Abstract
Verruciform proliferations of the vulva unrelated to HPV infection are rare. The term differentiated exophytic vulvar intraepithelial lesion (DEVIL) was recently proposed for these lesions, which harbor recurrent PIK3CA mutations. It is still unclear whether DEVIL is related to verrucous carcinoma, a neoplasm characterized by persistence and local recurrence but nil risk of distant spread. Specimens identified using the words “verruciform” and “verrucous” were reviewed. Diagnosis of DEVIL required verruciform acanthosis, hyper and/or parakeratosis, hypogranulosis, cytoplasmic pallor, and bland nuclei. Verrucous carcinoma required, in addition, discontinuous, bulbous, puzzle-like nests in the stroma. A targeted next-generation sequencing using a custom 11-gene panel was performed. Eighteen specimens corresponding to ten patients with DEVIL and/or verrucous carcinoma were included. Median age at presentation was 66 years for DEVIL and 70 years for verrucous carcinoma. A similar spectrum of prevalent mutations was found in both lesions involving HRAS, PIK3CA, and BRAF. DEVIL preceded verrucous carcinoma and/or was diagnosed concurrently or in subsequent follow-up in five patients. In four of these, the same mutation was identified in DEVIL and synchronous or metachronous carcinoma. All cases showed wild-type 53 staining and lacked pathogenic TP53 mutations. DEVIL is a rare form of squamous proliferation characterized by prevalent PIK3CA and HRAS mutations. Its temporal relationship with verrucous carcinoma and their shared mutational profile in some patients suggest that DEVIL is a precursor of verrucous carcinoma. Moreover, given their morphologic and molecular overlap and the nil risk of verrucous carcinoma for distant spread, it is conceivable that DEVIL and verrucous carcinoma represent a spectrum of the same entity.
Similar content being viewed by others
Introduction
Squamous cell carcinoma is the most common type of malignancy in the vulva, accounting for over 80% of vulvar malignancies [1]. From an etiology perspective, vulvar squamous cell carcinoma is classified as human papilloma virus (HPV) associated [2] and HPV independent [3, 4]. HPV-independent lesions are now divided in two main groups based on distinct clinical and morphologic features as well as p53 status [5]. Although the reported proportions of HPV associated versus HPV-independent vulvar squamous cell carcinoma varies depending on the particular study, it is accepted that the latter is more common [6]. With the utilization of HPV vaccination and the increase in life expectancy, the prevalence of HPV-independent squamous cell carcinoma is expected to increase.
One known precursor of vulvar squamous cell carcinoma, which is HPV-related, is known as high grade squamous intraepithelial lesion/usual vulvar intraepithelial neoplasia (uVIN). A second precursor lesion, which is HPV independent, is known as differentiated vulvar intraepithelial neoplasia (dVIN). dVIN tends to occur in older women, is associated with chronic inflammatory conditions such as lichen sclerosus, and harbors mutations in TP53. Nonetheless, ~30% of vulvar squamous cell carcinomas are not associated with any conventional precursor lesions at the time of diagnosis [7, 8]. It is still uncertain if a definite precursor exists for these lesions. In this regard, a spectrum of squamous lesions of the vulva, characterized by exophytic verruciform growth, has been described in the literature as potential precursors of vulvar squamous cell carcinoma. Formerly reported as vulvar acanthosis with altered differentiation (VAAD) [9], these lesions have been recently characterized under the term differentiated exophytic vulvar intraepithelial lesion (DEVIL) [10]. Unlike HPV-related squamous intraepithelial lesions and dVIN, DEVIL lacks significant nuclear atypia (basal or suprabasal), retains a high degree of squamous maturation, and has a characteristic verruciform appearance. Moreover, they are characterized by prevalent PIK3CA mutations and absence of TP53 mutations, which further supports the notion of DEVIL as a third type of precursor of vulvar squamous malignancy [10].
Verrucous carcinoma is a rare subtype of squamous carcinoma unrelated to HPV infection. Unlike conventional forms of HPV-independent squamous cell carcinoma, verrucous carcinoma has a relatively indolent behavior, characterized by local recurrence but a nil risk of distant spread [11]. While there is consensus in the literature that verrucous carcinoma is not related to HPV infection, little is known about its histogenesis. Previous studies have suggested a link between verrucous carcinoma and VAAD [9]. However, it remains unclear whether the more recently described DEVIL also has an association with verrucous carcinoma at the clinical, morphologic, and molecular levels. The purpose of this study was to document the clinical, histopathologic, and molecular alterations involved in DEVIL and verrucous carcinoma, in order to identify features that could explain their potential relationship and the histogenesis of verrucous carcinoma.
