglia functions as the major phagocyte in the central nervous system, which is responsible for myelin debris clearance and pruning synapsis.318 It has been reported that microglia phagocytose tissue debris in experimental ischemic stroke model, which contribute to tissue repair and neuronal network reconstruction.319,320 However, other studies have also pointed out that over-enhanced microglia engulfment exacerbates cerebral ischemia-induced brain injuries.321,322 Hence, microglial phagocytosis may play both beneficial or detrimental roles in ischemic stroke. Microglia could engulf a variety of dying cells and debris, in which a plethora of signaling pathways are involved. TMEM16F is expressed by stressed neurons in ischemic stroke, which induces neurons to expose phospholipid phosphatidylserine (PS), an ‘eat-me’ signal. Consistently, knockdown of TMEM16F hindered microglial phagocytosing viable neurons in the penumbra after experimental ischemic stroke.323 Besides, triggering receptor expressed on myeloid cells (TREM2) signaling pathways are deemed to be greatly involved in microglial phagocytosis in ischemic stroke. TREM2 deficiency dampens microglial phagocytosis of neurons, which further exacerbates ischemic brain injuries,319 indicated the neuroprotective role of Trem2 in ischemic stroke.324
Another part of phagocytosis is the complement system, including C1q and C3. Upon activation, C3 is cleaved into C3a and C3b, of which C3b as well as its receptor, CR3, function together to regulate dying cells clearance.325,326 Meanwhile, C1q, the biggest component of the C1 complex, has been reported to strengthen microglial clearance of apoptotic cells in ischemic stroke.327 After ischemic stroke, microglial phagocytosis of both synapses and neurons was directed by activation of complement, which eventually contributes to cognitive decline.328,329 Thus, with a variety of signaling pathways involved, activation of the complement system may also be closely interacted with microglial phagocytosis, which possibly, greatly influence the pathologies of ischemic stroke.
Therapeutic approaches targeting pathophysiological signaling pathways involved in ischemic stroke
So far, the only drug approved by FDA for treating ischemic stroke is tissue plasminogen activator (tPA), which breaks down the blood vessel clot.8 This therapy has several limitations, such as the therapeutic window is only 4.5 h, and treatment outside the therapeutic window could possibly result in cerebral hemorrhage.330 Progress have been made in discovering new therapeutic approaches against ischemic stroke. Current studies have shed lights on micro-RNA therapies, in which expression levels of miRNA are changed and apoptosis-related genes are subsequently mediated.331 Another potential treatment is cell therapy, which utilizes stem cells to differentiate.332 However, therapeutic approach is quite limited, and more research are need to discover new potential therapeutic strategy for ischemic stroke.
Given the pivotal roles the pathophysiology and signaling pathways play in ischemic stroke, numerous therapeutic approaches have been explored in both experimental and clinical studies, and several of them have been demonstrated to be effective in treatment of ischemic stroke (Table 2).
Therapeutic approaches targeting excitotoxicity and related signaling pathways in cerebral ischemic stroke
Targeting the GluN2B-PSD95-nNOS complex
The GluN2B-PSD95-nNOS complex plays a central role in regulating NMDAR activity and related signaling pathways; therefore, it could potentially serve as a therapeutic target for cerebral ischemic stroke. The Tat-NR2B9c peptide, which binds to either PSD95 or nNOS, was shown to prevent downstream neurotoxic pathways and superoxide production.333 Furthermore, Tat-NR2B9c administration reportedly improved behavioral deficits, reduced infarct volumes, and retained the gene transcription profiles in animal ischemic stroke models.334,335 Another study reported that TAT-NR2B9c alleviated neuronal death and p38-induced damage in ischemic injury,336 while a clinical study found that it significantly decreased infarcts in ischemic stroke patients.337 Another small molecule called ZL006 has been found to disrupt the interaction between PSD95 and nNOS in ischemia, without affecting the normal functions of NMDARs and nNOS.90,338 Similarly, IC87201 has been found to disrupt pathogenic interactions between PSD95 and nNOS but without impairing normal nNOS activities.27 Finally, a study has shed light on Neu2000, a sulfasalazine derivative and GluN2B antagonist that selectively blocks NMDARs and scavenges free radicals, which exerted a neuroprotective effect in ischemic stroke.339,340 All this experimental evidence highlights the potential of treating ischemic stroke by targeting the GluN2B-PSD95-nNOS complex and preventing its participation in excitotoxicity. However, several shortcomings still exist. Although overactivation of NMDARs is acknowledged to be important in the etiology of cerebrovascular insults, the importance in physiological function has made the current NMDAR antagonists ‘undruggable’ for clinical application in ischemic stroke.27,341 Also, the therapeutic time window is relatively short, and safety issues including nausea, vomiting, cardiovascular and psychomimetic effects, remain to be considered.342,343,344,345,346,347,348
Targeting the DAPK1 signaling pathway
DAPK1 phosphorylates p53, a tumor suppressor that serves as one of its substrates. The interfering peptide Tat-p53DM241–281 inhibits specifically the downstream targets of DAPK1, such as the pro-apoptotic genes Bax, Puma, and caspase-3, which are also regulated by p53.349 The administration of Tat-p53DM241–281 was observed to significantly reduce infarct area and alleviate behavioral deficits in experimental ischemic stroke models.350 Another drug, GluN2BCT1292–1304, dissociates DAPK1 from the GluN2B subunit and protects neurons from ischemic injury.351 However, it still remains controversial that McQueen et al. have found that genetic depletion of DAPK1 could not alleviate excitotoxic and ischemic injuries in neurons.351 With possible uncertainties, these results indicate that DAPK1 inhibition could potentially alleviate ischemic brain damage through decreasing cellular excitotoxicity.
Targeting the PTEN-induced signaling pathway
Based on the function of PTEN in inhibiting the PI3K/Akt signaling pathway and inducing apoptotic cell death via excitotoxicity, regulating PTEN could possibly help ameliorate excitotoxicity and, in turn, neurological deficits in ischemic stroke. Genetic knockdown of PTEN was found to retain PI3K/Akt signaling while downregulating the extra-synaptic NMDAR current, which exerted a neuroprotective effect on an experimental ischemic stroke model.74 Pharmacologically, an interfering peptide, Tat-K13, was utilized to disrupt the cell death signaling pathway activated by PTEN.75 Tat-K13 exerted a neuroprotective effect in rats suffering from experimental ischemic stroke by reducing the size of the infarct lesion.33,75 These findings suggest that, owing to its link to PI3K/Akt signaling, the PTEN-related pathway could serve as a potential therapeutic target in the treatment of ischemic stroke.
Targeting the AKT signaling pathway
The iridoid glycoside geniposide has been reported to protect neurons from ischemic damage by activating the GluN2A/AKT/ERK signaling pathway.352 Accordingly, pseudoginsenoside-F11 prevents calpain1 activation while promoting the GluN2A-mediated AKT/CREB pathway.353 Genes involved in the modulation of NMDAR expression along the Akt/ERK pathway could also potentially serve as therapeutic targets. TRPM2 knockout mice showed significantly smaller ischemic lesions, altered expression of GluN2A and GluN2B, and stimulation of pro-survival Akt and ERK signaling in an experimental ischemic stroke model.354 Overall, therapeutic approaches involving drugs, physical treatment, or gene modifications enhancing AKT-related signaling pathways and NMDAR activities could reinforce synaptic NMDAR activities and their neuroprotective effects in ischemic stroke.
Targeting the Panx1 signaling pathway
During ischemia, NMDAR activates Src kinases, which subsequently phosphorylate residue Y308 in the C-terminus of pannexin 1 (Panx1), leading to secondary ischemic currents.355,356 Preventing Panx1 phosphorylation may alleviate the symptoms and pathologies of ischemic stroke. Indeed, use of the interfering peptide Tat-Panx308 helped reduce infarct lesion size and alleviate sensorimotor deficit symptoms in middle cerebral artery occlusion (MCAO) rats, suggesting its effectiveness in treating ischemic stroke.356 In spite of the limited number of studies, regulation of Panx1 in excitotoxicity could represent a promising strategy for ischemic stroke treatment.
Therapeutic approaches targeting signaling pathways to alleviate symptoms and damage caused by oxidative stress in ischemic stroke
Nrf2/ARE signaling pathway
The Nrf2/ARE signaling pathway contributes to the generation of numerous protective factors, such as anti-inflammatory proteins, antioxidant enzymes, and growth factors. Its antioxidant target genes include those encoding for heme oxygenase 1 (HO1), NADP(H) quinone dehydrogenase 1 (NQO1), and glutathione S-transferase (GST).357 Thus, regulation of the Nrf2/ARE signaling pathway could potentially protect against oxidative stress-induced damage in ischemic stroke. It has been reported that injection of tBHQ, an Nrf2 inducer, alleviates the symptoms of experimental cerebral ischemic stroke.358 Similarly, administration of metformin in cerebral ischemic stroke models alleviated oxidative stress-induced BBB damage, possibly through activation of the NRF2/ARE signaling pathway.359 In contrast, higher vulnerability and exacerbated brain damage were observed in cerebral ischemic stroke models of Nrf2-knockout mice.360 Generally, activating the Nrf2/ARE signaling pathway may confer a neuroprotective effect in cerebral ischemic stroke, which is associated with mitigation of oxidative stress.
Sirtuin (SIRT)/forkhead box O (FOXO) signaling pathway
SIRT1–7 play important roles in oxidative stress during ischemic stroke. The SIRT/FOXO signaling pathway has been shown to prevent oxidative stress in cerebral ischemia-reperfusion. SIRT1 exerts an antioxidant effect by activating either the FOXO family or PPAR-γ coactivator-1 and, as such, could serve as a potential therapeutic target.361,362 SIRT3 has been reported to enhance SOD2 activity and decrease ROS levels.363 Moreover, transsodium crocetinate protected animals from oxidative stress induced by cerebral ischemia–reperfusion injury, probably by activating the SIRT3/FOXO3a/SOD2 signaling pathway.364 Similarly, genipin was found to regulate the UCP2/SIRT3 signaling pathway and alleviate oxidative stress induced by cerebral ischemia.365 These findings reveal the potential of SIRT signaling pathways in therapeutic approaches against oxidative stress and ischemic stroke.
Therapies targeting neuroinflammation-related signaling pathways
Chemokine-related signaling pathways
Therapeutic approaches regulating CCL2/CCR2 expression may alleviate the symptoms and pathologies of ischemic stroke. Whereas CCL2 gene disruption reduced infarct volume, CCR2 deletion reduced infarct size, while also improving locomotor ability of mice in an experimental ischemic stroke model.263,366 CCR knockout reduced infarct volumes and mortality of mice in experimental ischemic stroke models. However, it should be mentioned that hindering monocyte infiltration using an anti-CCR2 antibody delayed long-term behavioral recovery, along with decreased expression of anti-inflammatory genes in MCAO mice, suggesting a double-edged role of CCL2/CCR2 in ischemic stroke.367 Infarct size in rat MCAO models has been reduced also via inhibition of another chemokine, CCL23 (also known as MIP3α).265 Taken together, regulating chemokine expression, especially the CCL2/CCR2 signaling pathway, may serve as a potential therapeutic approach against cerebral ischemic stroke, although the harmful effects of such an intervention should be carefully considered.
TLR-associated signaling pathways
Considering the important role played by TLRs in neuroinflammation, several studies have demonstrated that TLR signaling could serve as a treatment target. Overexpression of miR-18a-5p downregulates the levels of TLR4 and TLR7, exerting a protective effect against ischemic injury in vitro.368 Resveratrol modulates microglial activity and improves ischemia-induced neurological symptoms by regulating the TLR4/NF-κB/STAT3 signaling pathway.369,370,371 Stevioside, a natural glycoside, protects against cerebral ischemia by inhibiting TLR/NF-κB pathway-mediated neuroinflammation.372 Moreover, treatment with progesterone and its metabolites has been shown to alleviate the symptoms of various cerebrovascular diseases by regulating the TLR4/NF-κB signaling pathway and inhibiting neuroinflammation.373,374,375 Similarly, dexmedetomidine has been proven effective against inflammatory reactions, oxidative stress, increased infarct volume, and brain edema in MCAO rats by inhibiting the HMGB1/TLR4/NF-κB signaling pathway.376 Interestingly, one study reported that activating TLR7 reduced infarct volume and neurological deficits by enhancing interferon expression.377 This observation is possibly associated with the dual effect of TLRs on neuroinflammation and ischemic stroke. In conclusion, regulation of TLR signaling has been revealed to attenuate neuroinflammation and, thus, protect against ischemic stroke. This therapeutic effect is possibly related to a variety of downstream molecules, including NF-κB and STAT3, whose modulation could promote the beneficial effects of TLRs in ischemic stroke.
Cytokine-related signaling pathways
Regulation of IL-1 and TNF cytokine families could also help attenuate ischemic stroke injuries. A study using a single intravenous dose of XPro1595 or etanercept, which targets TNFs, found that both compounds alleviated inflammatory reactions and enhanced locomotor abilities in a mouse model of focal cerebral ischemia; however, they did not decrease infarct volume.378 Another modified therapy, cTfRMAb-TNFR, which transfers TNFR across the BBB, has been reported to successfully reduce the infarct area and ameliorate neurological deficits.379,380 Similarly, a preclinical study demonstrated that sTNF-αR1 retained axonal plasticity in the cerebral cortex after stroke,381 which is in agreement with the results of another study showing that injection of solTNFR1 in dendritic cells alleviated infarct injury and inflammation after experimental stroke.382 However, it’s still worth mentioning that targeting both solTNF and tmTNF may concurrently raise the risk of cardiovascular and demyelinating disease.383 Due to the possible side effects of the anti-TNF therapies, more efforts should be made for more specific anti-TNF therapeutics.
