Abstract
Approximately 6% of deceased kidney donors (DKDs) are diabetic; their kidneys may be associated with worse allograft survival, but published studies suggest that recipient diabetes status has a greater impact on mortality and survival. Since biopsy findings are the most common reason for organ discard, we sought to understand histologic and clinical factors that influence graft survival in patients who receive a kidney from a diabetic DKD. We retrospectively reviewed our institutional experience from 2005 to 2019, and re-evaluated pre-implantation and earliest post-transplant biopsies. Histologic findings were compared against a control cohort of non-diabetic DKD. Of 829 adult DKD transplants, 37 (4.5%) came from diabetic donors. There was no significant difference in diabetic vs. non-diabetic DKD graft survival for all-comers; however, when stratified by duration of donor diabetes, donor diabetes ≥6 years was associated with graft failure. In 25 patients with post-transplant biopsies available, diabetic DKD allografts had significantly greater non-glomerular chronic injury than non-diabetic DKD allografts. Moderate arteriolar hyalinosis (in 24%), moderate tubular atrophy and interstitial fibrosis (IFTA, in 36%), and diabetic glomerulopathy (in 24%) on early post-transplant biopsy were associated with allograft failure. Pre-implantation frozen section discrepancies were more common in long-standing donor diabetes, and arteriolar hyalinosis and IFTA scores on frozen accurately prognosticated graft loss. There was no morphologic improvement in lesions of diabetic nephropathy on short-term follow-up. In conclusion, donor diabetes ≥6 years, and histologic findings on frozen section and early post-transplant biopsy are associated with diabetic DKD allograft loss.
Similar content being viewed by others
Introduction
The demand for allograft kidneys exceeds availability, leading to increased utilization of deceased kidney donors (DKDs) from diabetic and expanded criteria donors1,2,3,4,5. In considering use of kidneys from diabetic donors, results from previous large registry-based studies are varied, but suggest that donor or recipient diabetes can have statistically significant adverse impacts on mortality and graft survival, although absolute differences are often clinically small1,6,7,8,9. Although diabetic DKD allografts have been associated with higher mortality than non-diabetic DKD allografts, they offer survival benefit, demonstrating 9% lower mortality compared with remaining on the waitlist1. Very few studies have pathologic correlation, usually with no or mild histologic diabetic changes in diabetic DKD allografts10,11. In this histology-focused study, we sought to evaluate our institutional experience with diabetic DKD allografts, characterize pathologic findings on procurement and follow-up biopsies, and identify features associated with outcomes.
Materials/subjects and methods
After Institutional Review Board approval, we retrospectively reviewed our institutional experience with diabetic DKD from 2005 to 2019, and re-evaluated procurement and post-transplant biopsies. Donor and recipient clinical and laboratory data were obtained from United Network of Organ Sharing (UNOS) database with variables including donor height, weight, presence and duration of diabetes, insulin dependence, cause of death, kidney donor profile index (KDPI), cold ischemic time, donation after circulatory death, and use of kidney pump. For recipients, age, sex, weight, BMI, presence of diabetes, cause of end-stage kidney disease (ESKD), calculated panel reactive antibody, use of dialysis within first week of transplant, creatinine at discharge from transplant, graft outcome (functioning vs. failed/dialysis-dependent), most recent patient status (alive, dead, re-transplant, lost to follow-up), and most recent creatinine were assessed. In the overall cohort for graft survival analysis, there was no significant differences between diabetic vs. non-diabetic DKD for cold ischemic time, recipient age, gender, dialysis status at transplant, or creatinine at discharge. The cohort was not large enough to control for all contributory variables including histology, KDPI, rejection, hypertension, medications, or number of patients at the end of dialysis time.
