Abstract
Vulvar squamous cell carcinomas and their precursors are currently classified by the World Health Organization based on their association with high-risk human papillomavirus (HPV). HPV independent lesions often harbor driver alterations in TP53, usually seen in the setting of chronic vulvar inflammation. However, a group of pre-invasive vulvar squamous lesions is independent from both HPV and mutant TP53. The lesions described within this category feature marked acanthosis, verruciform growth and altered squamous maturation, and over the last two decades several studies have added to their characterization. They have a documented association with verrucous carcinoma and conventional squamous cell carcinoma of the vulva, suggesting a precursor role. They also harbor recurrent genomic alterations in several oncogenes, mainly PIK3CA and HRAS, indicating a neoplastic nature. In this review, we provide a historical perspective and a comprehensive description of these lesions. We also offer an appraisal of the terminology used over the years, going from Vulvar Acanthosis with Altered Differentiation and Verruciform Lichen Simplex Chronicus to Differentiated Exophytic Vulvar Intraepithelial Lesion and Vulvar Aberrant Maturation, the latter term having been recently proposed by the International Society for the Study of Vulvovaginal Diseases. In line with the recognition of these lesions by the 2020 World Health Organization Classification of Tumours as a neoplastic precursor, we herein propose the term HPV-independent, p53-wild-type verruciform acanthotic Vulvar Intraepithelial Neoplasia (HPVi(p53wt) vaVIN), which better conveys not only the pathology but also the neoplastic nature and the biologic risk inherent to these uncommon and challenging lesions. We outline strict morphologic and immunohistochemical criteria for its diagnosis and distinction from mimickers. Immunohistochemistry for p16 and p53 should be performed routinely in the diagnostic work-up of these lesions, and the morphologic alternative term vaVIN should be reserved for instances in which p16/HPV/p53 status is unknown. We also discuss management considerations and the need to further explore precursors within and beyond the spectrum of verruciform acanthotic vulvar intraepithelial neoplasia.
Similar content being viewed by others
Introduction
In line with other anatomic locations, the 5th edition of the World Health Organization (WHO) Classification of Female Genital Tumours now recognizes human papillomavirus (HPV)-associated and HPV-independent forms of squamous cell carcinoma of the vulva1. Likewise, vulvar intraepithelial squamous precursors are also separated according to HPV status. HPV-associated lesions, which are both readily recognizable and well-defined, are currently designated as high grade squamous intraepithelial lesion (HSIL) and have alternatively been known as usual-type vulvar intraepithelial neoplasia (uVIN, uVIN2–3). HPV-independent vulvar intraepithelial neoplasia (VIN), in contrast to HSIL, encompasses several entities, many of which have only recently been described. Thus, there is only partial consensus regarding their nomenclature as well as their place in the current classification schema.
The WHO acknowledges two types of HPV-independent VIN: (1) the differentiated type (dVIN), which is associated with TP53 alterations in the setting of chronic inflammatory dermatoses, and (2) precursors under the names of differentiated exophytic vulvar intraepithelial lesion (DEVIL) and vulvar acanthosis with altered differentiation (VAAD)1. The International Society of the Study of Vulvovaginal Diseases (ISSVD) Difficult Pathologic Diagnoses Committee also recognizes dVIN, but refers to DEVIL, VAAD and other related lesions as vulvar aberrant maturation (VAM)2. The College of American Pathologists Cancer Reporting Protocol only explicitly includes dVIN and HPV-associated lesions as additional pathologic findings and for margin assessment3.
About a third of HPV-independent vulvar squamous cell carcinomas are not associated with concurrent or preceding dVIN4. It has been argued that, in these instances, the precursor is obliterated by the invasive carcinoma by the time of diagnosis, as the progression from dVIN to invasive carcinoma occurs in a short interval (estimated to be 9–23 months)5,6. It has also been hypothesized that the carcinoma arises de novo out of pre-existing dermatoses such as lichen sclerosus, or that the precursor is underrecognized as it lacks conspicuous atypia7. This last hypothesis is particularly relevant to p53-wild-type tumors such as verrucous carcinoma. Mounting evidence has helped characterize an HPV-independent, p53-wild-type precursor characterized by verruciform and/or acanthotic growth and absence of nuclear atypia. This review presents the historical perspective and current knowledge of these HPV-independent, p53-wild-type lesions and their invasive counterparts. To this end, we discuss the pathology of VAAD, DEVIL and VAM, and propose the unifying term HPV-independent, p53-wild-type verruciform acanthotic Vulvar Intraepithelial Neoplasia to harmonize the nomenclature with the current WHO classification.
Historical perspective and nomenclature
In the process of characterizing HPV-independent, p53 wild-type lesions, different names have been assigned over the years. The first term, coined by Nascimento et al.8 in 2004, was vulvar acanthosis with altered differentiation (VAAD). In this seminal publication, a characteristic pattern of abnormal squamous maturation was described in the vicinity of seven cases of vulvar verrucous carcinoma. Morphologically, VAAD was defined by the presence of three features: marked acanthosis with variable verruciform architecture, loss of the granular cell layer with superficial epithelial cell pallor, and multilayered parakeratosis. Thus, VAAD was conceived as a descriptive diagnostic term. Additional work by Nooij et al.9 provided further evidence that VAAD was both a distinctive lesion and a putative precursor to verrucous carcinoma through the identification of frequent HRAS and NOTCH1 mutations in VAAD (71.4% and 28.6%, respectively) which are also observed in HPV-negative squamous cell carcinoma (31% and 41.4%, respectively)9. In support of this, recent work by Salama et al. has confirmed that NOTCH1 gene alterations, along with TERT, TP53, and CDKN2A alterations, are strongly associated with HPV-independent squamous cell carcinoma10.