IL-1Ra is the only therapeutic agent against IL-1-associated inflammation.226 Preclinical studies have shown that recombinant IL-1Ra protects against ischemia-induced injuries in rats384,385 and mice.386 Concomitantly, the first randomized, double-blind, placebo-controlled trial utilizing recombinant human IL-1Ra showed that patients receiving rhIL-1Ra displayed milder inflammatory reactions and nearly no disability 3 months after stroke.387 There’re several shortcomings that rhIL-1Ra crosses the BBB slowly and has relatively short half-life in the circulation to achieve effective and persistent therapeutic concentration.388,389 Also, there’re studies showing that IL-1Ra increased the possibility of poor mRS outcomes.390 Though that, IL-1Ra still has good perspectives in cerebral ischemic stroke treatment owing to its anti-inflammatory properties.
NLRP3 inflammasome
NLRP3 inflammasome regulation has been acknowledged as a potential therapeutic approach for ischemic stroke.391 Brilliant blue G, a P2X7R purinergic receptor antagonist, or MCC950, an NLRP3 inhibitor, not only attenuated cerebral infarct areas and neurological impairments but also inhibited caspase-3-associated neuronal apoptosis.392 Similarly, genistein, a natural phytoestrogen, has been reported to alleviate cerebral ischemia-induced injury in senescent mice by inhibiting NLRP3 inflammasome formation.393 An in vitro study revealed that treatment modulating the immunoproteasome/NF-κB/NLRP3 inflammasome signaling axis could work against hypoxia and ischemia, as well as prevent apoptosis.394 Therefore, inhibition of NLRP3 inflammasome formation could possibly attenuate ischemic stroke inflammatory processes and limit cell death.
Therapeutic approaches targeting the BBB in ischemic stroke
Sirt signaling pathways
Protecting the BBB could help alleviate ischemic stroke. In an experimental rat model of stroke, hyperbaric oxygen treatment helped protect the BBB, potentially by regulating the ATP/NAD+/Sirt1 signaling pathway.164 Similarly, quercetin has been shown to protect the BBB and alleviate ischemia–reperfusion-induced injuries via activation of Sirt1 signals in rats.395 Minocycline has also been shown to ameliorate hypoxia-induced BBB disruption. This effect was mediated by the Sirt3/proline hydroxylase-2 degradation pathway, together with decreased levels of MMP2, MMP9, and VEGF, as well as upregulation of tight junction proteins.396
MMP inhibition for BBB protection
Given the indispensable role of MMPs, inhibition of the MMP signaling pathway may be beneficial in anti-stroke therapy. Administration of hydrogen sulfide donors may help ameliorate cerebral BBB damage, most likely via MMP9 inhibition.397 In addition, vagus nerve stimulation could help protect the BBB in ischemic damage by inhibiting MMP2/9-mediated tight junction protein disruption.398 Similarly, hyperbaric oxygen has been reported to stabilize the BBB in an experimental ischemic stroke model, possibly by blocking MMP2 activation.399 Finally, intra-arterial norcantharidin alleviated cerebral BBB damage by decreasing MMP9 expression in an experimental ischemic stroke model.400 These results suggest that regulation of MMP-related signaling pathways protects the BBB from ischemic stroke injuries.
Cell death-related signaling pathways as targets for ischemic stroke treatment
Autophagy-related signaling pathways
A variety of signaling pathways related with autophagy, including Akt, AMPK, and others, has been shown to be potential therapeutic approaches against ischemic stroke. Fingolimod, a well-established sphingosine-1-phosphate receptor agonist, alleviates neurological deficits and reduces infarct areas by enhancing Akt signaling and ameliorating neuronal apoptosis,401,402 as well as regulating the mTOR/p70S6K autophagy signaling pathway in ischemic stroke models.403 Studies have also reported that selenium protects the BBB from ischemia-reperfusion injuries associated with PI3K/mTOR/AKT signaling pathway activation, which is possibly related to autophagy inhibition.152,404 As for the AMPK signaling pathway, SMXZF, a combination of Rb1, Rg1, schizandrin, and DT-13 (6:9:5:4), exerts a neuroprotective effect on cerebral ischemia-reperfusion injury, possibly by suppressing autophagy through regulation of the AMPK/mTOR and JNK signaling pathways, both in animals and oxygen-glucose deprivation/reperfusion models.405,406 Likewise, by activating AMPK-induced autophagy, ezetimibe ameliorates neuronal apoptosis and infarct volume, while improving neurological deficits in MCAO rat models.407 Finally, physical exercise induces AMPK activation and mTORC1 inhibition, thereby promoting autophagy, which consequently improves cerebral ischemia outcomes.408,409,410
Besides these two main target signals, additional autophagy-associated signaling pathways, related mainly to STAT, and SIRT, could also serve as targets for ischemic stroke therapies. Extracellular vesicles secreted by stem cells help mitigate ischemic brain damage, possibly by modulating STAT3-dependent autophagy, both in vivo and in vitro.411 In an experimental rat cerebral ischemia-reperfusion injury model, electroacupuncture mitigated neurological symptoms and related pathologies through inhibition of maladaptive autophagy and activation of the SIRT/FOXO1 signaling pathway.412,413 In addition, other signaling pathways involving SIRT, including SIRT3/AMPK/mTOR and SIRT1/BMAL1, are activated by luteolin and melatonin, respectively, and help protect against cerebral ischemia–reperfusion-induced injuries.414,415
Apoptosis-associated signaling pathways
Likewise, regulation of several signaling pathways, such as ERK/MAPK, AMPK and SIRT signaling pathways, are shown to mediate apoptosis in ischemic stroke. Beta-hydroxybutyrate ameliorates cerebral ischemic stroke injuries by suppressing apoptosis induced via oxidative stress and mitochondrial dysfunction, both in vivo and in vitro. The curative effects on apoptosis are probably associated with ERK/CREB/eNOS signaling pathway activation.416 Modulation of other ERK/MAPK signaling axes, including the MAPK/ERK/EGR1, CXCL13/ERK/MEK, and DAPK1/ERK signaling pathways, has also been shown to protect against ischemia-induced injuries both in vitro and in vivo.417,418,419 With respect to the AMPK signaling pathways, BML-275, an AMPK inhibitor, exerts a neuroprotective effect on cerebral ischemic stroke by downregulating cytochrome c and AIF expression, consequently blocking apoptosis.420 In addition, glycine was shown to attenuate cellular apoptosis and improve ischemic stroke damage by suppressing the AMPK/GSK3β/HO1 signaling pathway.421 SIRT signals are also possibly involved, as Rosuvastatin may exert protective effects on cerebral ischemia in rats through the Sirt1/NF-κB signaling pathway and inhibition of apoptosis.422 Stem cell therapies also attenuate ischemia-induced injuries, potentially through the SIRT/NF-κB signaling pathway.423 Finally, an in vitro study revealed that regulation of the miRNA-29b/SIRT1/PPAR-γ coactivator 1 alpha axis ameliorated oxygen-glucose deprivation-induced cell apoptosis, thus protecting cells from ischemia.424 All these reveal the potential of therapeutics against cellular apoptosis in ischemic stroke.
National clinical trials of therapeutic approaches targeting ischemic stroke and signaling pathways
Clinical trials targeting the pathophysiology and the related signaling pathways mentioned above have been implemented with respect to ischemic stroke. For instance, the value of targeting cellular excitotoxicity in ischemic stroke has been recognized by investigators pursuing clinical trials with nerinetine (NA-1), the inhibitor of GluN2B-PSD95-nNOS complex (NCT02930018, NCT04462536, NCT00728182, NCT02315443), Neu2000 (NCT04486430), and sofadil (NCT04453800). In addition, several clinical trials focused on neuroinflammation during ischemic stroke have also been implemented, including those targeted IL-1 (NCT04834388, NCT03737344), S1P receptors (NCT02002390), and Toll-like receptors (TLRs) (NCT04734548). Furthermore, therapeutic approaches targeting oxidative stress in ischemic stroke have also been tested in clinical trials, such as selenium (NCT02505295), astaxanthine (NCT03945526), and simavastatin (NCT03402204). Concurrently, stem cell therapy is attracting much attention due to its potential for exerting significant therapeutic effects on stroke patients.425 Various types of cells, including allogenic mesenchymal stem cells from adipose tissue(NCT01678534), bone-marrow-derived stem cell (NCT01501773), endothelial progenitor cells (NCT01468064), and autologous M2 macrophages (NCT018453500) have been tested in clinical trials as a reparative therapy for acute ischemic stroke. All these reveal prospects for targeting the pathophysiology and related signaling pathways in treating ischemic stroke.
Concluding remarks and future perspectives
Ischemic stroke is characterized by the blockade of cerebral blood flow caused by the presence of thrombi in the blood vessels and has an overwhelming effect on people’s health and their quality of life. In recent years, studies have sought to further elucidate the mechanisms of ischemic stroke. Nevertheless, the complex pathogenesis of ischemic stroke means that the participating signaling pathways need further comprehensive exploration. In this review, we summarized the signaling pathways involved in ischemic stroke and categorized them based on their specific pathophysiological roles in excitotoxicity, mitochondrial dysfunction, oxidative stress, neuroinflammation, and cell death. Because these signaling pathways are interconnected, combined therapeutic targets against ischemic stroke may be elucidated.
At present, recanalization of blood vessels via intravenous thrombolytic treatment or mechanical thrombectomy represents the major therapeutic approach for ischemic stroke. However, this is underscored by the lack of suitable pharmacological treatments, calling for the discovery of new therapeutic targets against ischemic stroke. In this review, we combed through existing therapeutic approaches and classified them according to their target signaling pathways. In conclusion, our review comprehensively elucidates the signaling pathways involved in the pathophysiology of ischemic stroke and also points out potential therapeutic approaches against ischemic stroke associated with those key signaling pathways.
Change history
12 August 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41392-022-01129-1
References
Moskowitz, M. A., Lo, E. H. & Iadecola, C. The science of stroke: mechanisms in search of treatments. Neuron 67, 181–198 (2010).
Feigin, V. L. et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 383, 245–255 (2014).
Kleindorfer, D. O. et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 52, E364–E467 (2021).
Adams, H. P. Jr. et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24, 35–41 (1993).
Hankey, G. J. Stroke. Lancet 389, 641–654 (2017).
Nguyen, T.-V. V. et al. Multiplex immunoassay characterization and species comparison of inflammation in acute and non-acute ischemic infarcts in human and mouse brain tissue. Acta Neuropathol. Comm. 4, (2016).
Park, J. Y. et al. Neuroprotective effect of human placental extract on hypoxic-ischemic brain injury in neonatal rats. Brain Dev. 35, 68–74 (2013).
Barthels, D. & Das, H. Current advances in ischemic stroke research and therapies. Biochimica Et Biophysica Acta-Mol. Basis Dis. 1866, (2020).
Yenari, M. A., Kauppinen, T. M. & Swanson, R. A. Microglial activation in stroke: therapeutic targets. Neurotherapeutics 7, 378–391 (2010).
Koyama, Y. Signaling molecules regulating phenotypic conversions of astrocytes and glial scar formation in damaged nerve tissues. Neurochem. Int. 78, 35–42 (2014).
Sims, N. R. & Yew, W. P. Reactive astrogliosis in stroke: Contributions of astrocytes to recovery of neurological function. Neurochem. Int. 107, 88–103 (2017).
Slujitoru, A.-S. et al. Clinical and morphological correlations in acute ischemic stroke. Rom. J. Morphol. Embryol. 53, 917–926 (2012).
Lakhan, S. E., Kirchgessner, A., Tepper, D. & Leonard, A. Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front. Neurol. 4, (2013).
Jin, R. et al. Role of inflammation and its mediators in acute ischemic stroke. J. Cardiovasc. Transl. Res. 6, 834–851 (2013).
Clausen, B. H. et al. Interleukin-1 beta and tumor necrosis factor-alpha are expressed by different subsets of microglia and macrophages after ischemic stroke in mice. J. Neuroinflammation. 5, (2008).
Gronberg, N. V., Johansen, F. F., Kristiansen, U. & Hasseldam, H. Leukocyte infiltration in experimental stroke. J. Neuroinflammation. 10, (2013).
Ritzel, R. M. et al. Functional differences between microglia and monocytes after ischemic stroke. J. Neuroinflammation. 12, (2015).
Sommer, C. J. Ischemic stroke: experimental models and reality. Acta Neuropathol. 133, 245–261 (2017).
Smith, H. K., Russell, J. M., Granger, D. N. & Gavins, F. N. E. Critical differences between two classical surgical approaches for middle cerebral artery occlusion-induced stroke in mice. J. Neurosci. Methods 249, 99–105 (2015).
Knowland, D. et al. Stepwise Recruitment Of Transcellular And Paracellular Pathways Underlies Blood-brain Barrier Breakdown In Stroke. Neuron 82, 603–617 (2014).