For post-transplant biopsies, slides were stained with H&E, Jones silver, and PAS, per protocol, and evaluated by a renal pathologist. Biopsies were performed for indication and usually for surveillance per institutional protocol, at 3 and 12 months. Of 25 patients, 18 had at least two post-transplant biopsies (median 2, range: 2–5) for review. To assess donor rather than recipient disease, the earliest available biopsy with adequate parenchyma and vasculature was analyzed for outcome purposes; of these, 16/25 (64%) were surveillance and 9 were for-cause. Cases were specifically evaluated for degree of global glomerulosclerosis (%), tubular atrophy and interstitial fibrosis (IFTA) (0, mild, moderate, severe), arteriosclerosis (0, mild, moderate, severe), arteriolar hyalinosis (0, mild, moderate, severe), and glomerular abnormalities, including mild or diffuse mesangial expansion with or without nodularity. Rejection and any other abnormalities were also recorded. Histologic findings were compared against a control cohort (n = 107) of all adult non-diabetic DKD allograft kidney biopsies (1 per patient) performed for surveillance purposes from 2018 to 2019, and matched for time interval from kidney transplant (all within first year, 60% within 3 months).
Frozen and permanent sections were evaluated with H&E using our institutional criteria for scoring deceased donor kidneys. Frozen section donor wedge pre-implantation biopsies were evaluated by surgical pathologists on call, and permanent sections were reviewed by renal pathologists; special stains including PAS and Jones were performed in a minority of cases, at pathologist discretion. For donor frozen evaluation, our institutional protocol from this time period used the following point-based scoring system: global glomerulosclerosis: 0: 0–5%; 1: 6–15%; 2: 16–25%; 3: >25%. Arteriosclerosis: 0: negligible (10% or less); 1: mild (11–20%); 2: moderate (21–50%); 3: severe (>50%). Arteriolar hyalinosis: 0: none; 1: focal; 2; multiple or circumferential deposits. IFTA: 0: negligible (10% or less); 1: mild (11–20%); 2: moderate (21–50%); 3: severe (>50%). Points from each compartment are added, with ≤2 points: ok to use for transplant; 3–4 points: correlate with clinical findings; and ≥5 points, or any category “severe”: do not use kidney. Major discrepancies were defined as those in which there was a change from “ok to use” to “do not use” or vice versa between the frozen and permanent section score.
Statistics were performed in GraphPad Prism 8 (La Jolla, CA) using non-parametric tests (Mann–Whitney and Fischer’s tests) for histologic variables; and outcome analyses were performed in Stata 13 (College Station, TX) and SPSS (Armonk, NY: IBM Corp) using Cox proportional hazards models.
Results
Overall clinical outcomes of allograft kidneys from diabetic deceased kidney donors
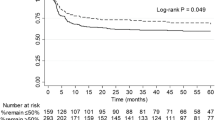
Of 829 adult DKD transplants performed between 2005 and 2019, 37 (4.5%) came from diabetic donors. There was no difference in death-censored kidney allograft survival between individuals receiving an allograft from a diabetic vs. non-diabetic DKDs, nor amongst diabetic recipients receiving a diabetic vs. non-diabetic DKD. Diabetic recipients had a non-significant trend toward shorter graft survival (p = 0.078), regardless of donor diabetes status. When stratified by duration of donor diabetes, recipients of kidneys from donors with diabetes ≥6 years had significantly shorter death-censored allograft survival (p = 0.04) (Fig. 1).
For recipients of an allograft kidney from a diabetic donor, there was no difference in death-censored graft survival for A all-comers, nor for B diabetic recipients. C Kidney allografts had a non-significant trend to fail earlier in a diabetic recipient, regardless of whether the donor was diabetic. D When stratified by duration of donor diabetes, recipients of allograft kidneys from donors with diabetes ≥6 years had significantly shorter death-censored allograft survival.