Materials and methods
This study was approved by the Research Ethics Boards at Sunnybrook Health Sciences Center in Toronto, Ontario, Canada.
Case selection and histological review
We performed a language structured query in the Sunnybrook Health Sciences Center laboratory information system. Search identified reports of vulvar specimens including the words “verruciform” and “verrucous”. Two cases were contributed by the University of Miami (AP). Full sets of Hematoxylin and eosin (H&E) stained material for each case were retrieved and reviewed by two gynecologic pathologists (CPH and JM) and one pathologist in training (AA). A review diagnosis was assigned to each case. Diagnosis of DEVIL and verrucous carcinoma was made using strict morphologic criteria. Diagnosis of DEVIL required verruciform acanthosis, hyperkeratosis and/or parakeratosis, hypogranulosis, cytoplasmic pallor, and bland nuclei, as described by Watkins et al. (Fig. 1a) [10]. Lesions falling under the definition of VAAD were also included under the DEVIL category (noting that VAAD and DEVIL have largely overlapping criteria). Diagnosis of verrucous carcinoma required the presence of discontinuous, bulbous, or puzzle-like nests in the dermis or submucosal stroma with a smooth, pushing border, as defined by the 2014 World Health Organization Classification of Tumors of the Female Genital Tract (Fig. 1b, c) [12]. The presence of any nuclear atypia including koilocytosis, nuclear hyperchromasia, high nuclear to cytoplasmic ratio or nuclear pleomorphism precluded the diagnosis of DEVIL or verrucous carcinoma [10]. Likewise, the presence of any irregular or frankly infiltrative stromal invasion excluded the diagnosis of verrucous carcinoma. Immunohistochemistry for p53 (clone Do-7, Roche Diagnostics) was performed in one section from a representative formalin-fixed paraffin-embedded block. Expression was interpreted as mutant type or wild type as recently described [13].
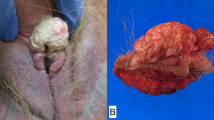
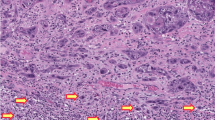
a Differentiated exophytic vulvar intraepithelial lesion (DEVIL) is characterized by verruciform acanthosis with hypogranulosis, cytoplasmic pallor and uniform, bland cytologic features. b Vulvar verrucous carcinoma is characterized by exophytic and endophytic growth. c Stromal invasion is seen histologically in the form of puzzle-like bulbous discontinuous nests of bland epithelium with retained differentiation.
DNA extraction and targeted gene sequencing
Samples were taken from formalin-fixed, paraffin-embedded tissue blocks. The most representative lesional areas were annotated by one pathologist (CPH) on H&E slides and subsequently on the corresponding unstained slides from formalin-fixed, paraffin-embedded tissue. Genomic DNA (gDNA) was extracted using the MagMAX FFPE DNA/RNA Ultra Kit (ThermoFisher Scientific). All procedures were carried out in accordance with the manufacturer’s instructions.
A custom targeted gene sequencing panel was designed based on previously published data on common somatic mutations in verrucous carcinoma and DEVIL [10, 14] by using Ion Ampliseq Designer (Thermofisher Scientific). The custom 11-gene panel contains 195 primer pairs and covers full coding sequence of TP53, CTNNB1, PIK3R1, FGFR2, and PTEN, and hotspot mutations in PIK3CA, HRAS, KRAS, AKT1, BRAF, and CDKN2A.
The amplicon library was constructed from 30 ng of gDNA by Ion Ampliseq Library Kit Plus with the custom panel. The targeted areas were amplified by polymerase chain reaction for 22 cycles. The resulting amplicons were treated with FuPa reagent to partially digest primers. Amplicons were ligated to Ion P1 and Ion Xpress barcode adapters and purified using Agencourt AMPure XP reagent (Beckman Coulter, Brea, CA, USA). Barcoded libraries were quantified using the Ion Library TaqMan Quantitation Kit (ThermoFisher Scientific) and diluted to a final concentration of 25 pM. The sequencing template preparation was done using the Ion Chef instrument with Ion 510 and Ion 520 and Ion 530 Chef Kits. Sequencing was performed for 500 flows on an Ion S5XL Sequencer with Ion 530 chip. For detection of somatic mutations, the sequencing was performed to at least 2500-fold mean depth coverage per sample.
