Abstract
Synovial hyperplasia, a profound alteration in the structure of synovial tissue, is the basis for cumulative joint destruction in rheumatoid arthritis (RA). It is generally accepted that controlling synovial hyperplasia can delay the progression of RA. As one of the most intensively studied isoforms of acid-sensing ion channels (ASICs), ASIC1a contributes to various physiopathologic conditions, including RA, due to its unique property of being permeable to Ca2+. However, the role and the regulatory mechanisms of ASIC1a in synovial hyperplasia are poorly understood. Here, rats induced with adjuvant arthritis (AA) and human primary synovial fibroblasts were used in vivo and in vitro to investigate the role of ASIC1a in the proliferation of RA synovial fibroblasts (RASFs). The results show that the expression of ASIC1a was significantly increased in synovial tissues and RASFs obtained from patients with RA as well as in the synovium of rats with AA. Moreover, extracellular acidification improved the ability of RASFs colony formation and increased the expression of proliferation cell nuclear antigen (PCNA) and Ki67, which was abrogated by the specific ASIC1a inhibitor psalmotoxin-1 (PcTX-1) or ASIC1a-short hairpin RNA (ASIC1a-shRNA), suggesting that extracellular acidification promotes the proliferation of RASFs by activating ASIC1a. In addition, the activation of c-Raf and extracellular signal-regulated protein kinases (ERKs) signaling was blocked with PcTX-1 or ASIC1a-shRNA and the proliferation of RASFs was further inhibited by the ERK inhibitor (U0126), indicating that ERK/MAPK signaling contributes to the proliferation process of RASFs promoted by the activation of ASIC1a. These findings gave us an insight into the role of ASIC1a in the proliferation of RASFs, which may provide solid foundation for ASIC1a as a potential target in the treatment of RA.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a common chronic autoimmune disease characterized by a marked synovial hyperplasia due to neovascularization, fibroblast proliferation, and the recruitment of inflammatory cells, which can severely affect the quality of life of patients through progressive destruction of cartilage and bone tissue [1]. In RA, a large number of inflammatory cells infiltrate the joint synovium. The synovial lining is normally 1–2 cell layers, but can increase to up to 15 layers in RA [2, 3]. During the pathological progression of RA, synovial fibroblasts can be activated in inflammatory environment and display a hyperplastic property and some tumor-like biological behavior such as proliferation, migration, invasion, and other aggressive phenotypes [4,5,6]. Therefore, new therapeutic targets must be identified to improve the management of symptoms of RA.
Many studies have shown that local extracellular acidification of synovial tissue infected with RA is similar to the microenvironment in tumor tissue, accompanied by pH reduction and hypoxia [7,8,9]. As a prominent feature of synoviocytes, the drop in extracellular pH as a consequence of lactic acid accumulation has been thought to stimulate synoviocyte proliferation and facilitate inflammatory process [10,11,12]. It is noteworthy that acidification of the synovial fluid may cause a drop in pH from 7.4 to <6.0 [13]. At the cellular level, a number of membrane channels or receptors can respond to extracellular protons, including acid-sensing ion channels (ASICs) [14, 15]. Previously, we demonstrated the function expression of ASIC 1a (ASIC1a) in primary articular chondrocytes obtained from adjuvant arthritis (AA) rat model and primary RA synovial fibroblasts (RASFs) from patients with RA, suggesting that ASIC1a may play a key regulatory role in the pathogenesis of RA [16, 17, 20].
As trimeric cation-selective and members of the epithelial sodium channel/degenerin superfamily of ion channels, ASICs are rapidly activated by protons and then contribute to various pathophysiological conditions because of their ability to mediate Ca2+ and Na+ influx [18, 19]. Seven ASIC subunit proteins (ASIC1a, ASIC1b, ASIC1b2, ASIC2a, ASIC2b, ASIC3, ASIC4), encoded by four genes (ACCN1, ACCN2, ACCN3, ACCN4), have been identified [20, 21]. The ASICs are widely distributed in mammalian central and peripheral nervous systems, in which they play a crucial role in pain, RA, neurological disease, tumor, and psychiatric diseases [22, 23]. Of the ASICs subunits, only ASIC1a has been found be permeable to calcium and has become a topic of interest in study for its powerful biological functions and pathological significance [24]. It is particularly striking that overexpression of ASIC1a (OE-ASIC1a) can activate several pathways related to cell proliferation by inducing Ca2+ overloading [25, 26]. Therefore, we explored whether ASIC1a is involved in the proliferation of RASFs and its potential molecular mechanisms.