In the subset of 25 patients with pathology data, 17 (68%) were male, with a median age of 58 years (range: 41–74 years). Eleven recipients (44%) had diabetes, the great majority of which (10/11, 91%) were male; diabetes was the cause of ESKD in 8. Duration of donor diabetes was: 0–5 years in 13 (52%), 6–10 years in 4 (16%) including 2 insulin-dependent, >10 years in 5 (20%) including 2 insulin-dependent, and unknown in 3 (12%). At a median follow-up time of 28 months (range: 3 months to 12 years), 3 experienced graft failure. Of examined clinical variables in this cohort with pathology available, donor diabetes >10 years was associated with graft failure (HR 11.5, p = 0.04). When combining graft failure (n = 3) and allograft CKD4 (n = 2), donor diabetes >10 years remained the only tested clinical variable associated with graft failure and/or allograft CKD4 (HR 7.9, p = 0.03). There were four deaths with a functioning allograft from a variety of etiologies, with no association with the presence of recipient diabetes or duration of donor diabetes.
Pathologic findings in allograft kidneys from diabetic donors, early post-transplant
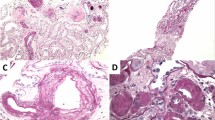
Of the 37 recipients of diabetic kidneys, 25 (68%, with 56 biopsies) had post-transplant biopsies at our intuition; 24 (96%) had at least one biopsy in the first year, including 88% within the first 3 months, for review. In attempt to capture donor rather than recipient disease and given known sampling pitfalls of procurement biopsies12,13,14,15,16,17, the first post-transplant biopsy was used for most comparisons. Vascular disease including arteriosclerosis (88%) and arteriolar hyalinosis (54%) (Fig. 2) was the most common lesion; one had atheroembolic disease. Eight (32%) had glomerular abnormalities including characteristic diabetic glomerulopathy in six recipients (24%), which usually manifest as mesangial sclerosis (17%), or nodular glomerulosclerosis (in two recipients from same donor); one also had focal segmental glomerulosclerosis (FSGS). Two (8%) had FSGS but without the underlying mesangial proliferative changes associated with diabetes (clinicopathologic findings are detailed in Supplementary Table). Compared against a control cohort of non-diabetic DKD (n = 107), kidneys from diabetic DKD had significantly greater global glomerulosclerosis (median 5% vs. 0%, p = 0.005), IFTA (mild vs. none, p < 0.0001), arteriolar hyalinosis (mild vs. none, p < 0.0001), and arteriosclerosis (mild-to-moderate vs. none, p < 0.0001) (Fig. 3). Based on the data distribution, we generated and tested potential thresholds for histologic findings on permanent sections associated with donor diabetes in DKDs, and found that arteriolar hyalinosis and arteriosclerosis—but not global glomerulosclerosis, IFTA, or classic diabetic glomerulopathy—were the most useful indicators of diabetes-related renal injury in our cohort (Table 1).
Post-transplant biopsies in kidney recipients from diabetic donors have frequent vascular disease. A Arteriolar hyalinosis (upper left, arrow) and glomerulus with mild segmental mesangial expansion (lower right, arrow) (PAS 400×). B Arteriosclerosis (arrowhead) and arteriolar hyalinosis (arrows), with classic diabetic nodular glomerulosclerosis (Jones 200×).
Examining relationships between histologic findings and outcomes in diabetic DKDs, moderate arteriolar hyalinosis (p = 0.01, seen in 24%), moderate IFTA (p = 0.02, seen in 36%), and diabetic glomerulopathy (p = 0.01, seen in 24%) on early post-transplant biopsy were associated with allograft failure. The degree of arteriolar hyalinosis was also associated with the combined outcome of graft failure or allograft CKD4 (p = 0.04). No other tested pathological variable on 3-month biopsy was associated with graft failure.