The Ion Torrent platform-specific pipeline software, Torrent Suite version 5.6 (ThermoFisher Scientific) was used to separate barcoded reads and to filter and remove poor signal reads. BAM format files were generated from the sequencing results and then exported to the Ion Reporter Server (ThermoFisher Scientific). The bioinformatics analysis of the sequencing data were performed with Ion Torrent platform-specific bioinformatics software, Ion Reporter v5.6 for detection of single nucleotide polymorphisms, insertions and deletions in the tumor and for calculation of a p value representing the statistical confidence of that call. The Ion Reporter also annotates the variants with information from dbSNP, 1000 genomes, Online Mendelian Inheritance in Man, Catalogue Of Somatic Mutations In Cancer (COSMIC), Polyphen, and ClinVar. Variants flagged as pathogenic or likely pathogenic were compared across the entire cohort and between samples from the same patient.
Results
Clinical and pathologic characteristics
A total of 44 surgical specimens were identified and reviewed. Eight cases did not have paraffin blocks available and were excluded. Upon review, 18 of the remaining 36 specimens were classified as condyloma (n = 4), lichen simplex chronicus (n = 3), dVIN (n = 2) or conventional squamous cell carcinoma (n = 9) and were also excluded. The remaining 18 specimens had a confirmed diagnosis of DEVIL and/or verrucous carcinoma. This set, which represents the final cohort of the study, included material corresponding to ten subjects: five with both DEVIL and verrucous carcinoma either synchronously or metachronously; three with DEVIL only and two with verrucous carcinoma only. Patients and their corresponding diagnoses are listed in Table 1. Nine of ten patients (90%) first presented with vulvar pruritus. Two patients had known history of lichen sclerosus. Similarly, two had history of lichen simplex chronicus. None of the patients had history of cervical or vulvar HPV-related lesions.
Among the eight women with DEVIL, the median age at presentation of the first lesion was 66 years (range 41–85). In three of these patients, DEVIL preceded a subsequent diagnosis of verrucous carcinoma with an interval of 65 months (patient A), 22 months (patient B), and 33 months (patient C). In another patient (patient D), DEVIL and verrucous carcinoma were diagnosed concurrently. One additional patient (E) had verrucous carcinoma resected and 2 months later developed a DEVIL lesion. Remaining subjects in this group (patients F, G, and H) only had lesions classified as DEVIL.
Among the seven women with verrucous carcinoma, median age at presentation of the carcinoma was 70 years (range 46–85). As mentioned above, five of these subjects also had specimens containing DEVIL (A–E). The remaining two (patients I and J) only had verrucous carcinoma. Tumor size, available in five out of seven carcinomas, ranged from 1.6 to 4.8 cm (median 3 cm). Depth of invasion ranged from 1.5 to 12 mm (median 4 mm). Upon resection, all tumors had negative resection margins. Lymphovascular or perineural invasion was not identified in any of the cases. Three (30%) subjects underwent lymph node sampling at the time of resection; all lymph nodes were negative for malignancy.
Follow-up was available in all ten patients. Median follow-up period, starting from the latest available DEVIL or verrucous carcinoma diagnosis, was 24 months (range 6–120). At the last time of follow-up, seven patients were alive free of disease. Three subjects (patients F, I, and J) were alive with a recurrent verruciform lesion clinically, managed with observation (pathologic confirmation was not performed or is unavailable).
Targeted genomic analysis
When analyzed as separate categories, DEVIL and verrucous carcinoma samples showed a similar spectrum of pathogenic mutations. The prevalence of gene mutations in each group is shown in Table 2. The most frequently affected genes were HRAS (37% DEVIL, 57% verrucous carcinoma), PIK3CA (37% DEVIL, 29% verrucous carcinoma), and BRAF (9% DEVIL, 14% verrucous carcinoma). Single nucleotide variants in TP53, classified as of unknown significance in the National Library of Medicine ClinVar repository, were identified in two (18%) DEVILs and two (28%) verrucous carcinomas. Immunohistochemistry for p53 showed a wild-type pattern in all 18 lesions, including those with TP53 variants. The expression was scattered in all; there was no mid-epithelial staining observed.