Lesions with verruciform growth and lack of basal atypia were revisited in 2017 by Watkins et al.11. They included cases with prominent acanthosis and verruciform architecture within the realm of VAAD (including lesions bordering on verrucous carcinoma) as well as verruciform lichen simplex chronicus (vLSC) and atypical verruciform hyperplasia. The study found frequent alterations in PIK3CA (73%), ARID2 (55%), and, less commonly, HRAS (18%)11. Importantly, no TP53 mutations were identified11. Based on the morphologic and molecular findings, the term differentiated exophytic vulvar intraepithelial lesion (DEVIL) was proposed. Independent series of cases fitting the morphologic definition of DEVIL (i.e., lesion with acanthotic/verruciform morphology, abnormal keratinocyte differentiation, absence of significant basal atypia, and absence of p53 abnormality) confirmed the frequent occurrence of PIK3CA and HRAS mutations12,13. An association between DEVIL and subsequent conventional squamous cell carcinoma11, and between DEVIL and subsequent, concurrent or preceding vulvar verrucous carcinoma12 was also described in these studies. Interestingly, the study by Akbari et al. showed that DEVIL and synchronous or metachronous verrucous carcinoma in the same patient often share the same mutational profiles, suggesting a precursor role and highlighting the overlap, not only morphologic but also molecular, between the two entities12. Of importance, a recent institutional reproducibility study by Neville et al. showed acceptable interobserver agreement among gynecologic pathologists in the distinction of DEVIL from HPV-associated lesions (condyloma and HSIL/uVIN) as well as dVIN, lichen simplex chronicus and psoriasis14. The reproducibility was better among practicing gynecologic pathologists than with trainees, suggesting that clinical experience with DEVIL and its mimickers is required to achieve optimal diagnostic consistency.
A recent study by Roy et al., which constitutes the largest series of verruciform and acanthotic squamous lesions of the vulva to date (n = 36), classified lesions as VAAD, DEVIL or vLSC15. Authors observed that there were no significant morphologic differences between these three categories other than the major features used for their categorization (flat acanthosis with hypogranulosis and epithelial pallor for VAAD, exophytic and/or acanthotic growth for DEVIL and hypergranulosis with lack of pallor for vLSC). Notably, they observed vLSC in the vicinity of 50% of VAAD and in 60% of DEVIL cases. Median patient age was 71 years (range 59–81 years), similar to previous studies, and the clinical presentation was also largely similar among categories. Lesion recurrence was frequent, documented in 64% of VAAD, 60% of DEVIL and 18% of vLSC cases. Importantly, they observed similar rates of subsequent invasive squamous cell carcinoma in all three categories (overall 37%; 46% in VAAD, 40% in DEVIL and 27% in vLSC), with a median progression to invasive disease of 43 months. Rate and time to lesion recurrence, as well as rate and time to progression to invasive carcinoma were all lower in vLSC than in VAAD & DEVIL, but the differences were not statistically significant. Based on this data, the authors recommend including vLSC within the spectrum of HPV-independent, p53 wild-type VIN instead of treating it as a variant of lichen simplex chronicus.
Given the significant overlap among these entities a third term, vulvar aberrant maturation (VAM), was recently proposed by the ISSVD2. VAM is defined as “an umbrella term for HPV-independent lesions combining aberrant maturation with minimal nuclear atypia”. Thus, VAM is proposed as an overarching diagnosis encompassing the many formerly proposed names for these lesions including DEVIL, VAAD, lichen sclerosus with acanthosis or hyperplasia, verruciform lichen simplex chronicus and squamous cell hyperplasia. ISSVD argues that the term VAM is unifying, emphasizing the commonalities in histopathologic findings. We counterargue that, while inclusive, the term VAM is vague and dilutes the documented biologic and molecular characteristics of VAAD and DEVIL. The term suggests a pathologic process rather than a discrete diagnostic entity. Like the terms VAAD and DEVIL, it also does not align with the VIN nomenclature adopted by the WHO, and thus, if applied, would not serve to acknowledge the neoplastic status of these lesions and the need for excision and close follow up by gynecologic oncology specialists. Most significantly, the current ISSVD approach allows p53-abnormal and p53-wild-type status in both dVIN and VAM categories, thereby blurring the molecular distinction between these lesions which are now increasingly understood as two separate pathogenic pathways. This constitutes a further departure from the effort made in the latest WHO classification to align morphology with underlying molecular pathogenesis across all female genital lesions.
The term DEVIL proposed previously11 aimed to go beyond the morphologic descriptive nature of VAAD to imply the neoplastic and cancer precursor nature of the lesion. Admittedly, its wording can cause confusion with other vulvar squamous precursors (differentiated VIN, high-grade squamous intraepithelial lesion), and does not align with the VIN terminology used in the current WHO classification. In order to overcome this, we propose a unifying term that acknowledges the biology of the lesion and incorporates it into the current WHO classification: HPV-independent, p53-wild-type, verruciform acanthotic Vulvar Intraepithelial Neoplasia (HPVi(p53wt) vaVIN). Admittedly, ancillary testing (e.g., immunohistochemistry) may not always be available, and as a result, pathologists may require a morphology-only descriptor. We think the best alternative is Verruciform Acanthotic Vulvar Intraepithelial Neoplasia (vaVIN), a term that may also be useful to separate these from other HPV-independent, p53-wild-type precursors without characteristic verruciform acanthosis, if such lesions are ever discovered. In the authors’ opinion the biggest shift in terminology needs to be towards the underlying biology of the lesion as the WHO currently acknowledges. Hence, we strongly advocate for the term HPVi(p53wt) vaVIN, both in academic discourse and routine clinical use, and recommend resorting to a morphologic alternative (vaVIN) only if p16/HPV/p53 status cannot be obtained (e.g., test is unavailable, stain is difficult to interpret). Using this term and its definition below also implies that the terms VAAD, DEVIL and vLSC are no longer needed and should be abandoned.