Huang, L. et al. Human neural stem cells rapidly ameliorate symptomatic inflammation in early-stage ischemic-reperfusion cerebral injury. Stem Cell. Res. Ther. 5, 129 (2014).
Qian, C. et al. Precise characterization of the penumbra revealed by MRI: a modified photothrombotic stroke model study. PLoS One 11, e153756 (2016).
Yang, L., Shah, K. K. & Abbruscato, T. J. In Astrocytes: Methods and Protocols Vol. 814 Methods in Molecular Biology (ed R. Milner) 451–466 (2012).
Li, D. et al. mTORC1 pathway disruption ameliorates brain inflammation following stroke via a shift in microglia phenotype from M1 type to M2 type. FASEB J. 30, 3388–3399 (2016).
Li, K. et al. Middle East Respiratory Syndrome Coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl Peptidase 4. J. Infect. Dis. 213, 712–722 (2016).
Xiong, X.-Y., Liu, L. & Yang, Q.-W. Refocusing neuroprotection in cerebral reperfusion era: new challenges and strategies. Front. Neurol. 9, 249 (2018).
Lai, T. W., Zhang, S. & Wang, Y. T. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. Neurobiol. 115, 157–188 (2014).
Jia, J. et al. New insights into targeting mitochondria in ischemic injury. Apoptosis 26, 163–183 (2021).
Macrez, R. et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. 10, 471–480 (2011).
Steliga, A. et al. Neurovascular unit as a source of ischemic stroke biomarkers-limitations of experimental studies and perspectives for clinical application. Transl. Stroke Res. 11, 553–579 (2020).
Datta, A. et al. Cell Death Pathways In Ischemic Stroke And Targeted Pharmacotherapy. Transl. Stroke Res. 11, 1185–1202 (2020).
Andrabi, S. S., Parvez, S. & Tabassum, H. Ischemic stroke and mitochondria: mechanisms and targets. Protoplasma 257, 335–343 (2020).
Wu, Q. J. & Tymianski, M. Targeting NMDA receptors in stroke: new hope in neuroprotection. Mol. Brain. 11, 15 (2018).
Olney, J. W. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science 164, 719–721 (1969).
Garthwaite, G., Williams, G. D. & Garthwaite, J. Glutamate toxicity: an experimental and theoretical analysis. Eur. J. Neurosci. 4, 353–360 (1992).
Choi, D. W., Koh, J. Y. & Peters, S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J. Neurosci. 8, 185–196 (1988).
Willard, S. S. & Koochekpour, S. Glutamate, glutamate receptors, and downstream signaling pathways. Int. J. Biol. Sci. 9, 948–959 (2013).
George, P. M. & Steinberg, G. K. Novel Stroke Therapeutics: Unraveling Stroke Pathophysiology And Its Impact On Clinical Treatments. Neuron 87, 297–309 (2015).
Barone, F. C. & Feuerstein, G. Z. Inflammatory mediators and stroke: New opportunities for novel therapeutics. J. Cereb. Blood Flow. Metab. 19, 819–834 (1999).
Lipton, P. Ischemic cell death in brain neurons. Physiol. Rev. 79, 1431–1568 (1999).
Mazala, D. A. G., Grange, R. W. & Chin, E. R. The role of proteases in excitation-contraction coupling failure in muscular dystrophy. Am. J. Physiol. Cell. Physiol. 308, C33–C40 (2015).
Weber, J. T. Altered calcium signaling following traumatic brain injury. Front. Pharmacol. 3, 60 (2012).
Xu, J. et al. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J. Neurosci. 29, 9330–9343 (2009).
Casas, A. I. et al. Calcium-dependent blood-brain barrier breakdown by NOX5 limits postreperfusion benefit in stroke. J. Clin. Invest. 129, 1772–1778 (2019).
Liu, F. et al. Mitochondria in Ischemic Stroke: New Insight and Implications. Aging Dis. 9, 924–937 (2018).
Dirnagl, U., Iadecola, C. & Moskowitz, M. A. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 22, 391–397 (1999).
Zhu, J. et al. Up-regulation of GluN2A-containing NMDA receptor protects cultured cortical neuron cells from oxidative stress. Heliyon 4, e00976 (2018).
Benveniste, H., Drejer, J., Schousboe, A. & Diemer, N. H. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J. Neurochem. 43, 1369–1374 (1984).
Joyal, J. L. et al. Communication - Calmodulin activates phosphatidylinositol 3-kinase. J. Biol. Chem. 272, 28183–28186 (1997).
Alessi, D. R. et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B alpha. Curr. Biol. 7, 261–269 (1997).
Zhang, F. X., Rubin, R. & Rooney, T. A. N-methyl-D-aspartate inhibits apoptosis through activation of phosphatidylinositol 3-kinase in cerebellar granule neurons - A role for insulin receptor substrate-1 in the neurotrophic action of N-methyl-D-aspartate and its inhibition by ethanol. J. Biol. Chem. 273, 26596–26602 (1998).
Jo, H. et al. Small molecule-induced cytosolic activation of protein kinase Akt rescues ischemia-elicited neuronal death. Proc. Natl Acad. Sci. USA 109, 10581–10586 (2012).
Soriano, F. X. et al. Preconditioning doses of NMDA promote neuroprotection by enhancing neuronal excitability. J. Neurosci. 26, 4509–4518 (2006).
Yamaguchi, A. et al. Akt activation protects hippocampal neurons from apoptosis by inhibiting transcriptional activity of p53. J. Biol. Chem. 276, 5256–5264 (2001).
Endo, H. et al. Activation of the Akt/GSK3 beta signaling pathway mediates survival of vulnerable hippocampal neurons after transient global cerebral ischemia in rats. J. Cereb. Blood Flow. Metab. 26, 1479–1489 (2006).
Kawano, T. et al. Neuroprotective effect of sodium orthovanadate on delayed neuronal death after transient forebrain ischemia in gerbil hippocampus. J. Cereb. Blood Flow. Metab. 21, 1268–1280 (2001).
Noshita, N. et al. Copper-zinc superoxide dismutase affects Akt activation after transient focal cerebral ischemia in mice. Stroke 34, 1513–1518 (2003).
Noshita, N., Lewen, A., Sugawara, T. & Chan, P. H. Evidence of phosphorylation of Akt and neuronal survival after transient focal cerebral ischemia in mice. J. Cereb. Blood Flow. Metab. 21, 1442–1450 (2001).
Yano, S. et al. Activation of Akt/protein kinase B contributes to induction of ischemic tolerance in the CA1 subfield of gerbil hippocampus. J. Cereb. Blood Flow. Metab. 21, 351–360 (2001).
Downward, J. & How, B. A. D. phosphorylation is good for survival. Nat. Cell Biol. 1, E33–E35 (1999).
Kim, A. H. et al. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol. Cell. Biol. 21, 893–901 (2001).
Wu, G. Y., Deisseroth, K. & Tsien, R. W. Activity-dependent CREB phosphorylation: Convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc. Natl Acad. Sci. USA 98, 2808–2813 (2001).
Impey, S. et al. Phosphorylation of CBP mediates transcriptional activation by neural activity and CaM kinase IV. Neuron 34, 235–244 (2002).
Favaron, M. et al. NMDA-stimulated expression of BDNF mRNA in cultured cerebellar granule neurones. Neuroreport 4, 1171–1174 (1993).
Hansen, H. H. et al. Mechanisms leading to disseminated apoptosis following NMDA receptor blockade in the developing rat brain. Neurobiol. Dis. 16, 440–453 (2004).
Shieh, P. B. et al. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron 20, 727–740 (1998).
Tao, X. et al. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 20, 709–726 (1998).
Ghosh, A., Carnahan, J. & Greenberg, M. E. Requirement for BDNF in activity-dependent survival of cortical neurons. Science 263, 1618–1623 (1994).
Zafra, F., Castren, E., Thoenen, H. & Lindholm, D. Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc. Natl Acad. Sci. USA 88, 10037–10041 (1991).
Chen, Q. et al. Differential roles of NR2A- and NR2B-containing NMDA receptors in activity-dependent brain-derived neurotrophic factor gene regulation and limbic epileptogenesis. J. Neurosci. 27, 542–552 (2007).
Hardingham, G. E., Fukunaga, Y. & Bading, H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 5, 405–414 (2002).
Comelli, M. C. et al. Photochemical stroke and brain-derived neurotrophic factor (BDNF) mRNA expression. Neuroreport 3, 473–476 (1992).
Chen, M. et al. Differential roles of NMDA Receptor Subtypes In Ischemic Neuronal Cell Death And Ischemic Tolerance. Stroke 39, 3042–3048 (2008).
Ning, K. et al. Dual neuroprotective signaling mediated by downregulating two distinct phosphatase activities of PTEN. J. Neurosci. 24, 4052–4060 (2004).
Zhang, S. et al. Critical role of increased PTEN nuclear translocation in excitotoxic and ischemic neuronal injuries. J. Neurosci. 33, 7997–8008 (2013).
Kyrylenko, S., Roschier, M., Korhonen, P. & Salminen, A. Regulation of PTEN expression in neuronal apoptosis. Mol. Brain Res. 73, 198–202 (1999).
Deiss, L. P. et al. Identification of a novel serine/threonine kinase and a novel 15-kD protein as potential mediators of the gamma interferon-induced cell death. Genes Dev. 9, 15–30 (1995).
Kissil, J. L. et al. DAP-kinase loss of expression in various carcinoma and B-cell lymphoma cell lines: possible implications for role as tumor suppressor gene. Oncogene 15, 403–407 (1997).
Marshall, J. et al. Calcium channel and NMDA receptor activities differentially regulate nuclear C/EBP beta levels to control neuronal survival. Neuron 39, 625–639 (2003).
Duval, X. et al. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann. Intern. Med. 152, 497–504 (2010). W175.
Shamloo, M. et al. Death-associated protein kinase is activated by dephosphorylation in response to cerebral ischemia. J. Biol. Chem. 280, 42290–42299 (2005).
Chen, C. H. et al. Bidirectional signals transduced by DAPK-ERK interaction promote the apoptotic effect of DAPK. EMBO J. 24, 294–304 (2005).
Garthwaite, J., Charles, S. L. & Chess-Williams, R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature 336, 385–388 (1988).
Brenman, J. E. et al. Cloning and characterization of postsynaptic density 93, a nitric oxide synthase interacting protein. J. Neurosci. 16, 7407–7415 (1996).
Kornau, H.-C., Schenker, L. T., Kennedy, M. B. & Seeburg, P. H. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science 269, 1737–1740 (1995).
Muller, B. M. et al. SAP102, a novel postsynaptic protein that interacts with NMDA receptor complexes in vivo. Neuron 17, 255–265 (1996).
Sattler, R. et al. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95. protein Sci. 284, 1845–1848 (1999).
Christopherson, K. S., Hillier, B. J., Lim, W. A. & Bredt, D. S. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J. Biol. Chem. 274, 27467–27473 (1999).
Huang, Z. et al. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science 265, 1883–1885 (1994).
Zhou, L. et al. Treatment of cerebral ischemia by disrupting ischemia-induced interaction of nNOS with PSD-95. Nat. Med. 16, 1439–U1123 (2010).
Liu, X. et al. Induction of apoptotic program in cell-free extracts: requirement for dATP and Cytochrome c. Cell 86, 147–157 (1996).
Murphy, A. N., Fiskum, G. & Flint Beal, M. Mitochondria in neurodegeneration: Bioenergetic function in cell life and death. J. Cereb. Blood Flow. Metab. 19, 231–245 (1999).
Hofmeijer, J. & van Putten, M. J. A. M. Ischemic cerebral damage an appraisal of synaptic failure. Stroke 43, 607–615 (2012).
Lee, J. M., Grabb, M. C., Zipfel, G. J. & Choi, D. W. Brain tissue responses to ischemia. J. Clin. Invest. 106, 723–731 (2000).
Dharmasaroja, P. A. Fluid intake related to brain edema in acute middle cerebral artery infarction. Transl. Stroke Res. 7, 49–53 (2016).
Huang, Y., Li, W., Su, Z.-Y. & Kong, A.-N. T. The complexity of the Nrf2 pathway: beyond the antioxidant response. J. Nutr. Biochem. 26, 1401–1413 (2015).
Xu, J. et al. Luteolin provides neuroprotection in models of traumatic brain injury via the Nrf2-ARE pathway. Free Radic. Biol. Med. 71, 186–195 (2014).
Buendia, I. et al. Nrf2-ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol. Ther. 157, 84–104 (2016).
Schinzel, A. C. et al. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc. Natl Acad. Sci. USA 102, 12005–12010 (2005).
Nakagawa, T. et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434, 652–658 (2005).
Baines, C. P. et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434, 658–662 (2005).
Lutz, J., Thuermel, K. & Heemann, U. Anti-inflammatory treatment strategies for ischemia/reperfusion injury in transplantation. J. Inflamm. 7, 27 (2010).
Hou, Y., Wang, J. & Feng, J. The neuroprotective effects of curcumin are associated with the regulation of the reciprocal function between autophagy and HIF-1α in cerebral ischemia-reperfusion injury. Drug Des., Dev. Ther. 13, 1135–1144 (2019).