Clinicopathologic correlation with pre-implantation frozen and permanent sections
Of the 25 diabetic donor kidneys, 17 (68%) had local pre-implantation frozen wedge biopsies available for review, which were correlated with permanent section results. This was performed in an effort to identify which clinically significant features were recognizable at frozen section, and to characterize type, rate, and impacts of frozen section discrepancies in kidneys from diabetic donors. Similar features were prognostic and identifiable prospectively at the time of frozen section: arteriolar hyalinosis (p = 0.029) and IFTA (p = 0.026) scores on frozen section were associated with diabetic DKD graft failure, but global glomerulosclerosis and arteriosclerosis were not. Diabetic glomerulopathy was difficult to identify on frozen section (Fig. 4), and contributed to all three major frozen-to-permanent discrepancies, where a major discrepancy was defined as one in which a frozen pre-implantation biopsy is scored as usable per our institutional protocol, when score from re-evaluation on permanent section would have placed it in an unusable category. This equates to a 18% major discrepancy rate for diabetic DKDs for frozen to permanent section, which may be due to innate deficiencies of frozen sections, wedge biopsy limitations, sampling site, or other factors. Of these discrepancies, all were in donors with diabetes for >10 years, and one recipient experienced early allograft failure at 1.5 years.
On frozen section, A glomeruli have diffuse mesangial expansion with subtle nodularity (H&E 200×) and B arteriolar hyalinosis may be focal or difficult to discern from surrounding tubules (H&E 400×). C Permanent sections from the same case have readily apparent severe arteriolar hyalinosis and nodular mesangial sclerosis (200×).
Given known inconsistencies in procurement biopsies12,13,14,15,16,17, we examined discordance rates between permanents from pre-implantation wedge biopsies to early post-transplant needle biopsies. In addition to the previously described frozen-to-permanent discrepancies, six recipient biopsies (35%) had a major discordance in the degree of chronic injury on 3-month biopsy compared with permanents from pre-implantation frozen sections, with overall scores that would have placed the kidney in a “do not use” category if seen on procurement biopsy. Increased arteriosclerosis, arteriolar hyaline, and IFTA (in five patients) on 3-month biopsies accounted for most of these discrepancies, whereas greater degrees of global glomerulosclerosis were seen in two, and only in conjunction with other chronic injury. Rather than representing frozen section limitations, these discrepancies are likely driven by differences in sample location and type, with more vascular disease observed in needle biopsies (post-transplant) compared with wedge biopsies (pre-implantation), as has been previously reported18. These procurement to 3-month discrepancies were unrelated to the duration of donor diabetes.
Evolution of pathologic lesions in repeat biopsies of kidneys from diabetic donors
At a median follow-up time of 5 months (range: 0.5–36 months), 18 of 25 patients had at least two post-transplant biopsies (median 2, range: 2–5) for review. After accounting for expected sampling-related variations and impact of other conditions such as rejection, repeat biopsies showed comparable degrees of global glomerulosclerosis, IFTA, and chronic vascular changes over the short time interval. Six of the eight recipients with donor-derived diabetes-associated glomerular changes (four diffuse mesangial sclerosis, two FSGS) had repeat biopsies. Two of these recipients were diabetic; one had stable diffuse mesangial sclerosis on repeat biopsy, and the other showed persistent FSGS with a two-fold increase in global glomerulosclerosis and IFTA with an increase in arteriosclerosis and arteriolar hyalinosis at 1 year, compared with 3-month biopsy. Four recipients were non-diabetic; repeat biopsies showed a subjectively similar degree of diffuse mesangial sclerosis, and one developed FSGS on repeat biopsy at 7 months. Thus, in a short time period, we observed subjectively stable donor-derived disease that appeared to increase in some patients over time, unrelated to recipient diabetes status. Although slow resolution of diabetes-associated glomerular injury is documented over 5–10 years19 donor-derived glomerular diabetic changes in our cohort had a year or less of histologic follow-up, limiting this assessment.
Discussion
In this study, we examined pathology and clinical outcomes for recipients of allograft kidneys from deceased diabetic donors, which account for 4.5% of the kidney transplants at our institution. We found no significant differences in diabetic vs. non-diabetic DKD graft survival, regardless of recipient diabetes status. However, when stratified by duration of donor diabetes, we observed a higher risk of loss in allografts from donors with diabetes for ≥6 years. Pathologically, the most common lesions identified in diabetic kidney donors were vascular rather than glomerular disease, and the degree of arteriolar hyalinosis and IFTA on both pre-implantation frozen section and early post-transplant biopsies were pathologic features associated with allograft loss.