HPV-independent, p53-wild-type verruciform acanthotic VIN—definition
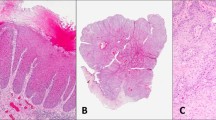
HPVi(p53wt) vaVIN, as defined below, presents in patients in their 6–9th decades of life as a discrete (most often single) lesion. The macroscopic appearance is of a raised white or erythematous mass, with irregular cauliflower-like or plaque-like exophytic surface (Fig. 1). Pruritus and pain are reported in a subset of patients15. The diagnosis of HPVi(p53wt) vaVIN is based on the presence of the following histopathologic features2,8,11 (Figs. 2, 3, Table 1):
-
1.
Acanthotic and/or verruciform architecture: Grossly, the lesion has verruciform growth (verruciform = in the shape of a verruca or wart, e.g., raised lesion with horn-like projections) or appears as a thick plaque. Microscopically, acanthosis is seen as thickening of the epidermis, whereas the verruciform architecture is imparted by visible elongation and irregularity of the rete ridges. The lesion is usually raised and discrete. It can have a flat plaque-like surface or be exophytic. In the latter case, the epithelial projections are variably sized and can have squared, bulbous, or pointed “church spire” shapes.
-
2.
Altered squamous differentiation: Squamous maturation is retained but altered, seen as hyper- and parakeratosis associated with either partial or complete hypogranulosis plus cytoplasmic pallor in mid and superficial layers (formerly defined as VAAD) or, less frequently, as hypergranulosis (formerly defined as vLSC).
-
3.
Absence of cytologic atypia: Atypia in this context is defined as that seen in HPV-related lesions (ranging from koilocytosis to the hyperchromasia, nuclear irregularity and loss of maturation seen in HSIL/uVIN) and in dVIN (enlarged basal nuclei, either hyperchromatic or with vesicular open chromatin and large nucleoli). Mitotic activity is usually low and always confined to the basal and parabasal epithelial layers.
-
4.
Negative or patchy p16 staining: This result is indicative of a lesion independent from high-risk HPV infection, which can be confirmed with HPV detection studies if such resources are available.
-
5.
Wild-type p53 expression: While the absence of significant basal atypia is evident in most cases, thus excluding dVIN on H&E examination alone, we acknowledge that this can be difficult to ascertain and therefore encourage routine use of p53 immunohistochemistry to confirm wild-type status.
Three examples displaying verruciform architecture are shown. At low power, the lesions are markedly acanthotic and have a prominent keratin layer (A–C); projections can be variably shaped (A), long and horn-like (B) or bulbous (C). Higher magnification of each of these three lesions shows abnormal maturation with hypogranulosis, cytoplasmic pallor of mid and upper epithelial layers and parakeratosis (D–F). Notice the absence of cytologic atypia (nuclear enlargement, pleomorphism and loss of polarity) both in the base and in upper epithelial layers.
Some examples show verruciform growth with a marked irregular convolution of the dermo-epidermal layer and a raised but otherwise flat surface (A–C). The epithelium is acanthotic and hyperkeratotic. Notice the hypogranulosis and cytoplasmic pallor. Other examples with evident verruciform exophytic appearance feature hypergranulosis, thus resembling lichen simplex chronicus (the so-called verruciform variant) (D–F).
We introduce p16 and p53 as biomarkers for routine use in this diagnosis given the known existence of acanthotic variants of both HSIL/uVIN and dVIN, underscoring the significant morphologic overlap between precursor lesions16,17. It is also foreseen that p16 and p53 testing becomes standard in the evaluation of all pre-invasive and invasive vulvar squamous neoplasms given not only the diagnostic but also the prognostic value of these markers4,18. Therefore, we recommend performing these stains on every lesion with a favored or suspected diagnosis of HPVi(p53wt) vaVIN. Likewise, performing HPV testing and next generation sequencing can be of value: finding PIK3CA, ARID2, and/or HRAS mutations would be supportive of HPVi(p53wt) vaVIN, with the caveat that absence of such alterations does not preclude this diagnosis. When performed, testing should reveal absence of both HPV DNA and TP53 alterations.
Differential diagnosis of HPVi(p53wt) vaVIN
The differential diagnosis of HPVi(p53wt) vaVIN includes several pre-malignant, malignant, and benign entities. Differentiated VIN (HPVi(p53mutant)) can have a warty exophytic appearance and display acanthosis, hyperkeratosis, and parakeratosis (Fig. 4). HSIL/uVIN can similarly have superimposed acanthosis and LSC-like changes (Fig. 5)19. These precursors can be distinguished from HPVi(p53wt) vaVIN by the presence of 1) cytologic atypia in the squamous population, 2) loss of maturation in HSIL/uVIN, and 3) abnormal p53 or p16 staining patterns. Of note, the loss of maturation in HSIL/uVIN may be less pronounced in cases with superimposed LSC. For this reason, immunohistochemistry may help confirm the diagnosis: p16 displays strong positivity in the low to mid epithelium, and p53 shows either scattered wild-type expression or a unique pattern of upregulation in the middle epithelial layers and complete sparing of the basal layer17,19. We have encountered patients with HPVi(p53wt) vaVIN without TP53 abnormalities that later present with verruciform HPVi(p53mutant) dVIN and TP53-mutant squamous cell carcinoma11. Thus, HPVi(p53wt) vaVIN can progress towards a more aggressive form of squamous neoplasia through the acquisition of functional TP53 and other cell cycle aberrations.