Extracellular signal-regulated protein kinase (ERK) cascades are essential pathways that are responsible for basic cellular processes, including cell growth and differentiation [27]. Among the most extensively studied groups of mitogen-activated protein kinases (MAPKs), the mammalian ERK1 and ERK2, known to be relevant to protein serine/threonine kinases, have been implicated as a pivotal regulator of cell proliferation [28, 29]. The c-Raf kinases regulate cell growth and division by signal transduction via the ERK cascade [30].
Therefore, this study aimed to investigate the role of ASIC1a in the regulation of RASFs proliferation and explore the effect of ERK/MAPK signal on this phenomenon.
Materials and methods
Reagents and antibodies
DMEM/high glucose medium, phosphate-buffered saline (PBS), and other cell culture products were purchased from Hyclone (MA, USA). Fetal bovine serum was obtained from BIO (Thermo Fisher Scientific, Australia). Mouse anti-β-actin antibodies were purchased from ZSGB (Beijing, China). Rabbit anti-ASIC1a antibodies were purchased from Bioss (Beijing, China). Rabbit anti-ERK1/2 and anti-p-ERK1/2 were purchased from Cell Signaling Technology (CST, USA). The specific inhibitor of ASIC1a, psalmotoxin-1 (PcTX-1), was obtained from Abcam (Cambridge, UK). U0126-EtOH was obtained from MedChemExpress (USA).
Collection of clinical synovial samples
This research was approved by the Human Research Ethics Committee of Anhui Medical University. All fresh synovial tissues (25 RA and 3 healthy) were provided by patients who underwent knee replacement surgery or knee synovial debridement in the First Affiliated Hospital of Anhui Medical University between 2018 and 2020.
Isolation and culture of synovial fibroblasts
Fresh synovial tissues were cut into small pieces (about 1 mm3) under sterile environment in the 20% DMEM medium and transferred to the cell culture flask using a Pasteur pipette. Subsequently, the tissue pieces were placed into the flask. The cells were then cultured in DMEM/high glucose medium. The cell culture flask was placed upright and incubated for 6 h at 37 °C in a 5% CO2 atmosphere and then flattened until the cell form the colony. The third to eighth passages of identified cells were used in subsequent experiments.
Identification of cultured cells and detection of protein expression
Flow cytometry was used to detect the expression of related protein and identify type B synovial fibroblasts. In brief, following digested and suspended into DMEM/high glucose medium, cells were fixed with 4% paraformaldehyde for 20 min at room temperature. Subsequently, cells were blocked for 30 min and then incubated with the anti-CD55 (1:200, Santa Cruz, USA), anti-ASIC1a (1:200, Proteintech, Wuhan, China), anti-PCNA (1:200, Bioss, Beijing, China), and anti-Ki67 (1:200, Bioss, Beijing, China) overnight at 4 °C. Each step was followed by extensive washing with PBS, and labeling was performed using 500 μl fluorescent secondary antibody (FITC-labeled goat anti-rabbit IgG, Zsbio) for 1 h in the dark. Lastly, the cells resuspended with PBS were detected by a flow cytometer (Beckman Coulter, USA) and the images were analyzed by the CytExpert software (Beckman Coulter, USA) to determine the fluorescence intensity.
Lentivirus packaging and cell transfection
Silencing or OE-ASIC1a (ASIC1a-short hairpin RNA (ASIC1a-shRNA) or OE-ASIC1a) was performed in a six-well plate. According to the manufacturer’s instructions, RASFs were transfected with ASIC1a-shRNA or ASIC1a gene lentivirus particles (Genechem, China) in 1 ml complete medium and other wells were transfected with control shRNA or empty plasmid lentivirus particles. After 10 h, the medium in each well was replaced with 1 ml of complete medium (without Polybrene), and 3 mg/ml puromycin dihydrochloride was used to screen stable expressing shRNA or the gene.
