Abstract
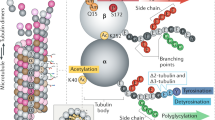
Microtubules play an important role in regulating several vital cellular activities, including cell division and tissue organization, through their dynamic protofilament network. In addition to forming the cytoskeleton, microtubules regulate the intracellular trafficking of cytoplasmic components and various signaling molecules, depending on the presence of post-transitional modifications (PTMs) and binding proteins. Accumulating evidence indicates the significant role of microtubule PTMs on cancer behavior. The PTMs that frequently occur on microtubules include acetylation, detyrosination, tyrosination, polyglutamylation, and polyglycylation. Alterations in these PTMs cause global effects on intracellular signal transduction, strongly linked to cancer pathogenesis. This review provides an update on the role of microtubule PTMs in cancer aggressiveness, particularly regarding cell death, sensitivity to chemotherapy, cell migration, and invasion. Additionally, it provides a mechanistic explanation of the molecular signaling pathways involved. This information might prove useful for predictive or therapeutic purposes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Nogales E. Structural insights into microtubule function. Annu Rev Biophys Biomol Struct. 2001;30:397–420.
Vicente JJ, Wordeman L. The quantification and regulation of microtubule dynamics in the mitotic spindle. Curr Opin Cell Biol. 2019;60:36–43.
Valiron O, Caudron N, Job D. Microtubule dynamics. Cell Mol Life Sci. 2001;58:2069–84.
Bodakuntla S, Jijumon AS, Villablanca C, Gonzalez-Billault C, Janke C. Microtubule-associated proteins: structuring the cytoskeleton. Trends Cell Biol. 2019;29:804–19.
Akhmanova A, Steinmetz MO. Control of microtubule organization and dynamics: two ends in the limelight. Nat Rev Mol Cell Biol. 2015;16:711–26.
Gadadhar S, Bodakuntla S, Natarajan K, Janke C. The tubulin code at a glance. J Cell Sci. 2017;130:1347–53.
Magiera MM, Janke C. Post-translational modifications of tubulin. Curr Biol. 2014;24:R351–R354.
Janke C. The tubulin code: molecular components, readout mechanisms, and functions. J Cell Biol. 2014;206:461–72.
Eshun-Wilson L, Zhang R, Portran D, Nachury MV, Toso DB, Löhr T, et al. Effects of α-tubulin acetylation on microtubule structure and stability. Proc Natl Acad Sci USA. 2019;116:10366–71.
Portran D, Schaedel L, Xu Z, Thery M, Nachury MV. Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nat Cell Biol. 2017;19:391–8.
Magiera MM, Singh P, Gadadhar S, Janke C. Tubulin posttranslational modifications and emerging links to human disease. Cell 2018;173:1323–7.
Sept D. Microtubule polymerization: one step at a time. Curr Biol. 2007;17:R764–R766.
Aranson IS, Tsimring LS. Pattern formation of microtubules and motors: Inelastic interaction of polar rods. Phys Rev E. 2005;71:050901.
Burbank KS, Mitchison TJ. Microtubule dynamic instability. Curr Biol. 2006;16:R516–R517.
Etienne-Manneville S. Microtubules in cell migration. Annu Rev Cell Dev Biol. 2013;29:471–99.
Jiang K, Hua S, Mohan R, Grigoriev I, Yau KW, Liu Q, et al. Microtubule minus-end stabilization by polymerization-driven CAMSAP deposition. Dev Cell. 2014;28:295–309.
Jiang K, Akhmanova A. Microtubule tip-interacting proteins: a view from both ends. Curr Opin Cell Biol. 2011;23:94–101.
Sharma N, Kosan ZA, Stallworth JE, Berbari NF, Yoder BK. Soluble levels of cytosolic tubulin regulate ciliary length control. Mol Biol Cell. 2011;22:806–16.
Schappi JM, Krbanjevic A, Rasenick MM. Tubulin, actin and heterotrimeric G proteins: coordination of signaling and structure. Biochim Biophys Acta. 2014;1838:674–81.
Ludueña RF, Banerjee A. The isotypes of tubulin. In The role of microtubules in cell biology, neurobiology, and oncology. Humana Press. 2008;123–75.
Mariani M, Karki R, Spennato M, Pandya D, He S, Andreoli M, et al. Class III β-tubulin in normal and cancer tissues. Gene 2015;563:109–14.
Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12:773–86.