Our finding of similar graft survival between diabetic and non-diabetic deceased donor kidneys is consistent with large registry-based studies1,6,8,9, and supports the use of diabetic DKDs. Although statistically powered to evaluate for confounding clinical variables, a limitation of registry-based studies is that pathologic correlation is not available; our findings reveal that the comparatively similar graft outcome of kidneys from diabetic donors can be explained, at least in part, by the relatively mild to moderate increase in chronic injury in kidneys from most diabetic DKDs. Specifically, kidneys from the majority of our diabetic DKDs had ≤10% global glomerulosclerosis (in 78%), zero or mild arteriolar hyalinosis (in 76%), zero or mild arterial intimal sclerosis (in 64%), and minimal to mild IFTA (in 64%); only a third had glomerular disease. Corroborating this, generally mild but occasionally severe injury related to diabetes and hypertension are documented in autopsy studies and those of non-neoplastic renal parenchyma from nephrectomies20,21,22. Greater acceptance of kidneys from DKDs who are older and have comorbidities, similar to European practice, has been modeled as an effective intervention for the long kidney waitlist in the US23. This highlights the role of pathology in identification and risk-stratification of clinically significant chronic injury, particularly as the transplant community considers kidney donors with more comorbidities to address organ shortage and long waitlist.
Donor diabetes ≥6 years was associated with graft loss in our study; similar impact on allograft survival dictated by duration of donor diabetes was reported in two large studies, including by Mohan et al.9 studying nearly 2000 diabetic standard criteria donors from the UNOS database. Using the USRDS database to examine outcomes for 2300 recipients of diabetic DKD6, Ahmad et al. found that long-term graft survival from diabetic DKDs was statistically significantly inferior to kidneys from non-diabetic donors, but that the absolute difference was clinically small (hazard ratio 1.11), particularly considering waitlist-associated mortality rates1. However, when stratified by duration of diabetes, the hazard ratio increased: kidneys from donors with diabetes for >5 years conferred a 22% increased risk of graft failure; death-censored graft failure was increased 33% for those from donors with diabetes duration 6–10 years6. Recipient mortality was increased by 27% in recipients of DKDs with donor diabetes >10 years, although death-censored graft survival was not6. Cohen et al.8 observed an increased risk of mortality and all-cause graft loss in recipients of diabetic vs. non-diabetic DKDs, but this was dependent on recipient diabetes status, and the potential impact of duration of donor diabetes is not described.
Procurement frozen section biopsies are performed in approximately 50% of deceased donor kidneys and 85% of extended criteria donors, and “biopsy findings” is the most commonly reported reason for discard (37%)13,16,17. Global glomerulosclerosis ≥20% has been the histologic factor most associated with organ discard17, but this variable does not reliably predict graft outcome24,25. Despite their common use in the US, the role of procurement biopsies and their ability to predict post-transplant outcomes is controversial; studies have shown significant limitations including inconsistent findings between different areas of the kidney, between needle core vs. wedge biopsies, and limited inter-observer reliability or association with graft outcome12,13,14,15,16,17,24,26,27. Frozen wedge biopsies may over-estimate subcapsular scarring, but often have a more tissue and glomeruli than core biopsies28,29, whereas core biopsies may be better for detection of arteriosclerosis18. The degree of global glomerulosclerosis is often reproducible among pathologists14,27, but not consistent if taken from different areas of the kidney13,28. Degree of IFTA and vascular disease are more consistent from different section of the kidney12,13; reproducibility among pathologists varies by study24, and may be low for arteriolar hyalinosis27. Most donor kidney frozen biopsies are interpreted by an on-call surgical pathologist, and some studies have suggested a tendency to over-call chronic injury14 and advocated for specific training in interpretation of donor kidney frozens14,27. However, poor reproducibility has been documented even for experts for some lesions27. Taken together, these inconsistencies can limit the utility of using frozen section diagnosis to predict graft outcome.