Liu, Y. Q. et al. Metformin attenuates blood-brain barrier disruption in mice following middle cerebral artery occlusion. J. Neuroinflammation. 11, (2014).
Dai, S. H. et al. Sirt3 confers protection against neuronal ischemia by inducing autophagy: Involvement of the AMPK-mTOR pathway. Free Radic. Biol. Med. 108, 345–353 (2017).
Semenza, G. L. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology 24, 97–106 (2009).
Ziello, J. E., Jovin, I. S. & Huang, Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J. Biol. Med. 80, 51–60 (2007).
Kim, J. W., Tchernyshyov, I., Semenza, G. L. & Dang, C. V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3, 177–185 (2006).
Hu, C. J. et al. Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1 alpha) and HIF-2 alpha in stem cells. Mol. Cell. Biol. 26, 3514–3526 (2006).
Guo, S., Miyake, M., Liu, K. J. & Shi, H. Specific inhibition of hypoxia inducible factor 1 exaggerates cell injury induced by in vitro ischemia through deteriorating cellular redox environment. J. Neurochem. 108, 1309–1321 (2009).
Itoh, K. et al. Emerging functional cross-talk between the Keap1-Nrf2 system and mitochondria. J. Clin. Biochem. Nutr. 56, 91–97 (2015).
Malhotra, D. et al. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. 38, 5718–5734 (2010).
Dinkova-Kostova, A. T. & Abramov, A. Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 88, 179–188 (2015).
Holmstrom, K. M. et al. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol. Open. 2, 761–770 (2013).
Xu, Y.-P., Han, F. & Tan, J. Edaravone protects the retina against ischemia/reperfusion-induced oxidative injury through the PI3K/Akt/Nrf2 pathway. Mol. Med. Report. 16, 9210–9216 (2017).
Kitagawa, H. et al. Immunoreactive Akt, PI3-K and ERK protein kinase expression in ischemic rat brain. Neurosci. Lett. 274, 45–48 (1999).
Cen, J. et al. Polyamine analogue QMA attenuated ischemic injury in MCAO rats via ERK and Akt activated Nrf2/HO-1 signaling pathway. Eur. J. Pharmacol. 844, 165–174 (2019).
Chang, C.-Y. et al. Tetramethylpyrazine inhibits neutrophil activation following permanent cerebral ischemia in rats. Biochem. Biophys. Res. Commun. 463, 421–427 (2015).
Innamorato, N. G. et al. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J. Immunol. 181, 680–689 (2008).
Kim, G. S. et al. Release of mitochondrial apoptogenic factors and cell death are mediated by CK2 and NADPH oxidase. J. Cereb. Blood Flow. Metab. 32, 720–730 (2012).
Diekmann, D. et al. Interaction of Rac with p67-phox and regulation of phagocytic NADPH oxidase activity. Sci. (Wash. D. C). 265, 531–533 (1994).
Sarfstein, R. et al. Dual role of Rac in the assembly of NADPH oxidase, tethering to the membrane and activation of p67(phox) - A study based on mutagenesis of p67(phox)-Rac1 chimeras. J. Biol. Chem. 279, 16007–16016 (2004).
Zimmer, S. et al. Inhibition of Rac1 GTPase decreases vascular oxidative stress, improves endothelial function, and attenuates atherosclerosis development in mice. Front. Cardiovasc. Med. 8, 680775 (2021).
Mandal, T. et al. Reduced phosphorylation of Stat3 at Ser-727 mediated by casein kinase 2-Protein phosphatase 2A enhances Stat3 Tyr-705 induced tumorigenic potential of glioma cells. Cell. Signal. 26, 1725–1734 (2014).
Jung, J. E., Kim, G. S. & Chan, P. H. Neuroprotection by Interleukin-6 is mediated by signal transducer and activator of Transcription 3 and antioxidative signaling in ischemic stroke. Stroke 42, 3574–U3371 (2011).
Chao, C. C., Ma, Y. L. & Lee, E. H. Y. Brain-derived neurotrophic factor enhances Bcl-xL expression through protein kinase Casein Kinase 2-activated and nuclear factor Kappa B-mediated pathway in Rat Hippocampus. Brain Pathol. 21, 150–162 (2011).
Afonyushkin, T., Oskolkova, O. V., Binder, B. R. & Bochkov, V. N. Involvement of CK2 in activation of electrophilic genes in endothelial cells by oxidized phospholipids. J. Lipid Res. 52, 98–103 (2011).
Baltan, S. et al. CK2 inhibition protects white matter from ischemic injury. Neurosci. Lett. 687, 37–42 (2018).
Bastian, C. et al. CK2 inhibition confers functional protection to young and aging axons against ischemia by differentially regulating the CDK5 and AKT signaling pathways. Neurobiol. Dis. 126, 47–61 (2019).
Meyer, D. A. et al. Ischemic stroke injury is mediated by Aberrant Cdk5. J. Neurosci. 34, 8259–8267 (2014).
Tan, X. et al. The inhibition of CDK5 activity after hypoxia/ischemia injury reduces infarct size and promotes functional recovery in neonatal rats. Neuroscience 290, 552–560 (2015).
Palikaras, K. & Tavernarakis, N. Mitochondrial homeostasis: The interplay between mitophagy and mitochondrial biogenesis. Exp. Gerontol. 56, 182–188 (2014).
Feng, J. et al. Inhibition of peroxynitrite-induced mitophagy activation attenuates cerebral ischemia-reperfusion injury. Mol. Neurobiol. 55, 6369–6386 (2018).
Di Sante, G. et al. Loss of Sirt1 promotes prostatic intraepithelial neoplasia, reduces mitophagy, and delays Park2 translocation to mitochondria. Am. J. Pathol. 185, 266–279 (2015).
Gustafsson, A. B. Bnip3 as a dual regulator of mitochondrial turnover and cell death in the myocardium. Pediatr. Cardiol. 32, 267–274 (2011).
Cui, T. et al. Silencing of PINK1 induces mitophagy via mitochondrial permeability transition in dopaminergic MN9D cells. Brain Res. 1394, 1–13 (2011).
Livingston, M. J. et al. Clearance of damaged mitochondria via mitophagy is important to the protective effect of ischemic preconditioning in kidneys. Autophagy 15, 2142–2162 (2019).
Fan, P. et al. Molecular regulation mechanisms and interactions between reactive oxygen species and mitophagy. DNA Cell Biol. 38, 10–22 (2019).
Bursch, W. et al. Active cell death induced by the anti-estrogens tamoxifen and ICI 164384 in human mammary carcinoma cells (MCF-7) in culture: The role of autophagy. Carcinogenesis 17, 1595–1607 (1996).
Clarke, P. G. Developmental cell death: morphological diversity and multiple mechanisms. Anat. Embryol. 181, 195–213 (1990).
Majno, G. & Joris, I. Apoptosis, oncosis, and necrosis: An overview of cell death. Am. J. Pathol. 146, 3–15 (1995).
He, C. & Klionsky, D. J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 43, 67–93 (2009).
Parzych, K. R. & Klionsky, D. J. An overview of autophagy: morphology, mechanism, and regulation. Antioxid. Redox Signal. 20, 460–473 (2014).
Balin, B., Abrams, J. T. & Schrogie, J. Toward a unifying hypothesis in the development of Alzheimer’s disease. CNS Neurosci. Ther. 17, 587–589 (2011).
Berezniuk, I. & Fricker, L. D. A defect in cytosolic carboxypeptidase 1 (Nna1) causes autophagy in Purkinje cell degeneration mouse brain. Autophagy 6, 558–559 (2010).
Medeiros, R., Baglietto-Vargas, D. & LaFerla, F. M. The role of Tau in Alzheimer’s disease and related disorders. CNS Neurosci. Ther. 17, 514–524 (2011).
Chu, C. T. Eaten alive - Autophagy and neuronal cell death after hypoxia-ischemia. Am. J. Pathol. 172, 284–287 (2008).
Wang, X. et al. An updated review of autophagy in ischemic stroke: From mechanisms to therapies. Exp. Neurol. 340, 113684 (2021).
Kim, Y. C. & Guan, K.-L. mTOR: a pharmacologic target for autophagy regulation. J. Clin. Investig. 125, 25–32 (2015).
Perez-Alvarez, M. J., Villa Gonzalez, M., Benito-Cuesta, I. & Wandosell, F. G. Role of mTORC1 controlling Proteostasis after Brain Ischemia. Front. Neurosci. 12, 60 (2018).
Fang, W. et al. CCR2-dependent monocytes/macrophages exacerbate acute brain injury but promote functional recovery after ischemic stroke in mice. Theranostics 8, 3530–3543 (2018).
Otxoa-de-Amezaga, A. et al. Microglial cell loss after ischemic stroke favors brain neutrophil accumulation. Acta Neuropathol. 137, 321–341 (2019).
Guo, Y.-Q. et al. Expression of CCL2 and CCR2 in the hippocampus and the interventional roles of propofol in rat cerebral ischemia/reperfusion. Exp. Ther. Med. 8, 657–661 (2014).
Wei, H. P. et al. cPKC gamma-modulated autophagy in neurons alleviates ischemic injury in brain of mice with ischemic stroke through Akt-mTOR pathway. Transl. Stroke Res. 7, 497–511 (2016).
Carloni, S., Buonocore, G. & Balduini, W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol. Dis. 32, 329–339 (2008).
Wang, M. Y. et al. Homocysteine enhances neural stem cell autophagy in in vivo and in vitro model of ischemic stroke. Cell Death Dis. 10, 561 (2019).
Jiang, T. et al. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br. J. Pharmacol. 171, 3146–3157 (2014).
Yu, Z. H. et al. Neuroprotective effects of Tongxinluo on focal cerebral ischemia and reperfusion injury in rats associated with the activation of the MEK1/2/ERK1/2/p90RSK signaling pathway. Brain Res. 1685, 9–18 (2018).
Bootman, M. D. et al. The regulation of autophagy by calcium signals: Do we have a consensus? Cell Calcium 70, 32–46 (2018).
Morselli, E. et al. Mutant p53 protein localized in the cytoplasm inhibits autophagy. Cell Cycle 7, 3056–3061 (2008).
Cuenda, A. & Rousseau, S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys. Acta 1773, 1358–1375 (2007).
Kalra, P., Khan, H., Kaur, A. & Singh, T. G. Mechanistic insight on autophagy modulated molecular pathways in cerebral ischemic injury: from preclinical to clinical perspective. Neurochem. Res., (2022).
Cui, D. R. et al. Propofol prevents cerebral ischemia-triggered autophagy activation and cell death in the rat hippocampus through the NF-kappaB/p53 signaling pathway. Neuroscience 246, 117–132 (2013).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
Xu, D. et al. Orexin-A protects against cerebral ischemia-reperfusion injury by inhibiting excessive autophagy through OX1R-mediated MAPK/ERK/mTOR pathway. Cell. Signal. 79, 109839 (2021).
Rami, A. Upregulation of Beclin 1 in the ischemic penumbra. Autophagy 4, 227–229 (2008).
Qi, Z. et al. Bcl-2 phosphorylation triggers autophagy switch and reduces mitochondrial damage in limb remote ischemic conditioned rats after ischemic stroke. Transl. Stroke Res. 6, 198–206 (2015).
Xu, F. et al. Peroxisome proliferator-activated receptor-gamma agonist 15d-prostaglandin J2 mediates neuronal autophagy after cerebral ischemia-reperfusion injury. PLoS One 8, e55080 (2013).
Qi, J. et al. Rab7b overexpression-ameliorated ischemic brain damage following tMCAO involves suppression of TLR4 and NF-kappaB p65. J. Mol. Neurosci. 68, 163–170 (2019).
Kerr, J. F., Wyllie, A. H. & Currie, A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26, 239–257 (1972).
Uzdensky, A. B. Apoptosis regulation in the penumbra after ischemic stroke: expression of pro- and antiapoptotic proteins. Apoptosis 24, 687–702 (2019).
Benn, S. C. & Woolf, C. J. Adult neuron survival strategies-slamming on the brakes. Nat. Rev. Neurosci. 5, 686–700 (2004).
Velier, J. J. et al. Caspase-8 and caspase-3 are expressed by different populations of cortical neurons undergoing delayed cell death after focal stroke in the rat. J. Neurosci. 19, 5932–5941 (1999).
Love, S. Apoptosis and brain ischaemia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 27, 267–282 (2003).
Plesnila, N. et al. BID mediates neuronal cell death after oxygen/ glucose deprivation and focal cerebral ischemia. Proc. Natl Acad. Sci. USA 98, 15318–15323 (2001).
Yin, X. M. et al. Bid-mediated mitochondrial pathway is critical to ischemic neuronal apoptosis and focal cerebral ischemia. J. Biol. Chem. 277, 42074–42081 (2002).
Sarmah, D. et al. Mitochondrial Dysfunction in Stroke: Implications of Stem Cell Therapy. Transl. Stroke Res., (2018).
Sekerdag, E., Solaroglu, I. & Gursoy-Ozdemir, Y. Cell death mechanisms in stroke and novel molecular and cellular treatment options. Curr. Neuropharmacol. 16, 1396–1415 (2018).
Scheinberg, P. Survival of the ischemic brain: a progress report. Circulation 60, 1600–1605 (1979).