HPVi(p53mutant) differentiated VIN can present with prominent exophytic growth (A) and verruciform acanthosis (B, C). Attention to the basal cell layers is imperative in this differential, as HPVi(p53mutant) dVIN is characterized by loss of basal organization, nuclear enlargement, and prominent nucleoli (D, E).
High grade squamous intraepithelial lesion / usual vulvar intraepithelial neoplasia can feature verruciform growth, often due to superimposed lichen simplex chronicus. Maturation in this setting is abrupt, and the lower epithelial layers still show an immature, basaloid and highly atypical appearance (A, B, higher magnification in insets). Vulvar condyloma, caused by low-risk HPV types, is characteristically verrucoid with bulbous papillary projections (C, D). Acanthosis and parakeratosis are usually not prominent, the granular cell layer is retained and there is absence of cytoplasmic pallor. In addition, viral koilocytic change can be appreciated (C, D insets).
The distinction between HPVi(p53wt) vaVIN and conventional invasive squamous cell carcinoma is usually straightforward as the latter features cytologic atypia and infiltrative invasion into the underlying stroma (Fig. 6). Verrucous carcinoma, an HPV-independent tumor, is an exception as it lacks nuclear atypia and features blunt, non-destructive invasive growth into the vulvar stroma15,16,17 (Fig. 6). Unlike conventional HPV-independent squamous cell carcinoma, verrucous carcinoma has a relatively indolent behavior with potential for local recurrence but no risk of nodal spread and distant metastases11. For this reason, it is conceivable that verrucous carcinoma represents in many instances just a florid version of HPVi(p53wt) vaVIN. Attention to the interface with the underlying vulvar stroma is important; HPVi(p53wt) vaVIN will have a smooth or only slightly convoluted demarcation at this level, whereas verrucous carcinoma features endophytic growth beyond the normal dermo-epidermal junction plane in the form of epithelial nests that are smoothly contoured, discontinuous from the epidermal surface component, and usually densely packed (puzzle-like). Appreciation of these features requires a well-oriented section; their presence in biopsy or poorly oriented tissue may represent tangential sectioning of the surface epithelium, and thus should be treated with caution. A clear distinction between HPVi(p53wt) vaVIN (an in-situ process) and verrucous carcinoma (by definition, invasive) cannot always be made with certainty. Designation as “at least HPVi(p53wt) vaVIN, cannot exclude verrucous carcinoma” may be prudent in these instances, providing a differential and recommending conservative complete surgical removal of the lesion.
Verrucous carcinoma overlaps morphologically with HPVi(p53wt) vaVIN. Its diagnosis requires endophytic “pushing” invasion into the underlying stroma in the form of packed nests of squamous epithelium, each with smooth convex borders and a bland squamous population (A, B). Conventional squamous cell carcinoma can also arise from HPVi(p53wt) vaVIN (C); notice the irregularity of the invasive nests (C, left aspect; higher magnification in inset) which facilitates the diagnosis. On occasion, HPVi(p53wt) vaVIN can have superimposed HPVi(p53mutant) dVIN changes, presumably via acquisition of TP53 alterations (D).
HPVi(p53wt) vaVIN can also be confused with vulvar exophytic condyloma, a lesion caused by low-risk HPV infection (typically types 6 and 11) (Fig. 5). Clinically, both lesions have warty or verrucoid appearance. Histologically, condyloma shows florid exophytic growth in the form of bulbous, convex projections of variable sizes. It usually has minimal to no growth into the underlying stroma. The epithelium is acanthotic and can show parakeratosis as well as a variable granular cell layer. Viral cytopathic effect is present but can be attenuated. The vanishingly rare term “giant condyloma of Buschke-Lowenstein” has been used historically to describe large tumors with condylomatous growth, blunt invasion into the underlying stroma and significant distortion of the local anatomy. They are also known to recur, and therefore are considered by many as a rare variant of squamous cell carcinoma. They are associated with low-risk HPV types20,21. However, we believe that older literature may have applied this term to lesions now understood as HPVi(p53wt) vaVIN, verrucous carcinomas and HPV-associated squamous cell carcinomas with warty growth, and these entities should thus be considered first, using modern criteria. We thus reserve the diagnosis of giant condyloma of Buschke-Lowenstein for lesions architecturally and cytologically compatible with a condyloma but that are large, distorting and feature blunt growth into the vulvar stroma. We also only make this diagnosis definitively with confirmatory HPV genotyping or in situ hybridization. Admittedly, the distinction between this tumor and verrucous carcinoma may not be possible if there is no access to low-risk HPV testing; in such cases, a differential should be provided.
