Collection |
Collections
Filters
-
Collection Type
-
-
Collection |
 Novel treatment paradigms for metastatic uveal melanoma
Novel treatment paradigms for metastatic uveal melanoma
Uveal melanoma (UM) is the most common primary intraocular malignancy, accounting for 85 to 95% of primary ocular malignancies and 3 to 5% of all melanoma cases. While patients with metastatic UM continue to represent a population in need of better outcomes, meaningful progress has been made with respect to deepening our understanding of the disease biology and development of novel therapeutics, including the first regulatory approval of a systemic agent shown to improve survival. Innovative molecular and immunotherapy approaches that leverage biologic vulnerabilities in UM cells and specific immunosuppressive pathways driving disease progression in the liver may support further advances. We hope this special issue of Cancer Gene Therapy proves to be a valuable resource for those in the scientific and medical communities committed to improving UM patient outcomes.
-
Collection |
 Reader's Choice: the best of Cancer Gene Therapy 2022
Reader's Choice: the best of Cancer Gene Therapy 2022
In this collection we highlight a selection of articles from 2022, which top the list of the journal’s most cited, downloaded and most shared (including press coverage, blogs, Twitter, Facebook and Weibo). The articles showcase the breadth of scope and coverage that the journal consistently delivers to its readers.
-
Collection |
 Long-term Impact of COVID in Cancer Patients
Long-term Impact of COVID in Cancer Patients
This collection is dedicated to the impact of the COVID-19 pandemic in cancer healthcare. We are particularly interested in research examining the long-term impact for patients with cancer. To increase the discoverability of scientific literature related to COVID-19 cancer research, here we bring together key articles from across a series of oncology journals. We welcome future submissions to expand this collection further.
Image: Gottschalk et al.Open for submissions -
Collection |
 CAR T-cell therapy in cancer
CAR T-cell therapy in cancer
Cancer therapy using chimeric antigen receptor (CAR)-T-cells is one of the most exciting recent developments in cancer therapy. To date CAR-T-cells have been successfully used to treat persons with hematologic cancers, especially acute lymphoblastic leukemia (ALL), lymphomas and plasma cell myeloma (PCM). Although most studies are in persons with advanced lymphomas, some controversial data suggest CAR-T-cell therapy might replace autologous hematopoietic cell transplants in persons failing conventional therapies. The CAR-T-cell constructs are directed against lineage-related targets such as CD19 and CD20. Although studies of using CAR-T-cells to treat other hematologic cancers such as acute myeloid leukemia (AML) and solid cancers are progressing, these targets have proved more elusive and no CAR-T-cell therapy is yet approved. In this collection we include key research published in the journals Leukemia, Blood Cancer Journal, Cancer Gene Therapy, Journal of Cancer Research and Clinical Oncology and Medical Oncology. The editors welcome future submissions to expand this collection further.
-
Collection |
 Regulatory T cells in cancer immunosuppression
Regulatory T cells in cancer immunosuppression
A paradox in tumor immunity is the co-existing of growing tumors with circulating or tissue-infiltrating tumor-reactive T cells. The inability of tumor-reactive T cells in control of tumor growth has been attributed to the presence of regulatory T cells (Treg) in cancer host. Since then, understanding the role of Treg cells has become one of the intensively studied areas in cancer immunotherapy. However, fewer options targeting Treg cells have been validated in clinical trials and therefore more preclinical and clinical studies are required to provide reliable therapeutic targets with respect to Treg cell biology. To that end, this selection will highlight recent progresses in Treg cell biology and provide new opportunities for developing new therapeutic approaches to target Treg cells. The scope and topics of this collection include but are not limited to: • The generation and accumulation of Treg cells in cancer host. • The regulatory function of Treg cells in shaping immune response to cancer. • The role of Treg cells in the prognosis of cancer and in response to immunotherapy. • Therapeutic targets in Treg cells that can promote antitumor immunity. In this collection we include key research published in the journals Leukemia, Cancer Gene Therapy and Cancer Immunology, Immunotherapy. The editors welcome future submissions to expand this collection further.