Western blotting
Total protein was isolated from RASFs using RIPA lysis buffer containing 1% protease inhibitor cocktail (Beyotime, Shanghai, China). The protein concentration was quantified with a BCA Protein Assay Kit (Beyotime, Shanghai, China). Equal amounts of protein samples were separated by 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore, USA), and then blocked with 5% skim milk in TBST (10 mM Tris, 150 mM Nacl, 0.05% Tween 20 [pH 8.3]) at room temperature for 2 h and then incubated overnight at 4 °C with the specific primary antibody, mouse anti-β-actin antibodies (1:1000, ZSGB, China), rabbit anti-ASIC1a antibodies (1:1000, Affinity, USA), rabbit anti-ERK1/2 (1:1000, CST, USA), or rabbit anti-p-ERK1/2 (1:2000, CST, USA). The membranes were washed three times with TBST for 15 min on each occasion and then incubated with anti-rabbit or anti-mouse antibodies conjugated with horseradish peroxidase (HRP) (1:5000, ZSGB, China) for 1 h at room temperature. Finally, ECL-chemiluminescence kit (Thermo Fisher Scientific) was used to expose the membranes and autoradiographs were developed by ImageJ software.
Immunofluorescence assay
The cultured RASFs were fixed with 4% paraformaldehyde for 20 min at room temperature. Following cell membrane permeabilization with 0.1% Triton X-100 for 10 min, cells were then blocked with 5% bovine serum albumin in PBS (Sigma) for 1 h. Subsequently, the treated cells were incubated with rabbit monoclonal anti-ASIC1a (Bioss, Beijing, China), rabbit monoclonal anti-Ki67 (Bioss, Beijing, China), and rabbit monoclonal anti-PCNA (Bioss, Beijing, China) overnight at 4 °C. After each step, extensive washing with PBS occurred, and subsequent staining was performed with the related FITC or AF488 fluorescent-labeled secondary antibody for 1 h in the dark. Lastly, the samples were counterstained with 1 µg/ml of 4′,6-diamidino-2-phenylindole or 5 min and images were captured using a confocal microscope (Zeiss, Germany).
Quantitative real-time polymerase chain reaction
Total RNA was extracted from cultured cells by TRIzol reagent (Invitrogen, USA), and then purified mRNA was reverse transcribed into complementary DNA (cDNA) using cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Quantitative real-time PCR analysis of ASIC1a and β-actin mRNA was performed with PIKO REAL RT-PCR kit (Thermo Fisher Scientific, Waltham, MA, USA). The primer sequences we used were shown as following:
ASIC1a, 5′-CGGCTGAAGACCATGAAAGG-3′ (forward);
ASIC1a, 5′-AAGGATGTCTCGTCGGTCTC-3′ (reverse);
β-actin, 5′-GCCAACACAGTGCTGTCTGG-3′ (forward);
β-actin, 5′-CTCAGGAGGAGCAATGATCTTG-3′ (reverse).
All primers were obtained from Sangon Biotech (Shanghai, China). All experiments were repeated at least three times. Ct values of the samples were calculated against β-actin as an endogenous control and the relative gene transcript levels were analyzed by the 2−ΔΔCT method.
Cell Counting Kit-8 (CCK-8) assay
To analyze cell proliferation, the cells were assayed using the CCK-8 (BestBio, China) according to the manufacturer’s instructions. Briefly, cells were seeded in 96-well plates at a density of 5000 cells per well and cultured for 24 h. After the different groups with pretreatments, the absorbance of each well was detected at 450 nm with a multimode reader (Synergy HTX, Bio TEK).
Colony formation assay
To observe the proliferation ability of RASFs, the cells were subjected to colony formation assay. First, RASFs were seeded in the dish at a density of 2000 cells. After culturing for 72 h, each group was subjected to different treatments separately for 12 h and then replaced with DMEM/high glucose medium supplemented with 20% FBS for culture about 3 weeks. Finally, the colonies were stained with 1% crystal violet and counted by Image J software. All experiments were repeated at least three times.
Animals, AA model induction, and treatment
Male Sprague-Dawley rats, weighing 120–140 g, were purchased from the Experimental Animal Center of Anhui Medical University (Hefei, China). All the animals were acclimated for 1 week and divided into two groups: control rats (n = 8) and rats with AA (n = 40). All the animal experimental procedures were performed in accordance with institutional ethics guidelines for laboratory animal care and use after approved by the University Animal Care and Use Committee.
The AA-induced model was achieved by intradermal injection of rats into the left hind metatarsal footpad (day 0) with 0.1 ml complete Freund’s adjuvant (Sigma, USA) containing 10 mg/ml of heat-inactivated mycobacteria. To investigate the pathological state of synovial proliferation in vivo, rats with AA were randomly divided into the following groups (n = 8 per group): AA model group, PcTX-1 high/medium/low-concentration groups (0.5, 1.0, and 2.0 μg/kg, respectively), and triamcinolone acetonide group (TA, positive drug, 1 mg/kg). On day 12 after immunization, PcTx-1 and TA were administered by intra-articular injection into joint cavities of rats with AA once every 3 days for a total of eight times. The AA model group was untreated.