Borys F, Joachimiak E, Krawczyk H, Fabczak H. Intrinsic and extrinsic factors affecting microtubule dynamics in normal and cancer cells. Molecules 2020;25:3705.
Souček K, Kamaid A, Phung AD, Kubala L, Bulinski JC, Harper RW, et al. Normal and prostate cancer cells display distinct molecular profiles of α‐tubulin posttranslational modifications. Prostate. 2006;66:954–65.
Wloga D, Joachimiak E, Fabczak H. Tubulin post-translational modifications and microtubule dynamics. Int J Mol Sci. 2017;18:2207.
Friedmann DR, Aguilar A, Fan J, Nachury MV, Marmorstein R. Structure of the α-tubulin acetyltransferase, αTAT1, and implications for tubulin-specific acetylation. Proc Natl Acad Sci USA. 2012;109:19655–60.
Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, et al. HDAC6 is a microtubule-associated deacetylase. Nature 2002;417:455–8.
North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–44.
Wloga D, Webster DM, Rogowski K, Bré MH, Levilliers N, Jerka-Dziadosz M, et al. TTLL3 is a tubulin glycine ligase that regulates the assembly of cilia. Dev Cell. 2009;16:867–76.
Janke C, Rogowski K, Wloga D, Regnard C, Kajava AV, Strub JM, et al. Tubulin polyglutamylase enzymes are members of the TTL domain protein family. Science 2005;308:1758–62.
Kimura Y, Kurabe N, Ikegami K, Tsutsumi K, Konishi Y, Kaplan OI, et al. Identification of tubulin deglutamylase among Caenorhabditis elegans and mammalian cytosolic carboxypeptidases (CCPs). J Biol Chem. 2010;285:22936–41.
Song Y, Kirkpatrick LL, Schilling AB, Helseth DL, Chabot N, Keillor JW, et al. Transglutaminase and polyamination of tubulin: posttranslational modification for stabilizing axonal microtubules. Neuron 2013;78:109–23.
Parker AL, Kavallaris M, McCarroll JA. Microtubules and their role in cellular stress in cancer. Front Oncol. 2014;4:153.
Ganguly A, Yang H, Sharma R, Patel KD, Cabral F. The role of microtubules and their dynamics in cell migration. J Biol Chem. 2012;287:43359–69.
Giannakakou P, Nakano M, Nicolaou KC, O’Brate A, Yu J, Blagosklonny MV, et al. Enhanced microtubule-dependent trafficking and p53 nuclear accumulation by suppression of microtubule dynamics. Proc Natl Acad Sci USA. 2002;99:10855–60.
Stewart ZA, Tang LJ, Pietenpol JA. Increased p53 phosphorylation after microtubule disruption is mediated in a microtubule inhibitor- and cell-specific manner. Oncogene 2001;20:113–24.
Nagae S, Meng W, Takeichi M. Non-centrosomal microtubules regulate F-actin organization through the suppression of GEF-H1 activity. Genes Cells. 2013;18:387–96.
Galigniana MD, Harrell JM, O’Hagen HM, Ljungman M, Pratt WB. Hsp90-binding immunophilins link p53 to dynein during p53 transport to the nucleus. J Biol Chem. 2004;279:22483–9.
Fife CM, McCarroll JA, Kavallaris M. Movers and shakers: cell cytoskeleton in cancer metastasis. Br J Pharm. 2014;171:5507–23.
Stehbens S, Wittmann T. Targeting and transport: how microtubules control focal adhesion dynamics. J Cell Biol. 2012;198:481–9.
Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenesis. Biochim Biophys Acta. 2009;1796:91–8.
Eitaki M, Yamamori T, Meike S, Yasui H, Inanami O. Vincristine enhances amoeboid-like motility via GEF-H1/RhoA/ROCK/Myosin light chain signaling in MKN45 cells. BMC Cancer. 2012;12:469.
Nalbant P, Chang YC, Birkenfeld J, Chang ZF, Bokoch GM. Guanine nucleotide exchange factor-H1 regulates cell migration via localized activation of RhoA at the leading edge. Mol Biol Cell 2009;20:4070–82.
Amin E, Dubey BN, Zhang SC, Gremer L, Dvorsky R, Moll JM, et al. Rho-kinase: regulation, (dys)function, and inhibition. Biol Chem. 2013;394:1399–410.