Leng, T., Shi, Y., Xiong, Z. G. & Sun, D. Proton-sensitive cation channels and ion exchangers in ischemic brain injury: new therapeutic targets for stroke? Prog. Neurobiol. 115, 189–209 (2014).
Chamorro, Á., Dirnagl, U., Urra, X. & Planas, A. M. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 15, 869–881 (2016).
Collingridge, G. L., Isaac, J. T. & Wang, Y. T. Receptor trafficking and synaptic plasticity. Nat. Rev. Neurosci. 5, 952–962 (2004).
Vats, K. et al. Inflammasomes in stroke: a triggering role for acid-sensing ion channels. Ann. N. Y. Acad. Sci. 1431, 14–24 (2018).
Li, H., Zhu, H., Xu, C. J. & Yuan, J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94, 491–501 (1998).
Bauer, T. M. & Murphy, E. Role of mitochondrial calcium and the permeability transition pore in regulating cell death. Circ. Res. 126, 280–293 (2020).
Fricker, M. et al. Neuronal cell death. Physiol. Rev. 98, 813–880 (2018).
Hollville, E., Romero, S. E. & Deshmukh, M. Apoptotic cell death regulation in neurons. FEBS J. 286, 3276–3298 (2019).
Leker, R. R., Aharonowiz, M., Greig, N. H. & Ovadia, H. The role of p53-induced apoptosis in cerebral ischemia: effects of the p53 inhibitor pifithrin alpha. Exp. Neurol. 187, 478–486 (2004).
Culmsee, C. & Krieglstein, J. Ischaemic brain damage after stroke: new insights into efficient therapeutic strategies. International Symposium on Neurodegeneration and Neuroprotection. EMBO Rep. 8, 129–133 (2007).
Morrison, R. S. et al. p53-dependent cell death signaling in neurons. Neurochem. Res. 28, 15–27 (2003).
Cregan, S. P. et al. p53 activation domain 1 is essential for PUMA upregulation and p53-mediated neuronal cell death. J. Neurosci. 24, 10003–10012 (2004).
Norbury, C. J. & Zhivotovsky, B. DNA damage-induced apoptosis. Oncogene 23, 2797–2808 (2004).
Balaganapathy, P. et al. Interplay between Notch and p53 promotes neuronal cell death in ischemic stroke. J. Cereb. Blood Flow. Metab. 38, 1781–1795 (2018).
Baik, S.-H. et al. Pin1 promotes neuronal death in stroke by stabilizing notch intracellular domain. Ann. Neurol. 77, 504–516 (2015).
Arumugam, T. V. et al. Evidence that gamma-Secretase-mediated notch signaling induces neuronal cell death via the nuclear Factor-kappa B-Bcl-2-interacting mediator of cell death pathway in ischemic stroke. Mol. Pharmacol. 80, 23–31 (2011).
Wei, Z. et al. Notch activation enhances the microglia-mediated inflammatory response associated with focal cerebral ischemia. Stroke 42, 2589–U2344 (2011).
Cheng, Y.-L. et al. Evidence that neuronal Notch-1 promotes JNK/c-Jun activation and cell death following ischemic stress. Brain Res. 1586, 193–202 (2014).
Cheng, Y.-L. et al. Evidence that collaboration between HIF-1 alpha and Notch-1 promotes neuronal cell death in ischemic stroke. Neurobiol. Dis. 62, 286–295 (2014).
Deb, P., Sharma, S. & Hassan, K. M. Pathophysiologic mechanisms of acute ischemic stroke: An overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiol.: Off. J. Int. Soc. Pathophysiol. 17, 197–218 (2010).
Festjens, N., Vanden Berghe, T. & Vandenabeele, P. Necrosis, a well-orchestrated form of cell demise: Signalling cascades, important mediators and concomitant immune response. Biochimica Et. Biophysica Acta-Bioenerg. 1757, 1371–1387 (2006).
Weber, K. et al. Nuclear RIPK3 and MLKL contribute to cytosolic necrosome formation and necroptosis. Commun. Biol 1, 6 (2018).
Vieira, M. et al. Ischemic insults induce necroptotic cell death in hippocampal neurons through the up-regulation of endogenous RIP3. Neurobiol. Dis. 68, 26–36 (2014).
Sun, L. et al. Mixed Lineage Kinase Domain-like Protein mediates necrosis signaling downstream of RIP3 Kinase. Cell 148, 213–227 (2012).
Vanden Berghe, T. et al. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 15, 134–146 (2014).
Dondelinger, Y. et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 7, 971–981 (2014).
Galluzzi, L., Kepp, O. & Kroemer, G. MLKL regulates necrotic plasma membrane permeabilization. Cell Res. 24, 139–140 (2014).
Dong, Z. et al. The possibility and molecular mechanisms of cell pyroptosis after cerebral ischemia. Neurosci. Bull. 34, 1131–1136 (2018).
Kono, H., Kimura, Y. & Latz, E. Inflammasome activation in response to dead cells and their metabolites. Curr. Opin. Immunol. 30, 91–98 (2014).
Poh, L. et al. Evidence that NLRC4 inflammasome mediates apoptotic and pyroptotic microglial death following ischemic stroke. Brain Behav. Immun. 75, 34–47 (2019).
Cao, J. Y. & Dixon, S. J. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 73, 2195–2209 (2016).
Tan, S. L. et al. The regulation of reactive oxygen species production during programmed cell death. J. Cell Biol. 141, 1423–1432 (1998).
Kenny, E. M. et al. Ferroptosis contributes to neuronal death and functional outcome after traumatic brain injury. Crit. Care Med. 47, 410–418 (2019).
Ren, J. X. et al. Crosstalk between oxidative stress and ferroptosis/oxytosis in ischemic stroke: possible targets and molecular mechanisms. Oxid. Med. Cell. Longev. 2021, 6643382 (2021).
Shichita, T. et al. MAFB prevents excess inflammation after ischemic stroke by accelerating clearance of damage signals through MSR1. Nat. Med. 23, 723 (2017).
Gadani, S. P., Walsh, J. T., Lukens, J. R. & Kipnis, J. Dealing with danger in the CNS: the response of the immune system to injury. Neuron 87, 47–62 (2015).
Gelderblom, M. et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 40, 1849–1857 (2009).
Iadecola, C. & Anrather, J. The immunology of stroke: from mechanisms to translation. Nat. Med. 17, 796–808 (2011).
Huang, J., Upadhyay, U. M. & Tamargo, R. J. Inflammation in stroke and focal cerebral ischemia. Surg. Neurol. 66, 232–245 (2006).
Zhang, R. L. et al. The expression of P- and E-selectins in three models of middle cerebral artery occlusion. Brain Res. 785, 207–214 (1998).
Liebner, S. et al. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol. 135, 311–336 (2018).
Stanimirovic, D. & Satoh, K. Inflammatory mediators of cerebral endothelium: A role in ischemic brain inflammation. Brain Pathol. 10, 113–126 (2000).
Gragnano, F. et al. The Role of von Willebrand Factor in vascular inflammation: from pathogenesis to targeted therapy. Mediators Inflamm. 2017, 5620314 (2017).
Ishitsuka, K. et al. Neurotrophin production in brain pericytes during hypoxia: A role of pericytes for neuroprotection. Microvasc. Res. 83, 352–359 (2012).
Badaut, T., Lasbennes, T., Magistretti, P. J. & Regli, L. Aquaporins in brain: Distribution, physiology, and pathophysiology. J. Cereb. Blood Flow. Metab. 22, 367–378 (2002).
Badaut, J. et al. Temporal and regional evolution of aquaporin-4 expression and magnetic resonance imaging in a rat pup model of neonatal stroke. Pediatr. Res. 62, 248–254 (2007).
Lambertsen, K. L., Finsen, B. & Clausen, B. H. Post-stroke inflammation-target or tool for therapy? Acta Neuropathol. 137, 693–714 (2019).
Lambertsen, K. L. et al. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J. Neurosci. 29, 1319–1330 (2009).
Clausen, B. H. et al. Cell therapy centered on IL-1Ra is neuroprotective in experimental stroke. Acta Neuropathol. 131, 775–791 (2016).
Kirchner, S. et al. LPS resistance in monocytic cells caused by reverse signaling through transmembrane TNF (mTNF) is mediated by the MAPK/ERK pathway. J. Leukoc. Biol. 75, 324–331 (2004).
Grell, M., Wajant, H., Zimmermann, G. & Scheurich, P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc. Natl Acad. Sci. USA 95, 570–575 (1998).
Grell, M. et al. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell 83, 793–802 (1995).
Um, J. Y., An, N. H. & Kim, H. M. TNF-alpha and TNF-beta gene polyrnorphisms in cerebral infarction. J. Mol. Neurosci. 21, 167–171 (2003).
Lambertsen, K. L., Biber, K. & Finsen, B. Inflammatory cytokines in experimental and human stroke. J. Cereb. Blood Flow. Metab. 32, 1677–1698 (2012).
Taoufik, E. et al. TNF receptor I sensitizes neurons to erythropoietin- and VEGF-mediated neuroprotection after ischemic and excitotoxic injury. Proc. Natl Acad. Sci. USA 105, 6185–6190 (2008).
Madsen, P. M. et al. Genetic ablation of soluble tumor necrosis factor with preservation of membrane tumor necrosis factor is associated with neuroprotection after focal cerebral ischemia. J. Cereb. Blood Flow. Metab. 36, 1553–1569 (2016).
Dinarello, C. A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117, 3720–3732 (2011).
Zou, L. et al. The association between three promoter polymorphisms of IL-1 and stroke: A meta-analysis. Gene 567, 36–44 (2015).
Bis, J. C. et al. Variation in inflammation-related genes and risk of incident nonfatal myocardial infarction or ischemic stroke. Atherosclerosis 198, 166–173 (2008).
Thornton, P. et al. Platelet interleukin-1 alpha drives cerebrovascular inflammation. Blood 115, 3632–3639 (2010).
Davies, C. A. et al. The progression and topographic distribution of interleukin-1 beta expression after permanent middle cerebral artery occlusion in the rat. J. Cereb. Blood Flow. Metab. 19, 87–98 (1999).
Spulber, S., Bartfai, T. & Schultzberg, M. IL-1/IL-1ra balance in the brain revisited - Evidence from transgenic mouse models. Brain Behav. Immun. 23, 573–579 (2009).
Sobowale, O. A. et al. Interleukin-1 in stroke from bench to bedside. Stroke 47, 2160–2167 (2016).
Boutin, H. et al. Role of IL-1 alpha and IL-1 beta in ischemic brain damage. J. Neurosci. 21, 5528–5534 (2001).
McColl, B. W., Rothwell, N. J. & Allan, S. M. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J. Neurosci. 27, 4403–4412 (2007).
Erta, M., Quintana, A. & Hidalgo, J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 8, 1254–1266 (2012).
Gronhoj, M. H. et al. Beneficial potential of intravenously administered IL-6 in improving outcome after murine experimental stroke. Brain Behav. Immun. 65, 296–311 (2017).
Riethmueller, S. et al. Cleavage site localization differentially controls interleukin-6 receptor proteolysis by ADAM10 and ADAM17. Sci. Rep. 6, 25550 (2016).
Wolf, J., Rose-John, S. & Garbers, C. Interleukin-6 and its receptors: A highly regulated and dynamic system. Cytokine 70, 11–20 (2014).
Rothaug, M., Becker-Pauly, C. & Rose-John, S. The role of interleukin-6 signaling in nervous tissue. Biochimica Et. Biophysica Acta-Mol. Cell Res. 1863, 1218–1227 (2016).
Scheller, J., Chalaris, A., Schmidt-Arras, D. & Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochimica Et. Biophysica Acta-Mol. Cell Res. 1813, 878–888 (2011).
Smith, C. J. et al. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 4, 2 (2004).
Beridze, M. et al. Selected acute phase CSF factors in ischemic stroke: findings and prognostic value. BMC Neurol. 11, 41 (2011).
Singh, N. et al. The effect of intravenous interleukin-1 receptor antagonist on inflammatory mediators in cerebrospinal fluid after subarachnoid haemorrhage: a phase II randomised controlled trial. J. Neuroinflammation. 11, 1 (2014).
Meng, C. et al. Inhibition of interleukin-6 abolishes the promoting effects of pair housing on post-stroke neurogenesis. Neuroscience 307, 160–170 (2015).
Sabat, R. et al. Biology of interleukin-10. Cytokine Growth Factor Rev. 21, 331–344 (2010).
Xu, L. et al. Astrocyte targeted overexpression of Hsp72 or SOD2 reduces neuronal vulnerability to forebrain ischemia. Glia 58, 1042–1049 (2010).
de Bilbao, F. et al. In vivo over-expression of interleukin-10 increases resistance to focal brain ischemia in mice. J. Neurochem. 110, 12–22 (2009).
Perez-de Puig, I. et al. IL-10 deficiency exacerbates the brain inflammatory response to permanent ischemia without preventing resolution of the lesion. J. Cereb. Blood Flow. Metab. 33, 1955–1966 (2013).
Vila, N. et al. Levels of anti-inflammatory cytokines and neurological worsening in acute ischemic stroke. Stroke 34, 671–675 (2003).
Protti, G. G., Gagliardi, R. J., Forte, W. C. N. & Sprovieri, S. R. S. Interleukin-10 may protect against progressing injury during the acute phase of ischemic stroke. Arq. Neuropsiquiatr. 71, 846–851 (2013).