Immunostaining analysis
The human synovial tissues and rat ankle joints were subjected to immunohistochemical staining (IHC) to detect the expression of related proteins. According to the manufacturer’s instructions, the rat contralateral joints were performed by the protocol in the IHC reagents kit (ZSGB, China) after soaked in ethylenediamine tetraacetic acid (EDTA) decalcifying solution. Briefly, after dried, deparaffinized, and rehydrated, the paraffin sections were heated with 10 mM citrate buffer in a microwave oven for antigen retrieval. For all samples, endogenous peroxidase activity was eliminated using 3% hydroperoxide–methanol solution to remove the background staining. The sections were blocked and incubated with primary rabbit monoclonal anti-ASIC1a antibody (Bioss, Beijing, China), anti-PCNA (Bioss, Beijing, China), and anti-Ki67 (Bioss, Beijing, China) overnight at 4 °C. Next, the sections were then incubated with the secondary antibody labeled with HRP for 10 min and then each sample image was captured by a 3DHISTECH pathology slide scanner. Finally, the positive areas of ASIC1a, proliferation cell nuclear antigen (PCNA) and Ki67 were analyzed by ImageJ software.
Hematoxylin–eosin (HE) staining histological examination
The rat ankle joints were performed according to the protocol in the HE staining kit (Beyotime, China). In brief, the rat contralateral joints were fixed in 4% paraformaldehyde, decalcified in 10% EDTA, and embedded in paraffin. Each section was stained with HE (Beyotime, China) and then scored for change in synovial hyperplasia with scores ranging from 0 to 4 (0 = no changes; 1 = mild; 2 = moderate; 3 = severe; 4 = very severe).
Statistical analysis
All the experiments were repeated three times, and the data are presented as the mean ± S.E.M. All analysis was performed using SPSS17.0 software. Differences were analyzed by Student’s t test between two groups or by one-way analysis of variance followed by a Dunnett’s post hoc test. Statistical significance was set at a P value < 0.05. Power calculations were performed using G*power 3.1 software.
Results
Pathological features and expression of ASIC1a in RA synovium
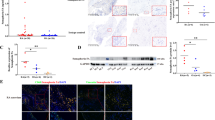
Figure 1A shows the pathophysiological changes of the synovial tissues obtained from patients with RA and healthy participants. HE staining results showed synovial hyperplasia and inflammatory cell infiltration in synovial membranes obtained from patients with RA. Meanwhile, IHC staining showed that the expression of PCNA (Fig. 1B) and Ki67 (Fig. 1C) in the synovial tissues obtained from patients with RA was significantly higher than that from healthy subjects. As shown in Fig. 1D, ASIC1a was highly expressed in synovium obtained from patients with RA. Post hoc power analyses demonstrated that sufficient power was available to distinguish the above significant differences (effect size: 3.007, power: 0.997).
The high expression of ASIC1a in RASFs
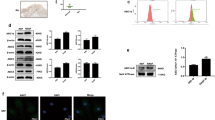
As mentioned in “Material and methods” sections, flow cytometry was performed to assess for synovial fibroblasts isolation. As evaluated by CD55 expression, NSFs and RASFs were successfully isolated and purified (Fig. 2A). As shown in Fig. 2B, C, the protein and mRNA expressions of ASIC1a in RASFs were significantly higher than levels in NSFs. Moreover, immunofluorescence revealed that the protein expression of ASIC1a in RASFs was higher than in NSFs (Fig. 2D).
A Identification of RASFs by flow cytometry. B, C Western blotting and quantitative real-time PCR analysis of ASIC1a expression in NSFs and RASFs. D Immunofluorescence analysis of ASIC1a expression in NSFs and RASFs. All the experiments were repeated three times. Data are presented as the mean ± S.E.M. for the three independent experiments. ***P < 0.001 compared to the normal synovial fibroblast (NSF) group.