Fortin Ensign SP, Mathews IT, Symons MH, Berens ME, Tran NL. Implications of Rho GTPase signaling in glioma cell invasion and tumor progression. Front Oncol. 2013;3:241.
Shieh AC. Biomechanical forces shape the tumor microenvironment. Ann Biomed Eng. 2011;39:1379–89.
Malik R, Lelkes PI, Cukierman E. Biomechanical and biochemical remodeling of stromal extracellular matrix in cancer. Trends Biotechnol. 2015;33:230–6.
Torrino S, Grasset EM, Audebert S, Belhadj I, Lacoux C, Haynes M, et al. Mechano-induced cell metabolism promotes microtubule glutamylation to force metastasis. Cell Metab. 2021;33:1342–57.
Tang K, Li S, Li P, Xia Q, Yang R, Li T, et al. Shear stress stimulates integrin β1 trafficking and increases directional migration of cancer cells via promoting deacetylation of microtubules. Biochim Biophys Acta Mol Cell Res. 2020;1867:118676.
Kevenaar JT, Bianchi S, Van Spronsen M, Olieric N, Lipka J, Frias CP, et al. Kinesin-binding protein controls microtubule dynamics and cargo trafficking by regulating kinesin motor activity. Curr Biol. 2016;26:849–61.
Liang YJ, Yang WX. Kinesins in MAPK cascade: how kinesin motors are involved in the MAPK pathway? Gene 2019;684:1–9.
Pu Y, Yi Q, Zhao F, Wang H, Cai W, Cai S. MiR-20a-5p represses multi-drug resistance in osteosarcoma by targeting the KIF26B gene. Cancer Cell Int. 2016;16:64.
Jiang YY, Shang L, Shi ZZ, Zhang TT, Ma S, Lu CC, et al. Microtubule-associated protein 4 is an important regulator of cell invasion/migration and a potential therapeutic target in esophageal squamous cell carcinoma. Oncogene 2016;35:4846–56.
Tang N, Lyu D, Chang JF, Liu ZT, Zhang Y, Liu HP. Enhanced expression of microtubule-associated protein 7 functioned as a contributor to cervical cancer cell migration and is predictive of adverse prognosis. Cancer Cell Int. 2020;20:1–16.
Zhou J, Gupta K, Yao J, Ye KQ, Panda D, Giannakakou P, et al. Paclitaxel-resistant human ovarian cancer cells undergo c-Jun NH2-terminal kinase-mediated apoptosis in response to noscapine. J Biol Chem. 2002;277:39777–85.
Geeraert C, Ratier A, Pfisterer SG, Perdiz D, Cantaloube I, Rouault A, et al. Starvation-induced hyperacetylation of tubulin is required for the stimulation of autophagy by nutrient deprivation. J Biol Chem. 2010;285:24184–94.
Jo H, Loison F, Luo HR. Microtubule dynamics regulates Akt signaling via dynactin p150. Cell Signal. 2014;26:1707–16.
Giustiniani J, Daire V, Cantaloube I, Durand G, Poüs C, Perdiz D, et al. Tubulin acetylation favors Hsp90 recruitment to microtubules and stimulates the signaling function of the Hsp90 clients Akt/PKB and p53. Cell Signal. 2009;21:529–39.
Korolchuk VI, Saiki S, Lichtenberg M, Siddiqi FH, Roberts EA, Imarisio S, et al. Lysosomal positioning coordinates cellular nutrient responses. Nat Cell Biol. 2011;13:453–60.
Di Bartolomeo S, Corazzari M, Nazio F, Oliverio S, Lisi G, Antonioli M, et al. The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J Cell Biol. 2010;191:155–68.
Hu JY, Chu ZG, Han J, Dang YM, Yan H, Zhang Q, et al. The p38/MAPK pathway regulates microtubule polymerization through phosphorylation of MAP4 and Op18 in hypoxic cells. Cell Mol Life Sci. 2010;67:321–33.
Carbonaro M, Escuin D, O’Brate A, Thadani-Mulero M, Giannakakou P. Microtubules regulate hypoxia-inducible factor-1α protein trafficking and activity: implications for taxane therapy. J Biol Chem. 2012;287:11859–69.
Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–75.
Oba T, Ono M, Matoba H, Uehara T, Hasegawa Y, Ito KI. HDAC6 inhibition enhances the anti-tumor effect of eribulin through tubulin acetylation in triple-negative breast cancer cells. Breast Cancer Res Treat. 2021;186:37–51.