Mennicken, F., Maki, R., de Souza, E. B. & Quirion, R. Chemokines and chemokine receptors in the CNS: a possible role in neuroinflammation and patterning. Trends Pharmacol. Sci. 20, 73–78 (1999).
Hughes, P. M. et al. Monocyte chemoattractant protein-1 deficiency is protective in a murine stroke model. J. Cereb. Blood Flow. Metab. 22, 308–317 (2002).
Dimitrijevic, O. B., Stamatovic, S. M., Keep, R. F. & Andjelkovic, A. V. Absence of the chemokine receptor CCR2 protects against cerebral Ischemia/reperfusion injury in mice. Stroke 38, 1345–1353 (2007).
Chen, Y. et al. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J. Cereb. Blood Flow. Metab. 23, 748–755 (2003).
Kim, J. S. et al. Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 after focal cerebral ischemia in the rat. J. Neuroimmunol. 56, 127–134 (1995).
Takami, S. et al. Chemokine receptor antagonist peptide, viral MIP-II, protects the brain against focal cerebral ischemia in mice. J. Cereb. Blood Flow. Metab. 21, 1430–1435 (2001).
Terao, S. et al. Blood cell-derived RANTES mediates cerebral microvascular dysfunction, inflammation, and tissue injury after focal ischemia-reperfusion. Stroke 39, 2560–2570 (2008).
Montecucco, F. et al. Systemic and intraplaque mediators of inflammation are increased in patients symptomatic for ischemic stroke. Stroke 41, 1394–1404 (2010).
Chen, C. et al. Chemokines play complex roles in cerebral ischemia. Neurochem. Int. 112, 146–158 (2018).
Glezer, I., Simard, A. R. & Rivest, S. Neuroprotective role of the innate immune system by microglia. Neuroscience 147, 867–883 (2007).
McGettrick, A. F. & O’Neill, L. A. J. Toll-like receptors: key activators of leucocytes and regulator of haematopoiesis. Br. J. Haematol. 139, 185–193 (2007).
Arancibia, S. A. et al. Toll-like receptors are key participants in innate immune responses. Biol. Res. 40, 97–112 (2007).
Manicassamy, S. & Pulendran, B. Modulation of adaptive immunity with Toll-like receptors. Semin. Immunol. 21, 185–193 (2009).
Wu, Y. & Zhou, B. P. TNF-alpha/NF-kappa B/Snail pathway in cancer cell migration and invasion. Br. J. Cancer 102, 639–644 (2010).
Chaturvedi, A. & Pierce, S. K. How location governs toll-like receptor signaling. Traffic 10, 621–628 (2009).
Balch, M. H. H., Nimjee, S. M., Rink, C. & Hannawi, Y. Beyond the brain: the systemic pathophysiological response to acute ischemic stroke. J. Stroke 22, 159 (2020).
Florez-Alvarez, L., Ruiz-Perez, L., Taborda, N. & Hernandez, J. C. Toll-like receptors as a therapeutic target in cancer, infections and inflammatory diseases. Immunotherapy 12, 311–322 (2020).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
Brien, J. D. et al. Interferon Regulatory Factor-1 (IRF-1) shapes both innate and CD8(+) T cell immune responses against West Nile virus infection. Plos Pathog. 7, e1002230 (2011).
Takahashi, H. & Nishibori, M. Current status and future prospects in HMGB1 and receptor researches. Nihon Rinsho. 74, 703–711 (2016).
Marsh, B. et al. Systemic Lipopolysaccharide protects the brain from ischemic injury by reprogramming the response of the brain to stroke: a critical role for IRF3. J. Neurosci. 29, 9839–9849 (2009).
Gesuete, R., Kohama, S. G. & Stenzel-Poore, M. P. Toll-like receptors and ischemic brain injury. J. Neuropathol. Exp. Neurol. 73, 378–386 (2014).
Vartanian, K. B. et al. LPS preconditioning redirects TLR signaling following stroke: TRIF-IRF3 plays a seminal role in mediating tolerance to ischemic injury. J. Neuroinflammation. 8, 140 (2011).
Kurita, N. et al. Metabolic endotoxemia promotes neuroinflammation after focal cerebral ischemia. J. Cereb. Blood Flow. Metab. 40, 2505–2520 (2020).
Singh, V., Roth, S., Veltkamp, R. & Liesz, A. HMGB1 as a key mediator of immune mechanisms in ischemic stroke. Antioxid. Redox Signal. 24, 635–651 (2016).
Magna, M. & Pisetsky, D. S. The role of HMGB1 in the pathogenesis of inflammatory and autoimmune diseases. Mol. Med. 20, 138–146 (2014).
Yang, H. & Tracey, K. J. Targeting HMGB1 in inflammation. Biochimica Et. Biophysica Acta-Gene Regul. Mech. 1799, 149–156 (2010).
Yang, H., Wang, H. C., Czura, C. J. & Tracey, K. J. The cytokine activity of HMGB1. J. Leukoc. Biol. 78, 1–8 (2005).
Qiu, J. et al. Early release of HMGB-1 from neurons after the onset of brain ischemia. J. Cereb. Blood Flow. Metab. 28, 927–938 (2008).
Deng, W. et al. Transcriptomic characterization of microglia activation in a rat model of ischemic stroke. J. Cereb. Blood Flow. Metab. 40, S34–S48 (2020).
Yang, H. et al. MD-2 is required for disulfide HMGB1-dependent TLR4 signaling. J. Exp. Med. 212, 5–14 (2015).
Kim, E. J. et al. HMGB1 increases IL-1 beta production in vascular smooth muscle cells via NLRP3 inflammasome. Front. Physiol. 9, 313 (2018).
Faraco, G. et al. High mobility group box I protein is released by neural cells upon different stresses and worsens ischemic neurodegeneration in vitro and in vivo. J. Neurochem. 103, 590–603 (2007).
Kim, E. K. & Choi, E.-J. Compromised MAPK signaling in human diseases: an update. Arch. Toxicol. 89, 867–882 (2015).
Sun, J. & Nan, G. The Mitogen-Activated Protein Kinase (MAPK) signaling pathway as a discovery target in stroke. J. Mol. Neurosci. 59, 90–98 (2016).
Choudhury, G. R. et al. Involvement of p38 MAPK in reactive astrogliosis induced by ischemic stroke. Brain Res. 1551, 45–58 (2014).
Zhang, T. S. et al. Excess salt intake promotes M1 microglia polarization via a p38/MAPK/AR-dependent pathway after cerebral ischemia in mice. Int. Immunopharmacol. 81, 106176 (2020).
Maddahi, A. & Edvinsson, L. Cerebral ischemia induces microvascular pro-inflammatory cytokine expression via the MEK/ERK pathway. J. Neuroinflammation. 7, 14 (2010).
Zhang, T. S. et al. Excess salt exacerbates blood-brain barrier disruption via a p38/MAPK/SGK1-dependent pathway in permanent cerebral ischemia. Sci. Rep. 5, 16548 (2015).
Rosenberg, G. A. & Yang, Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg. Focus. 22, E4–E4 (2007).
Pfefferkorn, T. & Rosenberg, G. A. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke 34, 2025–2030 (2003).
Bao Dang, Q. et al. High-density lipoproteins limit neutrophil-induced damage to the blood-brain barrier in vitro. J. Cereb. Blood Flow. Metab. 33, 575–582 (2013).
Barr, T. L. et al. Blood-brain barrier disruption in humans is independently associated with increased matrix metalloproteinase-9. Stroke 41, E123–E128 (2010).
Zhu, S. Z. et al. Hypothermia followed by rapid rewarming exacerbates ischemia-induced brain injury and augments inflammatory response in rats. Biochem. Biophys. Res. Commun. 474, 175–181 (2016).
Chelluboina, B. et al. Matrix metalloproteinase-12 induces blood-brain barrier damage after focal cerebral ischemia. Stroke 46, 3523–3531 (2015).
Chi, O. Z. et al. Hypoxic preconditioning increases blood-brain barrier disruption in the early stages of cerebral ischemia. Curr. Neurovasc. Res. 14, 26–31 (2017).
Shen, Y. et al. Inhibition of HIF-1 alpha reduced blood brain barrier damage by regulating MMP-2 and VEGF during acute cerebral ischemia. Front. Cell. Neurosci. 12, 288 (2018).
Spiegel, S. & Milstien, S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 4, 397–407 (2003).
Lv, M. et al. Sphingosine kinase 1/sphingosine-1-phosphate regulates the expression of interleukin-17A in activated microglia in cerebral ischemia/reperfusion. Inflamm. Res. 65, 551–562 (2016).
Hyakkoku, K. et al. Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience 171, 258–267 (2010).
Qin, C. et al. Fingolimod protects against ischemic white matter damage by modulating microglia toward M2 polarization via STAT3 pathway. Stroke 48, 3336–3346 (2017).
Schroder, K. & Tschopp, J. The Inflammasomes. Cell 140, 821–832 (2010).
Mohamed, I. N., Ishrat, T., Fagan, S. C. & El-Remessy, A. B. Role of inflammasome activation in the pathophysiology of vascular diseases of the neurovascular unit. Antioxid. Redox Signal. 22, 1188–1206 (2015).
Fann, D. Y.-W. et al. Pathogenesis of acute stroke and the role of inflammasomes. Ageing Res. Rev. 12, 941–966 (2013).
Yang, F. et al. NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J. Cereb. Blood Flow. Metab. 34, 660–667 (2014).
Chi, W. et al. HMGB1 promotes the activation of NLRP3 and caspase-8 inflammasomes via NF-kappa B pathway in acute glaucoma. J. Neuroinflammation. 12, 137 (2015).
Fann, D. Y. W. et al. Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis. 4, e790 (2013).
Wang, K. et al. Central nervous system diseases related to pathological microglial phagocytosis. CNS Neurosci. Ther. 27, 528–539 (2021).
Kawabori, M. et al. Triggering Receptor Expressed on Myeloid Cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke. J. Neurosci. 35, 3384–3396 (2015).
Ting, S.-M. et al. Brain cleanup as a potential target for poststroke recovery the role of RXR (Retinoic X Receptor) in phagocytes. Stroke 51, 958–966 (2020).
Brown, G. C. & Neher, J. J. Microglial phagocytosis of live neurons. Nat. Rev. Neurosci. 15, 209–216 (2014).
Neher, J. J. et al. Phagocytosis executes delayed neuronal death after focal brain ischemia. Proc. Natl Acad. Sci. USA 110, E4098–E4107 (2013).
Zhang, Y. et al. TMEM16F aggravates neuronal loss by mediating microglial phagocytosis of neurons in a rat experimental cerebral ischemia and reperfusion model. Front. Immunol. 11, 1144 (2020).
Gervois, P. & Lambrichts, I. The emerging role of triggering receptor expressed on myeloid Cells 2 as a target for immunomodulation in ischemic stroke. Front. Immunol. 10, 1668 (2019).
Campagne, M. V. L., Wiesmann, C. & Brown, E. J. Macrophage complement receptors and pathogen clearance. Cell. Microbiol. 9, 2095–2102 (2007).
Ma, Y., Liu, Y., Zhang, Z. & Yang, G.-Y. Significance of complement system in ischemic stroke: a comprehensive review. Aging Dis. 10, 429–462 (2019).
Fraser, D. A., Pisalyaput, K. & Tenner, A. J. C1q enhances microglial clearance of apoptotic neurons and neuronal blebs, and modulates subsequent inflammatory cytokine production. J. Neurochem. 112, 733–743 (2010).
Alawieh, A., Langley, E. F. & Tomlinson, S. Targeted complement inhibition salvages stressed neurons and inhibits neuroinflammation after stroke in mice. Sci. Transl. Med. 10, eaao6459 (2018).
Alawieh, A. M. et al. Complement-dependent synaptic uptake and cognitive decline after stroke and reperfusion therapy. J. Neurosci. 40, 4042–4058 (2020).
Fukuta, T. et al. Combination therapy with liposomal neuroprotectants and tissue plasminogen activator for treatment of ischemic stroke. FASEB J. 31, 1879–1890 (2017).
Rink, C. & Khanna, S. MicroRNA in ischemic stroke etiology and pathology. Physiol. Genomics. 43, 521–528 (2011).
Trounson, A. & McDonald, C. Stem cell therapies in clinical trials: progress and challenges. Cell. Stem Cell. 17, 11–22 (2015).
Chen, Y. et al. Tat-NR2B9c prevents excitotoxic neuronal superoxide production. J. Cereb. Blood Flow. Metab. 35, 739–742 (2015).
Cook, D. J., Teves, L. & Tymianski, M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature 483, 213–U112 (2012).
Teves, L. M., Cui, H. & Tymianski, M. Efficacy of the PSD95 inhibitor Tat-NR2B9c in mice requires dose translation between species. J. Cereb. Blood Flow. Metab. 36, 555–561 (2016).
Soriano, F. X. et al. Specific targeting of pro-death NMDA receptor signals with differing reliance on the NR2B PDZ Ligand. J. Neurosci. 28, 10696–10710 (2008).
Hill, M. D. et al. Safety and efficacy of NA-1 in patients with iatrogenic stroke after endovascular aneurysm repair (ENACT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 11, 942–950 (2012).