HPVi(p53wt) vaVIN also requires distinction from a variety of non-neoplastic lesions. Lichen simplex chronicus (LSC) is a histologic pattern secondary to chronic irritation of various causes. Florid examples of this process can overlap with HPVi(p53wt) vaVIN morphologically. Indeed, in the seminal publication of VAAD, Nascimento et al. mentioned LSC with verruciform architecture (identified by the presence of acanthosis, a prominent granular cell layer, and thick hyperkeratosis) within the periphery of verrucous carcinoma. The main distinguishing feature between VAAD and verruciform lichen simplex chronicus (vLSC) was the presence of hypergranulosis in the latter8. To this end, any lesion with morphologic features of LSC (hyperkeratosis, hypergranulosis, lichenoid dermal inflammatory infiltrates) but with exaggerated acanthosis and/or verruciform architecture should be strongly suspected to represent HPVi(p53wt) vaVIN. Presentation during the 7th decade of age or older as a clinically discrete lesion should also raise the possibility of HPVi(p53wt) vaVIN. As mentioned previously, the so-called vLSC can harbor alterations in PIK3CA11 and has a documented association with subsequent invasive squamous cell carcinoma15, and therefore should be regarded as HPVi(p53wt) vaVIN. The distinction between conventional LSC and HPVi(p53wt) vaVIN with hypergranulosis can be difficult, but appears to be possible once strict criteria for the diagnosis of HPVi(p53wt) vaVIN are established14.
Lichen sclerosus is an important inflammatory dermatosis with predilection for genital sites and is a known predisposing factor for HPV-independent vulvar squamous cell carcinoma. Remarkably, lichen sclerosus is documented as a preceding diagnosis in 15–33% of patients with VAAD/DEVIL in published series8,12,15. Conventional forms of lichen sclerosus show lichenoid inflammation with interface vacuolar change, as well as the characteristic homogenization of the dermal stroma with edema and/or collagen accumulation and flattening of the epithelium, making the diagnosis straightforward (Fig. 7). On occasion the epithelium can be acanthotic, and Weyer proposed the term hypertrophic lichen sclerosus in instances in which epithelial hyperplasia, individual necrotic keratinocytes, diminished granular layer and parakeratosis are observed22,23. Interestingly, Walton et al. noted the similarities between this definition of hypertrophic lichen sclerosus and VAAD24. Weyer initially portrayed it as a lesion without significant risk of malignancy. However, a recent series (in abstract form) of 36 consecutive vulvar squamous cell carcinomas by Campbell et al. showed changes consistent with hypertrophic lichen sclerosus in 10 (28%) of cases, most of these with wild-type p53 staining25. This evidence is scant. However, it suggests that, like vLSC, hypertrophic lichen sclerosus has not only morphologic overlap with HPVi(p53wt) vaVIN but also an association with vulvar squamous neoplasia, indicating a precursor role. These postulates require confirmation in future studies. From a practical perspective, the presence of acanthosis, epithelial pallor and other features of VAAD in a sample also showing changes of lichen sclerosus should be treated with caution, and the diagnosis of HPVi(p53wt) vaVIN should be considered in this setting.
Lichen sclerosus with superimposed acanthosis and hyperkeratosis can mimic HPVi(p53wt) vaVIN clinically and pathologically. However, the verruciform growth is not prominent, and the altered maturation characteristics of HPVi(p53wt) vaVIN are not observed (A). Confirmatory features of lichen sclerosus can be found in the superficial dermis (B). Like HPVi(p53wt) vaVIN, psoriasis features verruciform acanthosis and hypogranulosis. However, the acanthosis is uniform and associated with thinning of the supra-papillary epithelium (C). Cytoplasmic pallor is usually not observed (D).
Psoriasis is an important differential as it typically features verruciform acanthosis and hypogranulosis. Psoriasis only rarely involves the vulva, representing 1–3% of all vulvar dermatoses26,27. It is also generally an affliction of younger women, with an average age at presentation of 30 years28, although about 20% are 65 years-old or older29. Other anatomic sites are often also involved28; thus, correlation with the clinical history is critical. Histologically, the psoriasiform hyperplasia is associated with thinning of the skin above the dermal papillae (Fig. 7) and with neutrophilic infiltrates in the dermis and epidermis (forming characteristic micro-abscesses in corneal and spinous layers), features not seen in HPVi(p53wt) vaVIN.
Verruciform xanthoma, a rare lesion in the vulva, also has significant morphologic overlap given its typical low power acanthotic appearance. Unlike HPVi(p53wt) vaVIN, verruciform xanthoma typically presents in young, pre-menopausal women as a small (<2 cm) slow-growing mass30. The striking verruciform acanthosis is associated with collections of foamy histiocytes in the superficial dermis, often more prominent in the dermal papillae31. This finding, which can be obvious on H&E material or require histiocytic markers (PU1, CD68), confirms the diagnosis32.
Some common follicular-based diseases (i.e., hidradenitis suppurativa) have anecdotally demonstrated overlap with HPVi(p53wt) vaVIN. In particular, a recent case seen in consultation by one of the authors (JW) demonstrated classic features of folliculitis on resection, but the preceding small biopsy—which depicted only the epidermis within a follicle—had been interpreted as suspicious for VAAD given marked acanthosis, hyperkeratosis, and perceived premature keratinization in the form of keratohyaline granules. While an inflammatory disease such as folliculitis / perifolliculitis should rarely cause confusion with HPVi(p53wt) vaVIN, this does highlight the caution needed in diagnosing these entities in very limited samples.