Saba NF, Magliocca KR, Kim S, Muller S, Chen Z, Owonikoko TK, et al. Acetylated tubulin (AT) as a prognostic marker in squamous cell carcinoma of the head and neck. Head Neck Pathol. 2014;8:66–72.
Chien JY, Tsen SD, Chien CC, Liu HW, Tung CY, Lin CH. α-TAT1 downregulation induces mitotic catastrophe in HeLa and A549 cells. Cell Death Disco. 2016;2:1–9.
Wang Z, Hu P, Tang F, Lian H, Chen X, Zhang Y, et al. Hdac6 promotes cell proliferation and confers resistance to temozolomide in glioblastoma. Cancer Lett. 2016;379:134–42.
Gradilone SA, Radtke BN, Bogert PS, Huang BQ, Gajdos GB, LaRusso NF. Hdac6 inhibition restores ciliary expression and decreases tumor growth. Cancer Res. 2013;73:2259–70.
Wattanathamsan O, Thararattanobon R, Rodsiri R, Chanvorachote P, Vinayanuwattikun C, Pongrakhananon V. Tubulin acetylation enhances lung cancer resistance to paclitaxel-induced cell death through Mcl-1 stabilization. Cell Death Disco. 2021;7:1–13.
Wang G, He J, Zhao J, Yun W, Xie C, Taub JW, et al. Class I and class II histone deacetylases are potential therapeutic targets for treating pancreatic cancer. PloS one. 2012;7:e52095.
Zhang QC, Jiang SJ, Zhang S, Ma XB. Histone deacetylase inhibitor trichostatin A enhances antitumor effects of docetaxel or erlotinib in A549 cell line. Asian Pacific. J Cancer Prev. 2012;13:3471–6.
Cang S, Ma Y, Chiao J, Liu D. Phenethyl isothiocyanate and paclitaxel synergistically enhanced apoptosis and alpha-tubulin hyperacetylation in breast cancer cells. Exp Hematol Oncol. 2014;3:5.
Boggs AE, Vitolo MI, Whipple RA, Charpentier MS, Goloubeva OG, Ioffe OB, et al. α-Tubulin acetylation elevated in metastatic and basal-like breast cancer cells promotes microtentacle formation, adhesion, and invasive migration. Cancer Res. 2015;75:203–15.
Chakrabarti KR, Whipple RA, Boggs AE, Hessler LK, Bhandary L, Vitolo MI, et al. Pharmacologic regulation of AMPK in breast cancer affects cytoskeletal properties involved with microtentacle formation and re-attachment. Oncotarget 2015;6:36292–307.
Pongrakhananon V, Wattanathamsan O, Takeichi M, Chetprayoon P, Chanvorachote P. Loss of CAMSAP3 promotes EMT via the modification of microtubule-Akt machinery. J Cell Sci. 2018;131:jcs216168.
Oh S, You E, Ko P, Jeong J, Keum S, Rhee S. Genetic disruption of tubulin acetyltransferase, αTAT1, inhibits proliferation and invasion of colon cancer cells through decreases in Wnt1/β-catenin signaling. Biochem Biophys Res Commun. 2017;482:8–14.
Castro-Castro A, Janke C, Montagnac G, Paul-Gilloteaux P, Chavrier P. ATAT1/MEC-17 acetyltransferase and HDAC6 deacetylase control a balance of acetylation of alpha-tubulin and cortactin and regulate MT1-MMP trafficking and breast tumor cell invasion. Eur J Cell Biol. 2012;91:950–60.
Ko P, Choi JH, Song S, Keum S, Jeong J, Hwang YE, et al. Microtubule acetylation controls MDA-MB-231 breast cancer cell invasion through the modulation of endoplasmic reticulum stress. Int J Mol Sci. 2021;22:6018.
Gao YS, Hubbert CC, Yao TP. The microtubule-associated histone deacetylase 6 (HDAC6) regulates epidermal growth factor receptor (EGFR) endocytic trafficking and degradation. J Biol Chem. 2010;285:11219–26.
Asthana J, Kapoor S, Mohan R, Panda D. Inhibition of HDAC6 deacetylase activity increases its binding with microtubules and suppresses microtubule dynamic instability in MCF-7 cells. J Biol Chem. 2013;288:22516–26.
Mialhe A, Lafanechèere L, Treilleux I, Peloux N, Dumontet C, Brémond A, et al. Tubulin detyrosination is a frequent occurrence in breast cancers of poor prognosis. Cancer Res. 2001;61:5024–7.