Image: Getty Images -
Collection |
 Reader's Choice 2021
Reader's Choice 2021
In this collection we highlight a selection of articles from 2021, which top the list of the journal’s most cited, downloaded and most shared (including press coverage, blogs, Twitter, Facebook and Weibo). They showcase the breadth of scope and coverage that the journal consistently delivers to its readers.
-
Collection |
 CRISPR–Cas9 in cancer research and therapy
CRISPR–Cas9 in cancer research and therapy
Since its discovery as part of the adaptive immune system in bacteria, CRISPR-Cas9 has become an invaluable tool for genome editing with the potential to transform cancer therapies. The ability to alter specific bases in DNA enables comprehensive examination of molecular pathways and the part they play in cancer with incomparable resolution. The technology is also routinely applied in cancer modelling where it enables the production of faithful models of disease, capturing the specific molecular aberrancies of different cancers. As a therapy, CRISPR-Cas9 has demonstrated promises ex vivo, in oncolytic viruses and immunotherapies; however its use in vivo is also being explored by targeting genes essential for cancer cell viability, promoting apoptosis or cell cycle arrest in tumours and thereby improve patients’ survival. Overall, CRISPR-Cas9 technology has greatly advanced cancer research and has the potential to immensely transform cancer therapies. The below collection of articles represents key research in this area published in the journals Oncogene, Oncogenesis, Leukemia, Blood Cancer Journal and Cancer Gene Therapy. The editors welcome future submissions to expand this collection further.
-
Collection |
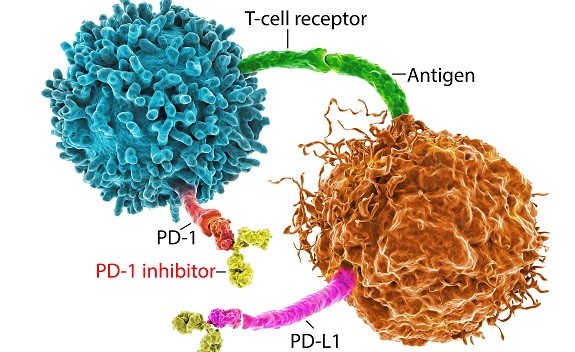 Immune checkpoint inhibitors in cancer treatment
Immune checkpoint inhibitors in cancer treatment
The advent and validation of immune checkpoint inhibitors targeting the T cell receptors PD1 and CTLA have been 'game changers' in clinical cancer therapy. Initially approved for melanoma, lung cancer, and kidney cancer these therapies have now gained substantial inroads in a diversity of tumor types as first or second-line therapy and promise to extend the lives of millions of more cancer patients. However, there is critical ongoing research attempting to optimize the therapeutic utility of these agents. Current investigations include analysis of tumor molecular profiles most apt to respond to specific checkpoint immunotherapy regimens, optimal timing of treatment and combination with chemotherapy, radiation, and molecularly targeted anti-cancer agents, and the potential to enhance responsiveness of checkpoint inhibition with concurrent use of novel therapies targeting innate or adaptive immunity. Research in all these areas has exploded at both the clinical and experimental levels. As such, these burgeoning reports comprise a valued addition and are a new cornerstone in Springer Nature's topical online collections in cancer research. The editors of the journals Oncogene, Blood Cancer Journal, Leukemia and Cancer Gene Therapy welcome future submissions to expand this collection further.