Analysis of the five subjects with synchronous and/or metachronous DEVIL and verrucous carcinoma revealed shared gene alterations, which are depicted in Table 1. Patient A first presented with a verruciform lesion meeting criteria for DEVIL. Sixty-five months later, she presented with a mass diagnosed as verrucous carcinoma; the adjacent epithelium was also verruciform and met criteria for DEVIL (Fig. 2a–c). The same PIK3CA mutation was detected in preceding DEVIL, verrucous carcinoma, and concurrent DEVIL. Patient B had a DEVIL, and 22 months later a larger lesion which upon resection corresponded to verrucous carcinoma and adjacent verruciform epithelium consistent with DEVIL (Fig. 2d–f). In this case, no shared mutations were identified across samples. In patient C, DEVIL preceded a subsequent diagnosis of verrucous carcinoma and concurrent DEVIL by an interval of 33 months (Fig. 2g–i). In this patient, all three lesions had mutations in HRAS; however, the mutation in the preceding DEVIL was different from the mutation in verrucous carcinoma and concurrent DEVIL. In patient D, a tumor diagnosed as verrucous carcinoma had adjacent areas of intraepithelial lesion consistent with DEVIL (Fig. 3a, b). Both lesions shared the same mutation in BRAF. Lastly, patient E presented with a mass, which was resected and diagnosed as verrucous carcinoma. Two months later, a new exophytic lesion appeared which upon resection corresponded to DEVIL (Fig. 3c, d). Both verrucous carcinoma and subsequent DEVIL shared the same mutation in HRAS.
Vulvar verruciform lesions from three patients (patient A a–c, patient B d–f, and patient C g–i). Each first presented with DEVIL (a, d, and g, respectively) and months later with a larger lesion histologically corresponding to verrucous carcinoma (b, e, and h, respectively) and adjacent DEVIL (c, f, and i, respectively). All figures were taken at ×50 magnification.
Vulvar verruciform lesions from two patients. Patient D presented with DEVIL (a) adjacent to verrucous carcinoma (b). Patient E first manifested with verrucous carcinoma (c) and months later with a recurrent intraepithelial lesion consistent with DEVIL (d). All figures were taken at ×50 magnification.
Discussion
Three distinct pathways are currently recognized in the carcinogenesis of vulvar squamous cell carcinoma, one related to high risk HPV infection, and the other two independent from it [5]. Regarding HPV-independent carcinomas, most are associated with chronic inflammatory dermatoses, early TP53 alterations, infiltrative stromal invasion, and more aggressive behavior compared with HPV-associated tumors. A less common category of HPV-independent lesions lacks driver TP53 alterations and have seemingly better behavior. Among this group, verrucous carcinoma is a rare morphological variant of HPV-independent carcinoma, which has a relatively indolent although often protracted clinical course [15,16,17]. This tumor type typically has potential for aggressive local growth and recurrence but does not metastasize to distant organs, indicating that verrucous carcinoma is different from conventional forms of squamous cell carcinoma.
About 33% of HPV-negative vulvar squamous cell carcinomas are not associated with either dVIN or high grade squamous intraepithelial lesion [7]. It is possible that, in these cases, the precursor is quickly obliterated by the carcinoma. Alternatively, it is possible that the precursor is not conventional, as it fails to show conspicuous atypia. This hypothesis applies especially to vulvar verrucous carcinoma. The characterization of a precursor lesion of vulvar verrucous carcinoma has been elusive, given the rarity of this carcinoma type and the absence of histopathologic features traditionally associated with malignancy.
dVIN is regarded as the precursor of TP53-mutant, HPV-independent vulvar squamous cell carcinoma. A separate, and less understood, type of HPV-independent vulvar intraepithelial neoplasia is characterized by verruciform growth, absence of conspicuous atypia and more indolent biology. The first description of verruciform intraepithelial lesions associated with verrucous carcinoma coined the term VAAD [9]. Interestingly, this lesion encompassed cases previously described as verruciform lichen simplex chronicus, not surprisingly given its bland cytomorphology and therefore potential for misdiagnosis as a nonneoplastic lesion. Watkins et al. expanded on the characterization of verruciform lesions under the umbrella of VAAD. Based on their shared morphologic features (acanthosis with verruciform growth, hypogranulosis, cytoplasmic pallor, and absence of atypia) and the finding of recurrent PIK3CA (73%) and ARID2 (55%) mutations, the novel term DEVIL was then proposed, as a way to acknowledge the neoplastic nature of these lesions [10]. Our aim was to expand on our understanding of these bland verruciform lesions following established definitions and their relationship with verrucous carcinoma. Our results support recent shifts toward classifying HPV-independent, verruciform, TP53 wild-type lesions as a third pathway of vulvar squamous preinvasive and invasive neoplasia. To this end, we prefer the term DEVIL, as its definition incorporates criteria formerly applied to VAAD and it better conveys the neoplastic nature of the lesion.