To this body of work, our study adds that pre-implantation frozen biopsies from diabetic DKDs have a discrepancy rates similar to those reported for other DKDs12,13,24. Major frozen discrepancies were from patients with diabetes duration >10 years, highlighting the importance of this detail during frozen section evaluation. Glomerular disease is difficult to identify on frozen section, but arteriolar hyalinosis is a hallmark of diabetes-related renal injury. Hyalinosis and degree of IFTA can be identified on pre-implantation frozen and are associated with graft outcomes, but other reported variables including global glomerulosclerosis and arteriosclerosis were not. Furthermore, we identified significant differences between pre-implantation permanent sections and early post-transplant biopsies, supporting previous reports that sampling plays a significant role in pathologic grading of lesions12,13.
Our findings complement the limited published data focusing on pathology and outcomes of diabetic donor kidneys. Similar to our study, in a cohort of post-perfusion and subsequent for-indication biopsies, Khan et al.10 describe mild mesangial sclerosis by light microscopy in only 5/26 (19%) of diabetic DKD post-perfusion biopsies; vascular disease was more common, with arterionephrosclerosis described in 40%, and IFTA in 15%. Compared with this study, we utilized both surveillance and for-cause biopsies, and observed a similar degree of glomerular disease in 32% of patients, with 17% having mesangial sclerosis, and nodular glomerulosclerosis seen in one additional donor. In addition, we observed FSGS without characteristic mesangial sclerosis in 8% of diabetic donors. Rather than relying on characteristic mesangial sclerosis as the hallmark of diabetes, our study suggests that arteriolar hyalinosis is a better marker of diabetes-related renal parenchymal injury in diabetic patients. We also document the prognostic importance of arteriolar hyalinosis and IFTA in diabetic donor graft outcomes. Arteriolar hyalinosis is multifactorial and likely also related to hypertension in these patients. Its presence has been correlated with graft outcome in other studies30.
Although we did not observe significant improvement in histologic findings in either diabetic or non-diabetic recipients, the biopsy follow-up in this subgroup was limited in duration (<1 year). Studies have demonstrated the potential for improvement in characteristic glomerular structural changes, including degree of basement membrane thickening and mesangial matrix expanison31, often after years of euglycemia19, but that may not be apparent at 1 year32. Additional evaluation over time or with electron microscopy may prove informative in future studies of diabetic DKDs, particularly those with mild glomerular disease.
Weaknesses in this study include small sample size, low rate of allograft failure, and single-institution retrospective design. Given the small sample size, we performed univariate rather than multivariable analyses, and could not control for potential clinical or pathologic confounders, including hypertension. Coexistant hypertension is common in patients with type II diabetes; our study and previous investigations of diabetic kidney donors10,11 have shown that vascular disease is the most common morphologic finding. We did not control for the presence or severity of hypertension in either diabetic or non-diabetic cohorts, and whether some features are due to diabetes or other comorbidities like hypertension is uncertain. However, the association between duration of donor diabetes and allograft loss is in line with registry-based studies summarized above, which are powered to examine numerous clinical variables that we could not control for, and provide external support for our findings. The potential additive effects of diabetes in the recipient and donor, and the mechanisms by which these influence graft survival, are not addressed in this study. Although diabetic DKDs may be included in other studies of pre-implantation and post-perfusion kidney biopsies, there are few studies that have examined histologic findings in kidneys from diabetic donors10,11. The comparative paucity of pathology data on diabetic DKDs is likely related to the historically low rate of use of kidneys from diabetic donors (3.5–6.4% of DKDs in published studies), and how or if this information is available to, or recorded by, the interpreting pathologist. Kidney transplant patients may also receive follow-up care at various centers, and complete donor information may not be readily available to the pathologist at the time of post-transplant kidney biopsy in the recipient. Thus, our study benefits from the integration of institutional pathologic and registry-based (UNOS) data from pre-implantation frozen sections to post-implantation 3-month biopsies, our institutional practice of surveillance biopsies in the first year of transplant, and additional short-term histologic follow-up.