Whipple RA, Balzer EM, Cho EH, Matrone MA, Yoon JR, Martin S. Vimentin filaments support extension of tubulin-based microtentacles in detached breast tumor cells. Cancer Res. 2008;68:5678–88.
Whipple RA, Vitolo MI, Boggs AE, Charpentier MS, Thompson K, Martin SS. Parthenolide and costunolide reduce microtentacles and tumor cell attachment by selectively targeting detyrosinated tubulin independent from NF-κB inhibition. Breast Cancer Res. 2013;15:1–12.
Balzer EM, Whipple RA, Cho EH, Matrone MA, Martin SS. Antimitotic chemotherapeutics promote adhesive responses in detached and circulating tumor cells. Breast Cancer Res Treat. 2010;121:65–78.
Nieuwenhuis J, Adamopoulos A, Bleijerveld OB, Mazouzi A, Stickel E, Celie P, et al. Vasohibins encode tubulin detyrosinating activity. Science 2017;358:1453–6.
Sato Y. The vasohibin family: a novel family for angiogenesis regulation. J Biochem. 2013;153:5–11.
Heishi T, Hosaka T, Suzuki Y, Miyashita H, Oike Y, Takahashi T, et al. Endogenous angiogenesis inhibitor vasohibin1 exhibits broad-spectrum antilymphangiogenic activity and suppresses lymph node metastasis. Am J Pathol. 2010;176:1950–8.
Hosaka T, Kimura H, Heishi T, Suzuki Y, Miyashita H, Ohta H, et al. Vasohibin-1 expression in endothelium of tumor blood vessels regulates angiogenesis. Am J Pathol. 2009;175:430–9.
Tu M, Liu X, Han B, Ge Q, Li Z, Lu Z, et al. Vasohibin-2 promotes proliferation in human breast cancer cells via upregulation of fibroblast growth factor-2 and growth/differentiation factor-15 expression. Mol Med Rep. 2014;10:663–9.
Kitahara S, Suzuki Y, Morishima M, Yoshii A, Kikuta S, Shimizu K, et al. Vasohibin-2 modulates tumor onset in the gastrointestinal tract by normalizing tumor angiogenesis. Mol Cancer. 2014;13:99.
Koyanagi T, Suzuki Y, Saga Y, Machida S, Takei Y, Fujiwara H, et al. In vivo delivery of sirna targeting vasohibin-2 decreases tumor angiogenesis and suppresses tumor growth in ovarian cancer. Cancer Sci. 2013;104:1705–10.
Takahashi Y, Koyanagi T, Suzuki Y, Saga Y, Kanomata N, Moriya T, et al. Vasohibin-2 expressed in human serous ovarian adenocarcinoma accelerates tumor growth by promoting angiogenesis. Mol Cancer Res. 2012;10:1135–46.
Koyanagi T, Saga Y, Takahashi Y, Tamura K, Yoshiba T, Takahashi S, et al. Knockout of vasohibin‐2 reduces tubulin carboxypeptidase activity and increases paclitaxel sensitivity in ovarian cancer. Cancer Med. 2021;10:2732–9.
Wang N, Bosc C, Ryul Choi S, Boulan B, Peris L, Olieric N, et al. Structural basis of tubulin detyrosination by the vasohibin-svbp enzyme complex. Nat Struct Mol Biol. 2019;26:571–82.
Liao S, Rajendraprasad G, Wang N, Eibes S, Gao J, Yu H, et al. Molecular basis of vasohibins-mediated detyrosination and its impact on spindle function and mitosis. Cell Res. 2019;29:533–47.
Iida‐Norita R, Kawamura M, Suzuki Y, Hamada S, Masamune A, Furukawa T, et al. Vasohibin‐2 plays an essential role in metastasis of pancreatic ductal adenocarcinoma. Cancer Sci. 2019;110:2296–308.
Barisic M, e Sousa RS, Tripathy SK, Magiera MM, Zaytsev AV, Pereira AL, et al. Microtubule detyrosination guides chromosomes during mitosis. Science 2015;348:799–803.
Owa M, Dynlacht B. A non-canonical function for centromere-associated protein-E controls centrosome integrity and orientation of cell division. Commun Biol. 2021;4:358.