-
Collection |
 KRAS-mutant cancers
KRAS-mutant cancers
We are still elucidating the signalling pathways and complex circuitry surrounding the most common mutated oncogene we observe in human malignancies. KRAS driver mutations underlie 20% of human malignancies and the journey here has been a true example of bench to bedside science. For example, in the last few years, we've observed that mutations in KRAS predict resistance to anti-EGFR therapeutics in colon cancer. Earlier this year, the US Food and Drug Administration approved the first ever KRAS inhibitor for use in patients, in this case non-small cell lung cancer. More KRAS inhibitors will be approved, each targeting specific amino acid changes in its structure, a true example of personalised, tailored precision medicine. The accumulation of knowledge here exemplifies how understanding basic science and mechanisms, ultimately can extend the quality and quantity of life of those living with cancer, and contribute to increasing the cure rate. The below collection of articles represents key research in this area published in the journals Oncogene, Oncogenesis, Leukemia and Cancer Gene Therapy. The editors welcome future submissions to expand this collection further.
-
Collection |
 Mechanisms of resistance to cancer therapy
Mechanisms of resistance to cancer therapy
Metastatic cancer is ultimately resistant to virtually all systemic therapies and continues to kill more than 10 million people per year around the world. This suggests a common mechanism for cancer resistance that evolves in millions of people each year, regardless of the instigating carcinogens, suite of mutations present in the tumor, or the tissue of origin. This therapeutic resistance has classically been attributed to genetic tumor cell heterogeneity that develops by stochastic chance, fueled by aneuploidy and genetic instability. In this classic view, resistance to each different therapy requires that the appropriate mutations that confer the different versions of resistance are acquired by at least one cell. Newer models have found potential evidence for the gradual, multifactorial adaptation to the inhibitors through acquisition of multiple cooperating genetic and epigenetic adaptive changes of multiple partially resistant clones as well as the presence of cancer stem cells in which a rare therapy-resistant population of cancer stem cells give rise to a recurrent, resistant population. The below collection of articles published in the journals Oncogene, Leukemia, Blood Cancer Journal, Oncogenesis, Cancer Gene Therapy and Clinical & Experimental Metastasis explore how treatment resistance arises in cancer. The editors welcome future submissions on this topic to expand this collection further.
-
Collection |
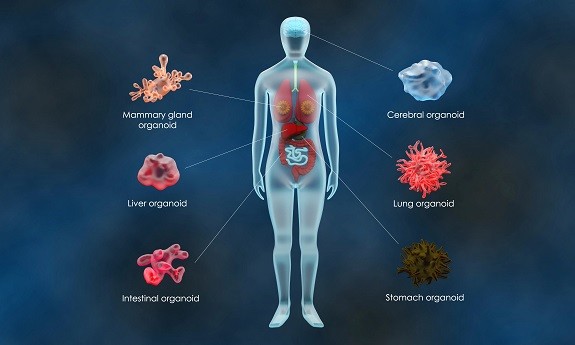 Patient-derived organoids as in vitro cancer models
Patient-derived organoids as in vitro cancer models
Cancer research has predominantly focused on two-dimensional (2D) cultured human cell lines during the past decades. Although these models have (i) improved our understanding of cellular signalling pathways, (ii) helped us identify potential drug targets and (iii) guided the design of candidate drugs for a wide range of cancers, still they have shown many limitations. The recent advances in in vitro 3D culture technologies, such as organoids, have opened new avenues to improve basic and clinical cancer research. Up to now, highly efficient establishment of organoids can be achieved from both normal and malignant patient tissues. Wild-type organoids, grown from embryonic and adult stem cells, can be mutated, through gene-editing technologies, into tumor organoids, which may emulate and give new insights into genetic alterations occurring during cancer initiation and progression. Organoids obtained from patient-derived tumour tissues represent an emerging approach for creating patient-derived in vitro cancer models that closely recapitulate the pathophysiological features of natural tumorigenesis and metastasis constituting a great tool for the discovery of personalized anti-cancer therapy and prognostic biomarkers. Taken together, organoids represent a promising model for cancer research and clinical translation. The below collection of articles represents key research published in the journals Oncogene, Oncogenesis, Cancer Gene Therapy and Cancer Cell International, focusing on the use of organoids in cancer research. The editors welcome future submissions to expand this collection further.