Luo, C.-X. et al. Interaction of nNOS with PSD-95 negatively controls regenerative repair after stroke. J. Neurosci. 34, 13535–13548 (2014).
Hong, J. M. et al. Safety and optimal neuroprotection of neu2000 in acute Ischemic stroke with reCanalization: study protocol for a randomized, double-blinded, placebo-controlled, phase-II trial. Trials 19, 375 (2018).
Cho, S. I., Park, U. J., Chung, J.-M. & Gwag, B. J. NEU2000, AN NR2B-Selective, moderate nmda receptor antagonist and potent spin trapping molecule for stroke. Drug N. Perspect. 23, 549–556 (2010).
Ginsberg, M. D. Neuroprotection for ischemic stroke: Past, present and future. Neuropharmacology 55, 363–389 (2008).
Takaoka, S. et al. Neuroprotective effect of NMDA receptor glycine recognition site antagonism persists when brain temperature is controlled. J. Cereb. Blood Flow. Metab. 17, 161–167 (1997).
Muir, K. W. & Lees, K. R. Clinical experience with excitatory amino acid antagonist drugs. Stroke 26, 503–513 (1995).
Lai, T. W., Shyu, W.-C. & Wang, Y. T. Stroke intervention pathways: NMDA receptors and beyond. Trends Mol. Med. 17, 266–275 (2011).
Wood, P. L. & Hawkinson, J. E. N-methyl-D-aspartate antagonists for stroke and head trauma. Expert Opin. Investig. Drugs 6, 389–397 (1997).
Albers, G. W., Atkinson, R. P., Kelley, R. E. & Rosenbaum, D. M. Safety, tolerability, and pharmacokinetics of the N-methyl-D-aspartate antagonist dextrorphan in patients with acute stroke. Stroke 26, 254–258 (1995).
Grotta, J. et al. Safety and tolerability of the glutamate antagonist CGS 19755 (Selfotel) in patients with acute ischemic stroke: Results of a phase IIa randomized trial. Stroke 26, 602–605 (1995).
Diener, H. C. et al. Treatment of acute ischaemic stroke with the low-affinity, use-dependent NMDA antagonist AR-R15896AR - A safety and tolerability study. J. Neurol. 249, 561–568 (2002).
Pei, L. et al. DAPK1-p53 interaction converges necrotic and apoptotic pathways of ischemic neuronal death. J. Neurosci. 34, 6546–6556 (2014).
Wang, X. et al. Intervention of death-associated protein Kinase 1-p53 interaction exerts the therapeutic effects against stroke. Stroke 45, 3089 (2014).
McQueen, J. et al. Pro-death NMDA receptor signaling is promoted by the GIuN2B C-terminus independently of Dapk1. Elife 6, e17161 (2017).
Huang, B. S. et al. Geniposide attenuates post-ischaemic neurovascular damage via GluN2A/AKT/ERK-dependent mechanism. Cell. Physiol. Biochem. 43, 705–716 (2017).
Liu, Y. et al. Neuroprotective effect of pseudoginsenoside-F11 on permanent cerebral ischemia in rats by regulating calpain activity and NR2A submit-mediated AKT-CREB signaling pathways. Phytomed: Int. J. Phytother. Phytopharmacol. 96, 153847 (1800).
Alim, I. et al. Modulation of NMDAR subunit expression by TRPM2 channels regulates neuronal vulnerability to ischemic cell death. J. Neurosci. 33, 17264–17277 (2013).
Thompson, R. J. Pannexin channels and ischaemia. J. Physiol. -Lond. 593, 3463–3470 (2015).
Weilinger, N. L. et al. Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat. Neurosci. 19, 432 (2016).
Alfieri, A. et al. Sulforaphane preconditioning of the Nrf2/HO-1 defense pathway protects the cerebral vasculature against blood-brain barrier disruption and neurological deficits in stroke. Free Radic. Biol. Med. 65, 1012–1022 (2013).
Hou, Y. et al. Nrf2 inhibits NLRP3 inflammasome activation through regulating Trx1/TXNIP complex in cerebral ischemia reperfusion injury. Behav. Brain Res. 336, 32–39 (2018).
Prasad, S. et al. Role of Nrf2 and protective effects of Metformin against tobacco smoke-induced cerebrovascular toxicity. Redox Biol. 12, 58–69 (2017).
Zhang, W. et al. Sirtuin 6 Protects the brain from cerebral ischemia/reperfusion injury through NRF2 activation. Neuroscience 366, 95–104 (2017).
Hou, J., Chong, Z. Z., Shang, Y. C. & Maiese, K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr. Neurovasc. Res. 7, 95–112 (2010).
Morris, K. C., Lin, H. W., Thompson, J. W. & Perez-Pinzon, M. A. Pathways for ischemic cytoprotection: Role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning. J. Cereb. Blood Flow. Metab. 31, 1003–1019 (2011).
Wang, R. et al. Curcumin attenuates IR-induced myocardial injury by activating SIRT3. Eur. Rev. Med. Pharmacol. Sci. 22, 1150–1160 (2018).
Chang, G., Chen, Y., Zhang, H. & Zhou, W. Trans sodium crocetinate alleviates ischemia/reperfusion-induced myocardial oxidative stress and apoptosis via the SIRT3/FOXO3a/SOD2 signaling pathway. Int. Immunopharmacol. 71, 361–371 (2019).
Zhao, B. et al. Genipin protects against cerebral ischemia-reperfusion injury by regulating the UCP2-SIRT3 signaling pathway. Eur. J. Pharmacol. 845, 56–64 (2019).
Hammond, M. D. et al. CCR2(+)Ly6C(hi) inflammatory monocyte recruitment exacerbates acute disability following intracerebral hemorrhage. J. Neurosci. 34, 3901–3909 (2014).
Wattananit, S. et al. Monocyte-derived macrophages contribute to spontaneous long-term functional recovery after stroke in mice. J. Neurosci. 36, 4182–4195 (2016).
Lu, Y.-Y., Ma, X.-J. & Yang, Y.-N. MicroRNA-18a-5p mitigates oxygen-glucose-deprivation/reoxygenation-induced injury through suppression of TLRs/NF-kappa B signaling by targeting TLR8 in PC12 cells. Biosci., Biotechnol. Biochem. 84, 2476–2483 (2020).
Ghazavi, H. et al. The role of resveratrol as a natural modulator in glia activation in experimental models of stroke. Avicenna J. Phytomed. 10, 557–573 (2020).
Rahimifard, M. et al. Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation. Ageing Res. Rev. 36, 11–19 (2017).
Zhang, X.-S. et al. Resveratrol attenuates acute inflammatory injury in experimental subarachnoid hemorrhage in rats via inhibition of TLR4 pathway. Int. J. Mol. Sci. 17, 1331 (2016).
Zan, J. et al. Isosteviol sodium injection improves outcomes by modulating TLRs/NF-B-dependent inflammatory responses following experimental traumatic brain injury in rats. Neuroreport 29, 794–803 (2018).
Hsieh, J. T. et al. Sex-specific effects of progesterone on early outcome of intracerebral hemorrhage. Neuroendocrinology 103, 518–530 (2016).
Li, X. et al. Progesterone reduces inflammation and apoptosis in neonatal rats with hypoxic ischemic brain damage through the PI3K/Akt pathway. Int. J. Clin. Exp. Med. 8, 8197–8203 (2015).
Wang, Z. et al. Progesterone administration modulates cortical TLR4/NF-kappa B signaling pathway after subarachnoid hemorrhage in male rats. Mediators Inflamm. 2011, 848309 (2011).
Zhai, Y. et al. Dexmedetomidine post-conditioning alleviates cerebral ischemia-reperfusion injury in rats by inhibiting high mobility group Protein B1 Group (HMGB1)/Toll-Like Receptor 4 (TLR4)/Nuclear Factor kappa B (NF-kappa B) Signaling Pathway. Med. Sci. Monit. 26, e918617 (2020).
Leung, P. Y. et al. Toll-like Receptor 7 preconditioning induces robust neuroprotection against stroke by a novel Type I interferon-mediated mechanism. Stroke 43, 1383–1389 (2012).
Clausen, B. H. et al. Systemically administered anti-TNF therapy ameliorates functional outcomes after focal cerebral ischemia. J. Neuroinflammation. 11, 203 (2014).
Zhou, Q.-H. et al. Brain-penetrating tumor necrosis factor decoy receptor in the mouse. Drug Metab. Disposition. 39, 71–76 (2011).
Sumbria, R. K., Boado, R. J. & Pardridge, W. M. Brain protection from stroke with intravenous TNF alpha decoy receptor-Trojan horse fusion protein. J. Cereb. Blood Flow. Metab. 32, 1933–1938 (2012).
Liguz-Lecznar, M., Zakrzewska, R. & Kossut, M. Inhibition of Tnf-alpha R1 signaling can rescue functional cortical plasticity impaired in early post-stroke period. Neurobiol. Aging 36, 2877–2884 (2015).
Works, M. G., Koenig, J. B. & Sapolsky, R. M. Soluble TNF receptor 1-secreting ex vivo-derived dendritic cells reduce injury after stroke. J. Cereb. Blood Flow. Metab. 33, 1376–1385 (2013).
Scheinfeld, N. A comprehensive review and evaluation of the side effects of the tumor necrosis factor-alpha blockers etanercept, infliximab, and adalimumab. J. Dermatol. Treat. 15, 280–294 (2004).
Relton, J. K., Martin, D., Thompson, R. C. & Russell, D. A. Peripheral administration of interleukin-1 receptor antagonist inhibits brain damage after focal cerebral ischemia in the rat. Exp. Neurol. 138, 206–213 (1996).
Loddick, S. A. & Rothwell, N. J. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J. Cereb. Blood Flow. Metab. 16, 932–940 (1996).
Nawashiro, H., Martin, D. & Hallenbeck, J. M. Neuroprotective effects of TNF binding protein in focal cerebral ischemia. Brain Res. 778, 265–271 (1997).
Emsley, H. C. A. et al. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J. Neurol. Neurosurg. Psychiatry 76, 1366–1372 (2005).
Gueorguieva, I. et al. Pharmacokinetic modelling of interleukin-1 receptor antagonist in plasma and cerebrospinal fluid of patients following subarachnoid haemorrhage. Br. J. Clin. Pharmacol. 65, 317–325 (2008).
Granowitz, E. V. et al. Pharmacokinetics, safety and immunomodulatory effects of human recombinant interleukin-1 receptor antagonist in healthy humans. Cytokine 4, 353–360 (1992).
Smith, C. J. et al. SCIL-STROKE (Subcutaneous Interleukin-1 Receptor Antagonist in Ischemic Stroke) a randomized controlled Phase 2 Trial. Stroke 49, 1210 (2018).
Hong, P. et al. NLRP3 inflammasome as a potential treatment in ischemic stroke concomitant with diabetes. J. Neuroinflammation. 16, (2019).
Ye, X. C. et al. Purinergic 2X7 receptor/NLRP3 pathway triggers neuronal apoptosis after ischemic stroke in the mouse. Exp. Neurol. 292, 46–55 (2017).
Wang, S. Q. et al. Genistein attenuates acute cerebral ischemic damage by inhibiting the NLRP3 inflammasome in reproductively senescent mice. Front. Aging Neurosci. 12, (2020).
Chen, X. Y., Wang, Y. Z., Yao, N. N. & Lin, Z. J. Immunoproteasome modulates NLRP3 inflammasome-mediated neuroinflammation under cerebral ischaemia and reperfusion conditions. J. Cell. Mol. Med. 26, 462–474 (2022).
Yang, R. et al. Quercetin attenuates ischemia reperfusion injury by protecting the blood-brain barrier through Sirt1 in MCAO rats. J. Asian Nat. Prod. Res. 24, 278–289 (2022).
Yang, F. et al. Minocycline ameliorates hypoxia-induced blood-brain barrier damage by inhibition of HIF-1 alpha through sirt-3/PHD-2 degradation pathway. Neuroscience 304, 250–259 (2015).
Liu, H. et al. Hydrogen sulfide attenuates tissue plasminogen activator-induced cerebral hemorrhage following experimental stroke. Transl. Stroke Res. 7, 209–219 (2016).
Yang, Y. et al. Non-invasive vagus nerve stimulation reduces blood-brain barrier disruption in a rat model of ischemic stroke. Brain Stimul. 11, 689–698 (2018).
Michalski, D. et al. Early outcome and blood-brain barrier integrity after co-administered thrombolysis and hyperbaric oxygenation in experimental stroke. Exp. Transl. Stroke Med. 3, 5–5 (2011).
Khan, I. S. et al. Intraarterial administration of norcantharidin attenuates ischemic stroke damage in rodents when given at the time of reperfusion: novel uses of endovascular capabilities. J. Neurosurg. 125, 152–159 (2016).
Hasegawa, Y. et al. Activation of Sphingosine 1-Phosphate Receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke 41, 368–374 (2010).
Wei, Y. et al. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann. Neurol. 69, 119–129 (2011).
Li, X. et al. Fingolimod suppresses neuronal autophagy through the mTOR/p70S6K pathway and alleviates ischemic brain damage in mice. PLoS One. 12, (2017).
Yang, B. A. et al. Selenium attenuates ischemia/reperfusion injury-induced damage to the blood-brain barrier in hyperglycemia through PI3K/AKT/mTOR pathway-mediated autophagy inhibition. Int. J. Mol. Med. 48, (2021).