In conclusion, there were no significant differences in graft survival for recipients of diabetic vs. non-diabetic kidney donors, but duration of donors’ diabetes for ≥6 years is associated with a higher risk of allograft loss. Pathologically, the most common lesions identified in diabetic kidney donors are vascular rather than glomerular disease. Pre-implantation frozen section discrepancies were more common in long-standing donor diabetes, but the degree of arteriolar hyalinosis and IFTA identified on frozen section as well as early post-transplant biopsies accurately prognosticated graft loss.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
References
Cohen, J. B. et al. Survival benefit of transplantation with a deceased diabetic donor kidney compared with remaining on the waitlist. Clin. J. Am. Soc. Nephrol. 12, 974–982 (2017).
Rege, A. et al. Trends in usage and outcomes for expanded criteria donor kidney transplantation in the United States characterized by kidney donor profile index. Cureus 8, e887 (2016).
Matas, A. J. et al. OPTN/SRTR 2013 Annual Data Report: kidney. Am. J. Transplant 15 (Suppl 2), 1–34 (2015).
Ojo, A. O. et al. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J. Am. Soc. Nephrol. 12, 589–597 (2001).
Rosengard, B. R. et al. Report of the Crystal City meeting to maximize the use of organs recovered from the cadaver donor. Am. J. Transplant 2, 701–711 (2002).
Ahmad, M. et al. Impact of deceased donor diabetes mellitus on kidney transplant outcomes: a propensity score-matched study. Transplantation 88, 251–260 (2009).
Ojo, A. O. et al. Impact of pre-existing donor hypertension and diabetes mellitus on cadaveric renal transplant outcomes. Am. J. Kidney Dis. 36, 153–159 (2000).
Cohen, J. B. et al. National outcomes of kidney transplantation from deceased diabetic donors. Kidney Int. 89, 636–647 (2016).
Mohan, S. et al. Availability, utilization and outcomes of deceased diabetic donor kidneys; analysis based on the UNOS registry. Am. J. Transplant 12, 2098–2105 (2012).
Khan, F. N. et al. Outcomes of kidney transplantation using deceased donors with history of diabetes. Clin. Transplant 34, e13775 (2020).
Truong, L. D., Suki, W. N., Gaber, L. W., Gaber, O. A. & Khan, F. Kidney donors with diabetes: renal biopsy findings at time of transplantation and their significance. Transplant. Direct 5, e465 (2019).
Husain, S. A. et al. Reproducibility of deceased donor kidney procurement biopsies. Clin. J. Am. Soc. Nephrol. 15, 257–264 (2020).
Carpenter, D. et al. Procurement biopsies in the evaluation of deceased donor kidneys. Clin. J. Am. Soc. Nephrol. 13, 1876–1885 (2018).
Azancot, M. A. et al. The reproducibility and predictive value on outcome of renal biopsies from expanded criteria donors. Kidney Int. 85, 1161–1168 (2014).
Haas, M. Donor kidney biopsies: pathology matters, and so does the pathologist. Kidney Int. 85, 1016–1019 (2014).
Angeletti, A. & Cravedi, P. Making procurement biopsies important again for kidney transplant allocation. Nephron 142, 34–39 (2019).
Kasiske, B. L. et al. The role of procurement biopsies in acceptance decisions for kidneys retrieved for transplant. Clin. J. Am. Soc. Nephrol. 9, 562–571 (2014).
Haas, M. et al. Arteriosclerosis in kidneys from healthy live donors: comparison of wedge and needle core perioperative biopsies. Arch. Pathol. Lab. Med. 132, 37–42 (2008).