To our knowledge, this is the first study documenting a temporal, morphologic, and molecular relationship between verrucous carcinoma and exophytic lesions defined as DEVIL. First, five of the seven (71%) verrucous carcinomas included were either preceded by DEVIL or had a concurrent or subsequent intraepithelial proliferation consistent with DEVIL. Second, there is morphologic overlap between DEVIL and verrucous carcinoma: both are by definition verruciform and lack cytologic atypia, and the main difference between the two is the presence of nondestructive puzzle-like stromal growth in verrucous carcinoma. Third, there is a shared mutational profile between the two entities including mutations in PIK3CA, HRAS, and BRAF, as well as absence of pathogenic TP53 mutations. Based on these considerations, and given the known potential for local recurrence but nil risk of distant spread of verrucous carcinoma, we postulate that DEVIL and verrucous carcinoma belong to the same spectrum of vulvar disease, with DEVIL being a precursor lesion still confined to the epithelial compartment and verrucous carcinoma representing a more florid proliferation showing endophytic growth into the underlying stroma.
It is important to note that in some of the subjects included, there was no mutation overlap in between lesions over time (for example, patient B). While it is still possible that such lesions are still clonally related based on gene mutations not covered by our panel of choice, we raise the possibility that, at least in some cases, DEVIL and verrucous carcinoma are independent processes manifesting in a synchronous or metachronous fashion. A “field” effect, known in other types of preinvasive vulvar squamous neoplasia, may thus play a role as well in the setting of verruciform vulvar lesions.
Amongst the genes included in our panel, PIK3CA and HRAS were the most frequently mutated. PIK3CA mutations were prevalent in the seminal description of DEVIL [10]. Conversely, PIK3CA mutation is only seen in 8% of human HPV-negative vulvar neoplasms [18], which suggests that DEVIL is indeed different from other HPV-independent lesions such as dVIN. HRAS and PIK3CA mutations were also identified in a recent series of vulvar verrucous carcinomas [14].
In the recently evolving classification of vulvar preinvasive and invasive squamous neoplasms, HPV-independent lesions are subdivided in TP53-mutant and TP53 wild-type categories. It is known that TP53-mutant vulvar squamous lesions have distinct biology and clinical behavior. Conversely, the group of TP53 wild-type lesions appears to have a more indolent behavior. In this shifting paradigm, verrucous carcinoma and DEVIL belong to the latter group. TP53 mutations were not observed in the series of DEVIL by Watkins et al. [10]. Of note, one study reported a TP53 mutation in one of seven lesions with histopathologic features consistent with DEVIL [19]. In our series, we identified TP53 single nucleotide variants in four lesions (two DEVIL and two verrucous carcinomas). These are classified as pathogenic as per FATHMM prediction in the COSMIC repository, but in ClinVar are classified as variants of unknown significance or with conflicting interpretations of pathogenicity. Therefore, it appears that TP53 mutations in DEVIL and verrucous carcinoma do not have a significant biologic role. The fact that the variants identified in our series did not correlate with abnormal p53 expression by immunohistochemistry supports this statement. A recent comprehensive analysis of in situ and invasive squamous cell carcinomas of the vulva also showed absence of TP53 mutations and wild-type p53 expression in all their DEVIL and verrucous carcinoma cases [13]. Moreover, there is evidence that TP53 alterations do not correlate with the risk of local recurrence in verrucous carcinomas [14].