Guo, Z. et al. A combination of four active compounds alleviates cerebral ischemia-reperfusion injury in correlation with inhibition of autophagy and modulation of AMPK/mTOR and JNK pathways. J. Neurosci. Res. 92, 1295–1306 (2014).
Wang, G. et al. Schizandrin protects against OGD/R-induced neuronal injury by suppressing autophagy: involvement of the AMPK/mTOR pathway. Molecules. 24, (2019).
Yu, J. et al. Ezetimibe, a NPC1L1 inhibitor, attenuates neuronal apoptosis through AMPK dependent autophagy activation after MCAO in rats. Exp. Neurol. 307, 12–23 (2018).
Schwalm, C. et al. Activation of autophagy in human skeletal muscle is dependent on exercise intensity and AMPK activation. Faseb. J. 29, 3515–3526 (2015).
Batatinha, H. A. P. et al. Regulation of autophagy as a therapy for immunosenescence-driven cancer and neurodegenerative diseases: The role of exercise. J. Cell. Physiol. 234, 14883–14895 (2019).
Pan, G. et al. Treadmill exercise improves neurological function by inhibiting autophagy and the binding of HMGB1 to Beclin1 in MCAO juvenile rats. Life Sci. 243, 117279 (2020).
Xia, Y. et al. Small extracellular vesicles secreted by human iPSC-derived MSC enhance angiogenesis through inhibiting STAT3-dependent autophagy in ischemic stroke. Stem Cell. Res. Ther. 11, (2020).
Mei, Z.-G. et al. Electroacupuncture ameliorates cerebral ischemia/reperfusion injury by suppressing autophagy via the SIRT1-FOXO1 signaling pathway. Aging-Us. 12, 13187–13205 (2020).
Xu, S. Y. et al. Electroacupuncture alleviates cerebral ischemia/reperfusion injury in rats by Histone H4 Lysine 16 acetylation-mediated autophagy. Front. Psychiatry 11, 576539 (2020).
Liu, S. et al. Luteolin protects against CIRI, potentially via regulation of the SIRT3/AMPK/mTOR signaling pathway. Neurochem. Res. 45, 2499–2515 (2020).
Liu, L. et al. Melatonin ameliorates cerebral ischemia-reperfusion injury in diabetic mice by enhancing autophagy via the SIRT1-BMAL1 pathway. FASEB J. 35, e22040 (2021).
Li, Y., Zhang, X. P., Ma, A. J. & Kang, Y. Rational application of beta-Hydroxybutyrate attenuates ischemic stroke by suppressing oxidative stress and mitochondrial-dependent apoptosis via activation of the Erk/CREB/eNOS pathway. ACS Chem. Neurosci. 12, 1219–1227 (2021).
Yong, Y.-X. et al. Up-regulated microRNA-199b-3p represses the apoptosis of cerebral microvascular endothelial cells in ischemic stroke through down-regulation of MAPK/ERK/EGR1 axis. Cell Cycle 18, 1868–1881 (2019).
Xiong, W. et al. DAPK1-ERK signal mediates oxygen glucose deprivation reperfusion induced apoptosis in mouse N2a cells. J. Neurol. Sci. 387, 210–219 (2018).
Guo, T. et al. Promoting role of long non-coding RNA small nucleolar RNA Host Gene 15 (SNHG15) in neuronal injury following ischemic stroke via the MicroRNA-18a/CXC Chemokine Ligand 13 (CXCL13)/ERK/MEK Axis. Med. Sci. Monit. 26, e923610 (2020).
Hu, Y. et al. AMPK inhibitor BML-275 induces neuroprotection through decreasing cyt c and AIF expression after transient brain ischemia. Biorg. Med. Chem. 52, 116522 (2021).
Chen, Z. J. et al. Glycine improves ischemic stroke through miR-19a-3p/AMPK/GSK-3 beta/HO-1 pathway. Drug Des. Devel. Ther. 14, 2021–2031 (2020).
Yan, L. & Zhu, T. Effects of rosuvastatin on neuronal apoptosis in cerebral ischemic stroke rats via Sirt1/NF-kappa B signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 23, 5449–5455 (2019).
Sarmah, D. et al. Sirtuin-1 - Mediated NF-κB pathway modulation to mitigate inflammasome signaling and cellular apoptosis is one of the neuroprotective effects of intra-arterial mesenchymal stem cell therapy following ischemic stroke. Stem. Cell. Rev. Rep. 18, 821–838 (2022).
Xu, J., Wang, C., Meng, F. & Xu, P. Long non-coding RNA H19 inhibition ameliorates oxygen-glucose deprivation-induced cell apoptosis and inflammatory cytokine expression by regulating the microRNA-29b/SIRT1/PGC-1 alpha axis. Mol. Med. Rep. 23, 131 (2021).
Kawabori, M., Shichinohe, H., Kuroda, S. & Houkin, K. Clinical trials of stem cell therapy for cerebral ischemic stroke. Int. J. Mol. Sci. 21, 7380 (2020).
Zhou, L. et al. Treatment of cerebral ischemia by disrupting ischemia-induced interaction of nNOS with PSD-95. Nat. Med. 16, 1439–1443 (2010).
Bach, A. et al. Biochemical investigations of the mechanism of action of small molecules ZL006 and IC87201 as potential inhibitors of the nNOS-PDZ/PSD-95-PDZ interactions. Sci. Rep. 5, 12157 (2015).
Kim, Y. R. et al. Electroacupuncture confers beneficial effects through ionotropic glutamate receptors involving phosphatidylinositol-3 kinase/Akt signaling pathway in focal cerebral ischemia in rats. Eur. J. Int. Med. 4, E413–E420 (2012).
Weilinger, N. L., Tang, P. L. & Thompson, R. J. Anoxia-induced NMDA receptor activation opens pannexin channels via Src Family Kinases. J. Neurosci. 32, 12579–12588 (2012).
Abdul, Y. et al. Inhibition of Toll-Like Receptor-4 (TLR-4) improves neurobehavioral outcomes after acute ischemic stroke in diabetic rats: possible role of vascular endothelial TLR-4. Mol. Neurobiol. 56, 1607–1617 (2019).
Shi, H. et al. Role of Toll-like receptor-mediated signaling in traumatic brain injury. Neuropharmacology 145, 259–267 (2019).
Wang, Y., Ge, P. & Zhu, Y. TLR2 and TLR4 in the brain injury caused by cerebral ischemia and reperfusion. Mediators Inflamm. 2013, 124614 (2013).
Gui, B. et al. Neuroprotective effects of pretreatment with Propofol in LPS-induced BV-2 microglia cells: role of TLR4 and GSK-3 beta. Inflammation 35, 1632–1640 (2012).
Marik, P. E. Propofol: An immunomodulating agent. Pharmacotherapy 25, 28S–33S (2005).
Yang, R. et al. Quercetin attenuates ischemia reperfusion injury by protecting the blood-brain barrier through Sirt1 in MCAO rats. J. Asian Nat. Prod. Res. 24, 278–289 (2021).
Wei, L. L. et al. Patchouli alcohol protects against ischemia/reperfusion-induced brain injury via inhibiting neuroinflammation in normal and obese mice. Brain Res. 1682, 61–70 (2018).
Mamtilahun, M. et al. DL-3n-Butylphthalide improves blood-brain barrier integrity in rat after middle cerebral artery occlusion. Front. Cell. Neurosci. 14, 610714 (2021).
Qi, Z. F. et al. AKT-related autophagy contributes to the neuroprotective efficacy of Hydroxysafflor Yellow A against ischemic stroke in rats. Transl. Stroke Res. 5, 501–509 (2014).
Wang, M. M. et al. Electroacupuncture inhibits neuronal autophagy and apoptosis via the PI3K/AKT pathway following ischemic stroke. Front. Cell. Neurosci. 14, 134 (2020).
Meng, J., Ma, H., Zhu, Y. & Zhao, Q. Dehydrocostuslactone attenuated oxygen and glucose deprivation/reperfusion-induced PC12 cell injury through inhibition of apoptosis and autophagy by activating the PI3K/AKT/mTOR pathway. Eur. J. Pharmacol. 911, 174554 (2021).
Zhang, X. et al. Alterations of brain quantitative proteomics profiling revealed the molecular mechanisms of diosgenin against cerebral lschemia reperfusion effects. J. Proteome Res. 19, 1154–1168 (2020).
Yang, B. et al. Melatonin plays a protective role by regulating miR-26a-5p-NRSF and JAK2-STAT3 pathway to improve autophagy, inflammation and oxidative stress of cerebral ischemia-reperfusion injury. Drug Des. Dev. Ther. 14, 3177–3188 (2020).
Pan, Q. et al. MTMR14 protects against cerebral stroke through suppressing PTEN-regulated autophagy. Biochem. Biophy. Res. Commun. 529, 1045–1052 (2020).
Xue, H. et al. Sevoflurane post-conditioning alleviates neonatal rat hypoxic-ischemic cerebral injury via Ezh2-regulated autophagy. Drug Des. Dev. Ther. 13, 1691–1706 (2019).
Zhong, S.-J. et al. MicroRNA-144 promotes remote limb ischemic preconditioning-mediated neuroprotection against ischemic stroke via PTEN/Akt pathway. Acta Neurol. Belg. 121, 95–106 (2021).
Mu, Q. et al. NPD1 inhibits excessive autophagy by targeting RNF146 and wnt/beta-catenin pathway in cerebral ischemia-reperfusion injury. J. Recept. Signal Transduct. 40, 456–463 (2020).
Chen, C. et al. Electroacupuncture pretreatment prevents ischemic stroke and inhibits Wnt signaling-mediated autophagy through the regulation of GSK-3 beta phosphorylation. Brain Res. Bull. 158, 90–98 (2020).
He, H. et al. Puerarin provides a neuroprotection against transient cerebral ischemia by attenuating autophagy at the ischemic penumbra in neurons but not in astrocytes. Neurosci. Lett. 643, 45–51 (2017).
Fu, K. et al. Grape seed proanthocyanidins attenuate apoptosis in ischemic stroke. Acta Neurol. Belg. 121, 357–364 (2021).
Yang, Y. et al. Apelin-13 protects against apoptosis by activating AMP-activated protein kinase pathway in ischemia stroke. Peptides 75, 96–100 (2016).
Gao, J. F., Qian, T. T. & Wang, W. CTRP3 Activates the AMPK/SIRT1-PGC-1 alpha pathway to protect mitochondrial biogenesis and functions in cerebral ischemic stroke. Neurochem. Res. 45, 3045–3058 (2020).
Lv, H. et al. Salvianolic acid B attenuates apoptosis and inflammation via SIRT1 activation in experimental stroke rats. Brain Res. Bull. 115, 30–36 (2015).
Sarmah, D. et al. Sirtuin-1-mediated NF-kappa B pathway modulation to mitigate inflammasome signaling and cellular apoptosis is one of the neuroprotective effects of intra-arterial mesenchymal stem cell therapy following ischemic stroke. Stem. Cell. Rev. Rep. 18, 821–838 (2022).
Zhou, M. et al. A DNA nanostructure-based neuroprotectant against neuronal apoptosis via inhibiting toll-like Receptor 2 signaling pathway in acute ischemic stroke. ACS Nano. 16, 1456–1470 (2022).
Gu, J. et al. Anti-inflammatory and anti-apoptotic effects of the combination of Ligusticum chuanxiong and Radix Paeoniae against focal cerebral ischaemia via TLR4/MyD88/MAPK/NF-kappa B signalling pathway in MCAO rats. J. Pharm. Pharmacol. 70, 268–277 (2018).
Acknowledgements
This work was funded by National Natural Science Foundation of China (Grants: 82071380, 81873743, 81801223), and Tongji Hospital (HUST) Foundation for Excellent Young Scientist (Grant No. 2020YQ06).
Author information
Authors and Affiliations
Contributions
All authors have read and approved the article. Concept and design: C.Q., D.S.T., and W.W. Drafting of the manuscript: C.Q., S.Y., Y.H.C., H.Z., X.W.P., L.C., L.Q.Z., and M.C. Critical revision of the manuscript for important intellectual content: D.S.T. and W.W. Obtained funding: C.Q. and D.S.T. Supervision: D.S.T. and W.W.
Corresponding authors
Ethics declarations
Competing interests
The author declares no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qin, C., Yang, S., Chu, YH. et al. Signaling pathways involved in ischemic stroke: molecular mechanisms and therapeutic interventions. Sig Transduct Target Ther 7, 215 (2022). https://doi.org/10.1038/s41392-022-01064-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-022-01064-1
This article is cited by
-
Mitochondrial stress: a key role of neuroinflammation in stroke
Journal of Neuroinflammation (2024)
-
Efficacy and safety of hyperbaric oxygen therapy in acute ischaemic stroke: a systematic review and meta-analysis
BMC Neurology (2024)
-
Anti-aging Factor GRSF1 Attenuates Cerebral Ischemia-Reperfusion Injury in Mice by Inhibiting GPX4-Mediated Ferroptosis
Molecular Neurobiology (2024)
-
Ripks and Neuroinflammation
Molecular Neurobiology (2024)
-
Progress of Ferroptosis in Ischemic Stroke and Therapeutic Targets
Cellular and Molecular Neurobiology (2024)