Fioretto, P., Steffes, M. W., Sutherland, D. E., Goetz, F. C. & Mauer, M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N. Engl. J. Med. 339, 69–75 (1998).
Henriksen, K. J., Meehan, S. M. & Chang, A. Nonneoplastic kidney diseases in adult tumor nephrectomy and nephroureterectomy specimens: common, harmful, yet underappreciated. Arch. Pathol. Lab. Med. 133, 1012–1025 (2009).
Henriksen, K. J., Meehan, S. M. & Chang, A. Non-neoplastic renal diseases are often unrecognized in adult tumor nephrectomy specimens: a review of 246 cases. Am. J. Surg. Pathol. 31, 1703–1708 (2007).
Perrone, M. E., Chang, A. & Henriksen, K. J. Medical renal diseases are frequent but often unrecognized in adult autopsies. Mod. Pathol. 31, 365–373 (2018).
Aubert, O. et al. Disparities in acceptance of deceased donor kidneys between the United States and France and estimated effects of increased US acceptance. JAMA Intern. Med. 179, 1365–1374 (2019). https://doi.org/10.1001/jamainternmed.2019.2322.
Wang, C. J., Wetmore, J. B., Crary, G. S. & Kasiske, B. L. The donor kidney biopsy and its implications in predicting graft outcomes: a systematic review. Am. J. Transplant 15, 1903–1914 (2015).
Sung, R. S. et al. Determinants of discard of expanded criteria donor kidneys: impact of biopsy and machine perfusion. Am. J. Transplant 8, 783–792 (2008).
Goumenos, D. S. et al. The prognostic value of frozen section preimplantation graft biopsy in the outcome of renal transplantation. Ren. Fail. 32, 434–439 (2010).
Liapis, H. et al. Banff histopathological consensus criteria for preimplantation kidney biopsies. Am. J. Transplant 17, 140–150 (2017).
Mazzucco, G., Magnani, C., Fortunato, M., Todesco, A. & Monga, G. The reliability of pre-transplant donor renal biopsies (PTDB) in predicting the kidney state. A comparative single-centre study on 154 untransplanted kidneys. Nephrol. Dial. Transplant 25, 3401–3408 (2010).
Yong, Z. Z., Kipgen, D., Aitken, E. L., Khan, K. H. & Kingsmore, D. B. Wedge versus core biopsy at time zero: which provides better predictive value for delayed graft function with the Remuzzi Histological Scoring System? Transplant Proc. 47, 1605–1609 (2015).
Wazna, E., Pazik, J., Perkowska-Ptasinska, A. & Durlik, M. Does histopathology of implanted kidney according to Banff 07 help predict long-term transplantation outcome? Transplant Proc. 50, 1765–1768 (2018).
Pichaiwong, W. et al. Reversibility of structural and functional damage in a model of advanced diabetic nephropathy. J. Am. Soc. Nephrol. 24, 1088–1102 (2013).
Hsu, C. T. et al. Ongoing donor-transmitted diabetic kidney disease in kidney transplant recipients with fair sugar control: a single center retrospective study. BMC Nephrol. 21, 458 (2020).
Acknowledgements
A portion of this work was presented in abstract form at United States and Canadian Academy of Pathologists meeting in 2021.
Author information
Authors and Affiliations
Contributions
A. G., D. S., and N. K. A. performed study concept and design; A. G, D. S., and N. K. A provided acquisition, analysis and interpretation of data, and statistical analysis; all authors performed development of methodology and writing, and review and revision of the paper; all authors have read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the Institutional Review Board (IRB) at Oregon Health & Science University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gilbert, A., Scott, D., Stack, M. et al. Long-standing donor diabetes and pathologic findings are associated with shorter allograft survival in recipients of kidney transplants from diabetic donors. Mod Pathol 35, 128–134 (2022). https://doi.org/10.1038/s41379-021-00927-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00927-2