Lastly, certain rare forms of epidermal hyperplasia lacking epithelial atypia should be considered. Keratoacanthoma, although extremely rare in the vulva33,34,35, deserves consideration in the diagnostic work-up of HPVi(p53wt) vaVIN. Keratoacanthoma can develop rapidly over time and undergo spontaneous regression. Morphologically, the lesion is acanthotic and raised, thus mimicking HPVi(p53wt) vaVIN. However, keratoacanthoma has a classic cup-shaped appearance with a depressed center occupied by a dense keratin plug. Pseudoepitheliomatous hyperplasia is also by definition acanthotic and presents clinically as a raised lesion. It typically displays a convoluted stromal interface with irregular elongation of the rete ridges. Importantly, an underlying inflammatory or infectious condition is often present, such as granular cell tumor, lichen sclerosus, Herpes Simplex Virus and granuloma annulare36,37,38,39. In both keratoacanthoma and pseudoepitheliomatous hyperplasia, there should be absence of well-developed verruciform architecture, plaque-like parakeratosis, hypogranulosis and cytoplasmic pallor. Complete excision may be necessary to appreciate the features classic of these entities, although if suspected the diagnosis of keratoacanthoma or pseudoepitheliomatous hyperplasia can be raised on biopsy material, allowing for a conservative initial approach.
A modern classification scheme for intraepithelial squamous neoplasia of the vulva is presented in Table 2, based on the evidence and rationale discussed above. A schematic representation of the terminology historically used for HPV-independent, p53-wild-type vulvar squamous intraepithelial lesions is presented in Fig. 8, emphasizing the unifying aspect of the HPVi(p53wt) vaVIN nomenclature.
HPVi(p53wt) vaVIN, as defined, includes lesions formerly described as differentiated exophytic vulvar intraepithelial lesion (DEVIL). DEVIL, in turn, was characterized on cohorts formerly classified as vulvar acanthosis with altered differentiation (VAAD) and verruciform lichen simplex chronicus (vLSC). It also includes other lesions with verruciform atypia, which along with VAAD and vLSC harbor recurrent PIK3CA and HRAS mutations. All these lesions have a risk for concurrent or subsequent invasive squamous cell carcinoma. The so-called hypertrophic lichen sclerosus has overlapping features with HPVi(p53wt) vaVIN, but it remains to be documented whether it represents a distinct precursor or belongs to the HPVi(p53wt) vaVIN spectrum. The term vulvar aberrant maturation (VAM) is recommended by the International Society of the Study of Vulvovaginal Diseases. This term could be considered, but only when faced with a lesion that does not fit in the clinical and pathologic definition of HPVi(p53wt) vaVIN and after exclusion of HSIL/uVIN and HPVi(p53mutant) dVIN, with an explanatory note. Lastly, this category may include lesions that are not acanthotic/verruciform, which requires further study.
Management of HPVi(p53wt) vaVIN
The rarity and scarce literature on the lesions falling within the morphologic spectrum of HPVi(p53wt) vaVIN precludes evidence-based recommendations on the management of this disease. Based on our experience, as well as the close temporal and spatial association between HPVi(p53wt) vaVIN and verrucous carcinoma8,12 and the 37% rate of progression towards invasive squamous cell carcinoma15, it is prudent to consider excision of the lesion with negative margins, or alternatively close surveillance with follow-up biopsies if surgical management is not feasible.
We recommend replacing previous terminology (VAAD, DEVIL, others) with HPVi(p53wt) vaVIN in both biopsy and excision material. In both specimen types, but most importantly on biopsy, the diagnosis of HPVi(p53wt) vaVIN should be made only after carefully considering the differential diagnoses described above and in light of the clinical context. Patient age in the 7–9th decades and presentation as a discrete raised mass are the most helpful clinical clues to the diagnosis of HPVi(p53wt) vaVIN. If definitive classification is not possible, sign out as “atypical verruciform lesion “ is recommended, followed by a comment discussing HPVi(p53wt) vaVIN plus all other pertinent differentials and suggesting continued monitoring and consideration for excisional sampling.
Since both recurrence rate of HPVi(p53wt) vaVIN (44%) and rate of subsequent invasive carcinoma (37%) are significant15, follow-up is indicated after complete surgical removal. It is unclear whether margin status or other parameters impact recurrence or malignant progression; in the series by Roy et al, most cases were diagnosed on biopsy only (67%)15.
In summary, the current classification of vulvar squamous lesions is based on the presence of HPV infection and TP53 mutational status. The group of precursors that lead to HPV-independent, p53-wild-type squamous cell carcinoma is still largely unexplored. Nonetheless, a subset of lesions with verruciform acanthosis and altered squamous maturation has been characterized over the years using diverse nomenclature such as VAAD, vLSC, DEVIL, and VAM. Currently, we know that these lesions are associated with invasive squamous cell carcinoma and verrucous carcinoma of the vulva, and that they harbor recurrent alterations in oncogenes like PIK3CA, HRAS, and NOTCH1. Moreover, reproducibility in their distinction from other squamous vulvar precursors and mimickers with acanthosis or verruciform growth can be achieved. For these reasons, and to align with the WHO classification, we propose the unifying term of HPVi(p53wt) vaVIN. This diagnosis requires strict clinical, morhologic and immunohistochemical criteria. The morphology-only equivalent vaVIN should be reserved to instances where p16 and p53 cannot be performed. Confirmation of the documented observations on this disease thus far is still needed in larger series, to which end the use of a single diagnostic name will likely aid in case identification and surveillance. Equally needed is the study of lesions with apparent (but still not fully documented) overlap with HPVi(p53wt) vaVIN such as hypertrophic lichen sclerosus and atypical verruciform lesions that defy classification. Lastly, it is imperative to explore the existence of other HPV-independent, p53-wild-type lesions beyond the acanthotic/verruciform (vaVIN) spectrum.