Ohashi A, Ohori M, Iwai K, Nambu T, Miyamoto M, Kawamoto T, et al. A novel time-dependent CENP-E inhibitor with potent antitumor activity. PLoS One. 2015;10:e0144675.
Banerjee A. Increased levels of tyrosinated α-, βIII-, and βIV-tubulin isotypes in paclitaxel-resistant MCF-7 breast cancer cells. Biochem Biophys Res Commun. 2002;293:598–601.
Gadau SD. Morphological and quantitative analysis on α-tubulin modifications in glioblastoma cells. Neurosci Lett. 2018;687:111–8.
Yao Q, An Y, Hou W, Cao YN, Yao MF, Ma NN, et al. LRP6 promotes invasion and metastasis of colorectal cancer through cytoskeleton dynamics. Oncotarget 2017;8:109632–45.
Yu I, Garnham CP, Roll-Mecak A. Writing and reading the tubulin code. J Biol Chem. 2015;290:17163–72.
Thazhath R, Liu C, Gaertig J. Polyglycylation domain of β-tubulin maintains axonemal architecture and affects cytokinesis in Tetrahymena. Nat Cell Biol. 2002;4:256–9.
Rogowski K, Juge F, Van Dijk J, Wloga D, Strub JM, Levilliers N, et al. Evolutionary divergence of enzymatic mechanisms for posttranslational polyglycylation. Cell 2009;137:1076–87.
Van Dijk J, Rogowski K, Miro J, Lacroix B, Edde B, Janke C. A targeted multienzyme mechanism for selective microtubule polyglutamylation. Mol Cell. 2007;26:437–48.
Wasylyk C, Zambrano A, Zhao C, Brants J, Abecassis J, Schalken JA, et al. Tubulin tyrosine ligase like 12 links to prostate cancer through tubulin posttranslational modification and chromosome ploidy. Int J Cancer. 2010;127:2542–53.
Yang S, Liang Y, Qian H, Li Q. TTLL12 expression in ovarian cancer correlates with a poor outcome. Int J Clin Exp Pathol. 2020;13:239–47.
Arnold J, Schattschneider J, Blechner C, Krisp C, Schlüter H, Schweizer M, et al. Tubulin tyrosine ligase like 4 (TTLL4) overexpression in breast cancer cells is associated with brain metastasis and alters exosome biogenesis. J Exp Clin Cancer Res. 2020;39:1–15.
Rocha C, Papon L, Cacheux W, Marques Sousa P, Lascano V, Tort O, et al. Tubulin glycylases are required for primary cilia, control of cell proliferation and tumor development in colon. EMBO J. 2014;33:2247–60.
Froidevaux-Klipfel L, Targa B, Cantaloube I, Ahmed-Zaïd H, Poüs C, Baillet A. Septin cooperation with tubulin polyglutamylation contributes to cancer cell adaptation to taxanes. Oncotarget 2015;6:36063–80.
Targa B, Klipfel L, Cantaloube I, Salameh J, Benoit B, Poüs C, et al. Septin filament coalignment with microtubules depends on SEPT9_i1 and tubulin polyglutamylation, and is an early feature of acquired cell resistance to paclitaxel. Cell Death Dis. 2019;10:1–14.
Funding
This work was supported by the National Research Council of Thailand (NRCT; N41A640133 to VP) and the Second Century Fund (C2F to OW), Chulalongkorn University.
Author information
Authors and Affiliations
Contributions
All authors researched data, performed an extensive literature survey, and provided substantial contribution to the content of this article equally. All authors reviewed, edited, and approved the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wattanathamsan, O., Pongrakhananon, V. Post-translational modifications of tubulin: their role in cancers and the regulation of signaling molecules. Cancer Gene Ther 30, 521–528 (2023). https://doi.org/10.1038/s41417-021-00396-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-021-00396-4
This article is cited by
-
Physiological roles of chloride ions in bodily and cellular functions
The Journal of Physiological Sciences (2023)
-
Inhibition of histone deacetylase 6 destabilizes ERK phosphorylation and suppresses cancer proliferation via modulation of the tubulin acetylation-GRP78 interaction
Journal of Biomedical Science (2023)
-
Dynamics of TUBB protein with five majorly occurring natural variants: a risk of cortical dysplasia
Journal of Molecular Modeling (2023)
-
Understanding the impact of structural modifications at the NNAT gene’s post-translational acetylation site: in silico approach for predicting its drug-interaction role in anorexia nervosa
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2023)