HRAS is an oncogene that belongs to the RAS family and is involved in the RTK/RAS/PI3K pathway [20]. It is implicated in several cancers including head and neck squamous cell carcinoma where mutations lead to aberrant activation of mTOR signaling and tumor development and progression [21]. In our study HRAS mutations were seen in 8/18 specimens (44%) and more frequently occurred in cases without PIK3CA mutations suggesting that HRAS is an additional key gene in the biology of DEVIL and verrucous carcinoma. Similar results were reported by Nooij et al. where HRAS mutations were seen in HPV-independent vulvar carcinoma precursor lesions independent of TP53 mutations [19]. In this study, 70% of HPV-independent squamous cell carcinomas that lacked TP53 mutations carried HRAS and/or NOTCH mutations, suggesting that HPV-independent tumors could potentially be separated into two molecular subtypes: (1) TP53 abnormal and (2) TP53 wild type, HRAS/NOTCH abnormal. Under this logic, verrucous carcinoma and DEVIL could belong to the latter category. Interestingly, HRAS mutations in HPV-independent squamous cell carcinomas of the vulva have been shown to confer a worse prognosis [18].
Our study is limited by the rarity of DEVIL and verrucous carcinoma, reflected by the small number of subjects identified. In addition, our panel included a limited number of genes. Identification of other key genetic aberrations requires more comprehensive sequencing platforms, as well as exploring gene expression and methylation analysis. Exploring further mechanisms is important as evidenced by the fact that some of our cases did not harbor any alterations in the genes surveyed. Follow-up was limited in some of the subjects included, particularly those with DEVIL lesions only. As seen in the patients with longer follow-up, the temporal evolution of DEVIL and verrucous carcinoma can span months to years. We did not assess the intra and interobserver reproducibility of the pathologic diagnosis of DEVIL and verrucous carcinoma. While we abided by strict criteria for inclusion, it remains to be determined if the proposed definition of DEVIL is reproducible among pathologists. Lastly, the diagnosis and management of verrucous carcinoma and DEVIL are still poorly understood. DEVIL is also associated with conventional forms of vulvar squamous cell carcinoma [10], and progression from verrucous carcinoma to poorly differentiated carcinoma has also been reported [22]. This highlights the need for additional studies of these entities and the mechanisms underlying progression to more aggressive forms of malignancy.
In conclusion, DEVIL is a rare vulvar lesion with prevalent PIK3CA and HRAS mutations, absence of TP53 alterations and an association with subsequent and/or concurrent verrucous carcinoma. Based on this evidence, we postulate that DEVIL represents a third form of vulvar intraepithelial neoplasia and likely a precursor lesion for verrucous carcinoma. Furthermore, verrucous carcinoma and DEVIL may represent morphologic ends of the same spectrum of disease, one defined by exophytic verruciform growth and potential for local recurrence but negligible risk of distant spread.
References
Kosary CL. Cancer of the vulva. In: Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J, editors. SEER survival monograph: cancer survival among adults: U.S. SEER Program, 1988–2001, patient and tumor characteristics. Bethesda MD: National Cancer Institute, SEER Program, National Institute of Health, Pub. No. 07-6215; 2007. p. 147–54.
Kurman RJ, Toki T, Schiffman MH. Basaloid and warty carcinomas of the vulva. distinctive types of squamous cell carcinoma frequently associated with human papillomaviruses. Am J Surg Pathol. 1993;17:133–45.
Hording U, Junge J, Daugaard S, Lundvall F, Poulsen H, Bock JE. Vulvar squamous cell carcinoma and papillomaviruses: indications for two different etiologies. Gynecol Oncol. 1994;52:241–6.
Bigby SM, Eva LJ, Jones RW. Spindle cell carcinoma of the vulva: a series of 4 cases and review of the literature. Int J Gynecol Pathol. 2014;33:203–12.
Singh N, Gilks CB. Vulval squamous cell carcinoma and its precursors. Histopathology. 2020;76:128–38.
McAlpine JN, Leung SCY, Cheng A, Miller D, Talhouk A, Gilks CB, et al. Human papillomavirus (HPV)-independent vulvar squamous cell carcinoma has a worse prognosis than HPV-associated disease: a retrospective cohort study. Histopathology. 2017;71:238–46.
Leibowitch M, Neill S, Pelisse M, Moyal-Baracco M. The epithelial changes associated with squamous cell carcinoma of the vulva: a review of the clinical, histological and viral findings in 78 women. Br J Obstet Gynaecol. 1990;97:1135–9.
Allo G, Yap ML, Cuartero J, Milosevic M, Ferguson S, Mackay H, et al. HPV-independent vulvar squamous cell carcinoma is associated with significantly worse prognosis compared with HPV-associated tumors. Int J Gynecol Pathol. 2019. https://doi.org/10.1097/PGP.0000000000000620.