References
International Agency for Research on Cancer. World Health Organization Classification of Tumours—Female Genital Tumours. 5th edition. IARC: Lyon, 2020.
Heller DS, Day T, Allbritton JI, Scurry J, Radici G, Welch K, et al. Diagnostic criteria for differentiated vulvar intraepithelial neoplasia and vulvar aberrant maturation. J. Low Genit Tract Dis 25, 57–70 (2021).
Movahedi-Lankarani S, Krishnamurti U, Bell D, Birdsong G, Biscotti C, Chapman Jr C, et al. Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Vulva. Version 4.1.0.1. 2020. https://documents.cap.org/protocols/cp-femalereproductive-vulva-20-4101.pdf (accessed 9 Jun 2021).
Singh N, Gilks CB. Vulval squamous cell carcinoma and its precursors. Histopathology 76, 128–138 (2020).
Voss FO, Thuijs NB, Vermeulen RFM, Wilthagen EA, van Beurden M, Bleeker MCG. The vulvar cancer risk in differentiated vulvar intraepithelial neoplasia: a systematic review. Cancers 13, 6170 (2021).
Leibowitch M, Neill S, Pelisse M, Moyal-Baracco M. The epithelial changes associated with squamous cell carcinoma of the vulva: a review of the clinical, histological and viral findings in 78 women. Br J Obstet Gynaecol 97, 1135–1139 (1990).
van de Nieuwenhof HP, Bulten J, Hollema H, Dommerholt RG, Massuger LFAG, van der Zee AGJ, et al. Differentiated vulvar intraepithelial neoplasia is often found in lesions, previously diagnosed as lichen sclerosus, which have progressed to vulvar squamous cell carcinoma. Mod Pathol 24, 297–305 (2011).
Nascimento AF, Granter SR, Cviko A., Yuan L, Hecht JL, Crum CP. Vulvar acanthosis with altered differentiation: a precursor to verrucous carcinoma? Am J Surg Pathol 28, 638–643 (2004).
Nooij LS, Ter Haar NT, Ruano D, Rakislova N, van Wezel T, Smit VTHBM, et al. Genomic characterization of vulvar (pre)cancers identifies distinct molecular subtypes with prognostic significance. Clin Cancer Res 23, 6781–6789 (2017).
Salama AM, Momeni-Boroujeni A, Vanderbilt C, Ladanyi M, Soslow R. Molecular landscape of vulvovaginal squamous cell carcinoma: new insights into molecular mechanisms of HPV-associated and HPV-independent squamous cell carcinoma. Mod Pathol 35, 274–282 (2022).
Watkins JC, Howitt BE, Horowitz NS, Ritterhouse LL, Dong F, MacConaill LE, et al. Differentiated exophytic vulvar intraepithelial lesions are genetically distinct from keratinizing squamous cell carcinomas and contain mutations in PIK3CA. Mod Pathol 30, 448–458 (2017).
Akbari A, Pinto A, Amemiya Y, Seth A, Mirkovic J, Parra-Herran C. Differentiated exophytic vulvar intraepithelial lesion: clinicopathologic and molecular analysis documenting its relationship with verrucous carcinoma of the vulva. Mod. Pathol. 33, 2011–2018 (2020).
Tessier-Cloutier B, Pors J, Thompson E, Ho J, Prentice L, McConechy M, et al. Molecular characterization of invasive and in situ squamous neoplasia of the vulva and implications for morphologic diagnosis and outcome. Mod Pathol 34, 508-518 (2021).
Neville G, Chapel DB, Crum CP, Song SJ, Yoon J-Y, Lee KR, et al. Interobserver reproducibility of differentiated exophytic vulvar intraepithelial lesion (DEVIL) and the distinction from its mimics. Histopathology 79, 957–965 (2021).
Roy SF, Wong J, Le Page C, Tran-Thanh D, Barkati M, Pina A, et al. DEVIL, VAAD and vLSC constitute a spectrum of HPV-independent, p53-independent intraepithelial neoplasia of the vulva. Histopathology 79, 975–988 (2021).
Rakislova N, Alemany L, Clavero O, Del Pino M, Saco A, Marimon L, et al. HPV-independent precursors mimicking high-grade squamous intraepithelial lesions (HSIL) of the vulva. Am J Surg Pathol 44, 1506–1514 (2020).
Rakislova N, Alemany L, Clavero O, Del Pino M, Saco A, Quirós B, et al. Differentiated vulvar intraepithelial neoplasia-like and lichen sclerosus-like lesions in HPV-associated squamous cell carcinomas of the vulva. Am J Surg Pathol 42, 828–835 (2018).
Kortekaas KE, Bastiaannet E, van Doorn HC, de Vos van Steenwijk PJ, Ewing-Graham PC, Creutzberg CL, et al. Vulvar cancer subclassification by HPV and p53 status results in three clinically distinct subtypes. Gynecol Oncol 159, 649–656 (2020).
Watkins JC, Yang E, Crum CP, Herfs M, Gheit T, Tommasino M, et al. Classic vulvar intraepithelial neoplasia with superimposed lichen simplex chronicus: a unique variant mimicking differentiated vulvar intraepithelial neoplasia. Int J Gynecol Pathol 38, 175–182 (2019).