Nascimento AF, Granter SR, Cviko A, Yuan L, Hecht JL, Crum CP. Vulvar acanthosis with altered differentiation: A precursor to verrucous carcinoma? Am J Surg Pathol. 2004;28:638–43.
Watkins JC, Howitt BE, Horowitz NS, Ritterhouse LL, Dong F, MacConaill LE, et al. Differentiated exophytic vulvar intraepithelial lesions are genetically distinct from keratinizing squamous cell carcinomas and contain mutations in PIK3CA. Mod Pathol. 2017;30:448–58.
Liu G, Li Q, Shang X, Qi Z, Han C, Wang Y, et al. Verrucous carcinoma of the vulva: A 20 year retrospective study and literature review. J Low Genit Trac Dis. 2016;20:114–8.
Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO classification of tumours of female reproductive organs. 4th ed. Lyon, France: IARC WHO Classification of Tumours, No 6; 2014. p. 234.
Tessier-Cloutier B, Kortekaas KE, Thompson E, Pors J, Chen J, Ho J, et al. Major p53 immunohistochemical patterns in in situ and invasive squamous cell carcinomas of the vulva and correlation with TP53 mutation status. Mod Pathol. 2020. https://doi.org/10.1038/s41379-020-0524-1.
Pors J, Tessier-Cloutier B, Ho J, Thompson E, Wong R, Trevisan G, et al. Verrucous carcinomas of the vulva: targeted next generation sequencing (NGS) and p53 immunohistochemistry (IHC) reveals p53 aberrations in 50% of cases. Mod Pathol. 2019;32 S2:1102A.
Kondi-Paphitis A, Deligeorgi-Politi H, Liapis A, Plemenou-Frangou M. Human papilloma virus in verrucus carcinoma of the vulva: an immunopathological study of three cases. Eur J Gynaecol Oncol. 1998;19:319–20.
Mehta V, Durga L, Balachandran C, Rao L. Verrucous growth on the vulva. Indian J Sex Transm Dis AIDS. 2009;30:125–6.
Robertson DI, Maung R, Duggan MA. Verrucous carcinoma of the genital tract: is it a distinct entity? Can J Surg. 1993;36:147–51.
Trietsch MD, Spaans VM, ter Haar NT, Osse EM, Peters AA, Gaarenstroom KN, et al. CDKN2A(p16) and HRAS are frequently mutated in vulvar squamous cell carcinoma. Gynecol Oncol. 2014;135:149–55.
Nooij LS, Ter Haar NT, Ruano D, Rakislova N, van Wezel T, Smit VTHBM, et al. Genomic characterization of vulvar (pre)cancers identifies distinct molecular subtypes with prognostic significance. Clin Cancer Res. 2017;23:6781–9.
Filmus J, Robles AI, Shi W, Wong MJ, Colombo LL, Conti CJ. Induction of cyclin D1 overexpression by activated ras. Oncogene. 1994;9:3627–33.
Kiaris H, Spandidos D. Analysis of h-ras, k-ras and N-ras genes for expression, mutation and amplification in laryngeal tumors. Int J Oncol. 1995;7:75–80.
Al-Bannai R, Miller D, Sadownik L, Gilks CB. Vulvar acanthosis with altered differentiation (VAAD): report of a case with progression to poorly differentiated carcinoma over a 5-yr period. Int J Gynecol Pathol. 2015;34:385–9.
Acknowledgements
This study was funded by the Strategic Innovation Fund at the Department of Laboratory Medicine and Molecular Diagnostics, Sunnybrook Health Sciences Center (Toronto, ON, Canada).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akbari, A., Pinto, A., Amemiya, Y. et al. Differentiated exophytic vulvar intraepithelial lesion: Clinicopathologic and molecular analysis documenting its relationship with verrucous carcinoma of the vulva. Mod Pathol 33, 2011–2018 (2020). https://doi.org/10.1038/s41379-020-0573-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-020-0573-5
This article is cited by
-
HPV-independent, p53-wild-type vulvar intraepithelial neoplasia: a review of nomenclature and the journey to characterize verruciform and acanthotic precursor lesions of the vulva
Modern Pathology (2022)
-
Molecular landscape of vulvovaginal squamous cell carcinoma: new insights into molecular mechanisms of HPV-associated and HPV-independent squamous cell carcinoma
Modern Pathology (2022)
-
Molecular characterization of invasive and in situ squamous neoplasia of the vulva and implications for morphologic diagnosis and outcome
Modern Pathology (2021)