Dietl J, Fierlbeck G [Giant condyloma (Buschke-Löwenstein) of the vulva]. Geburtshilfe Frauenheilkd 50, 819–821 (1990).
Ergün SS, Kural YB, Büyükbabani N, Verim L, Akbulut H, Gürkan L. Giant condyloma acuminatum. Dermatol Surg 29, 300–303 (2003).
Weyers W. Hypertrophic lichen sclerosus with dyskeratosis and parakeratosis-a common presentation of vulvar lichen sclerosus not associated with a significant risk of malignancy. Am J Dermatopathol 35, 713–721 (2013).
Weyers W. Hypertrophic lichen sclerosus sine sclerosis: clues to histopathologic diagnosis when presenting as psoriasiform lichenoid dermatitis. J Cutan Pathol 42, 118–129 (2015).
Walton DB, Stearns L, Fillman EP, Banks N, Dalton S.R. Vulvar acanthosis with altered differentiation: is this entity a variant of hypertrophic lichen sclerosus? J Cutan Pathol 42, 1038–1042 (2015).
Campbell K, Shalin S, Quick C. Hypertrophic lichen sclerosus: a distinct precursor to squamous cell carcinoma in the vulva. Mod Pathol 33, 1022–1023 (2020).
Chan MP, Zimarowski MJ. Vulvar dermatoses: a histopathologic review and classification of 183 cases. J Cutan Pathol 42, 510–518 (2015).
Joehlin-Price AS, Mully TW. Review of 189 consecutive female genital skin and mucosal biopsies submitted to an academic dermatopathology practice. Am J Clin Pathol 155, 418–427 (2021).
Kapila S, Bradford J, Fischer G. Vulvar psoriasis in adults and children: a clinical audit of 194 cases and review of the literature. J Low Genit Tract Dis 16, 364–371 (2012).
da Silva N, Augustin M, Langenbruch A, Mrowietz U, Reich K, Thaçi D, et al. Disease burden and treatment needs of patients with psoriasis in sexually-sensitive and visible body areas: results from a large-scale survey in routine care. Eur J Dermatol 30, 267–278 (2020).
Fite C, Plantier F, Dupin N, Avril M-F, Moyal-Barracco M. Vulvar verruciform xanthoma: ten cases associated with lichen sclerosus, lichen planus, or other conditions. Arch Dermatol 147, 1087–1092 (2011).
Santa Cruz DJ, Martin SA. Verruciform xanthoma of the vulva. Report of two cases. Am J Clin Pathol 71, 224–228 (1979).
Muirhead D, Stone MS, Syrbu SI. The utility of PU.1 as an immunohistochemical marker for histiocytic and dendritic lesions of the skin. Am J Dermatopathol 31, 432–435 (2009).
Rhatigan RM, Nuss RC. Keratoacanthoma of the vulva. Gynecol Oncol 21, 118–123 (1985).
Chen W, Koenig C. Vulvar keratoacanthoma: a report of two cases. Int J Gynecol Pathol 23, 284–286 (2004).
Gilbey S, Moore, DH, Look KY, Sutton GP. Vulvar keratoacanthoma. Obstet Gynecol 89, 848–850 (1997).
Lee ES, Allen D, Scurry J. Pseudoepitheliomatous hyperplasia in lichen sclerosus of the vulva. Int J Gynecol Pathol 22, 57–62 (2003).
Frimer M, Chudnoff S, Hebert T, Shahabi S. Pseudoepitheliomatous hyperplasia mimicking vulvar cancer in a patient with AIDS. J Low Genit Tract Dis 15, 66–68 (2011).
Tangjitgamol S, Loharamtaweethong K, Thawaramara T, Chanpanitkitchot S. Vulvar pseudoepitheliomatous hyperplasia associated with herpes simplex virus type II mimicking cancer in an immunocompromised patient. J Obstet Gynaecol Res 40, 255–258 (2014).
Vera-Sirera B, Zabala P, Aviño-Mira C, Vera-Sempere FJ. Multiple granular cell tumors with metachronous occurrence in tongue and vulva. Clinicopathological and immunohistochemical study. J Oral Maxillofac Pathol 18, 437–441 (2014).
Acknowledgements
The authors thank Dr. Christopher P Crum (Brigham and Women’s Hospital) for his insightful comments on the preliminary versions of this manuscript and, most importantly, for pioneering the work that has led to our current understanding of HPVi(p53wt) vaVIN.
Funding
There is no external funding to disclose.
Author information
Authors and Affiliations
Contributions
CPH, MN and JW participated in the conception and initial writing of the review article. CPH led the literature review including reference citations, and prepared figures and tables. NS, NR, BH, LH, TB, and BG participated in the discussion, editing and final consensus on the contents of the review article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Parra-Herran, C., Nucci, M.R., Singh, N. et al. HPV-independent, p53-wild-type vulvar intraepithelial neoplasia: a review of nomenclature and the journey to characterize verruciform and acanthotic precursor lesions of the vulva. Mod Pathol 35, 1317–1326 (2022). https://doi.org/10.1038/s41379-022-01079-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-022-01079-7
This article is cited by
-
Molekulare Klassifikation des Vulvakarzinoms
Die Onkologie (2024)
-
Comment on HPV-independent, p53-wild-type vulvar intraepithelial neoplasia: a review of nomenclature and the journey to characterize acanthotic precursor lesions of the vulva. Parra-Herran C. et al Mod Pathol 2022 Apr 18 doi: 10.1038/s41379-022-01079-7
Modern Pathology (2022)