Effect of acidification on proliferation of RASFs
To explore the possible roles of acidification on the proliferation of RASFs, cells were pretreated with acid at different time intervals (1.5, 3, 6, 12, and 24 h) and the nonacid stimulation was marked as 0 h or control group. As shown in Fig. 3A, the results of CCK-8 assay demonstrated that the proliferation rate of RASFs treated with acid stimulation was significantly increased compared with the nonacid stimulation. As shown in Fig. 3B, C, flow cytometry demonstrated that proliferation of RASFs was significantly increased after the acidification at 12 and 24 h. In addition, the results of immunofluorescence in Fig. 3D, E showed that the positive staining for the cell proliferation protein markers (PCNA and Ki67) increased at 12 or 24 h compared with the control group. These results indicated that acidification promoted the proliferation of RASFs.
A Cell Counting Kit-8 assay results of the cell proliferation rate of RASFs divided into acid stimulation and nonacid stimulation. B, C Flow cytometry analysis for the expression of PCNA and Ki67 in RASFs with pretreatment of acid (pH 6.0) at 1.5, 3, 6, 12, and 24 h or nonacid stimulation. D, E Immunofluorescence analysis of PCNA and Ki67 expressions in RASFs after pretreatment with acid (pH 6.0) at different time points. All the experiments were repeated three times. Data are presented as the mean ± S.E.M. for the three independent experiments. ***P < 0.001 compared to the 0 h group.
Effect of ASIC1a inhibition on the proliferation of RASFs
As depicted in Fig. 4A, the expression of ASIC1a was decreased in ASIC1a-shRNA. Likewise, Fig. 4B, C shows that the protein and mRNA levels of ASIC1a in ASIC1a-shRNA group were significantly lower as compared to the control shRNA group (negative control), suggesting that the ASIC1a-silencing model was successfully constructed. As shown in Fig. 4D, E, the fluorescence intensity of PCNA and Ki67 decreased by pretreatment with ASIC1a-shRNA in comparison to the control shRNA group. Interestingly, the increase of both PCNA and Ki67 staining could be reversed after pretreatment of the cells with PcTX-1 (an ASC1a specific inhibitor). Figure 4F shows that the ability of cells to form colonies significantly decreased after knocking down the expression of ASIC1a as compared as control shRNA group. Similarly, PcTX-1 distinctly reversed the effect of acidification on colony formation. Based on the above experimental results, it was believed that ASIC1a promoted the acidification-induced proliferation of RASFs.
A Immunofluorescence results of ASIC1a expression in RASFs without transfection, transfected with negative control (shRNA), and ASIC1a-shRNA. B, C Western blotting and qRT-PCR analysis of ASIC1a expression levels in RASFs, control shRNA, and ASIC1a-shRNA group. β-actin served as an endogenous control. The results of gray density were analyzed by ImageJ software and the relative gene transcript levels were analyzed using the 2−ΔΔCT method. D, E Immunofluorescence analysis of PCNA and Ki67 expression in RASFs treated with control, pH 6.0, control shRNA, ASIC1a-shRNA, and PcTx-1 (100 nM) group for 12 h. F The proliferation ability of RASFs in different treatments for 12 h was analyzed using the colony formation assay. All data are presented as the mean ± S.E.M. for the three independent experiments. **P < 0.01, ***P < 0.001 compared to the shRNA or pH 6.0 group.
Effect of ASIC1a on the proliferation of RASFs
As shown in Fig. 5A, the immunofluorescence assay showed that the expression of ASIC1a in OE-ASIC1a group was increased compared with the vector group (negative control). Figure 5B, C depicts that the protein and mRNA expressions of ASIC1a were significantly increased compared with the empty vector group, suggesting the ASIC1a overexpression model was successfully constructed. Moreover, Fig. 5D, E shows that the cell positive staining cells of proliferation marker proteins (PCNA and Ki67) were increased following pretreatment with lentivirus containing ASIC1a gene compared with the empty vector. As shown in Fig. 5F, acidification promoted an increase in the number and size of cell colonies. Furthermore, OE-ASIC1a gene could significantly improve the ability of cells to growth independently.
A Immunofluorescence results of ASIC1a expression in RASFs without transfection, transfected with negative control (shRNA), and ASIC1a-shRNA; the results of gray density were analyzed by ImageJ software. B, C Western blotting and qRT-PCR analysis of ASIC1a expression in RASFs, shRNA, and ASIC1a-shRNA group. β-actin served as an endogenous control. The results of gray density were analyzed using ImageJ software and the relative gene transcript levels were analyzed using the 2−ΔΔCT method. D, E Immunofluorescence analysis of PCNA and Ki67 expression in RASFs divided into control, pH 6.0, vector, and OE-ASIC1a group for 12 h. F The colony formation assay was to analyze the proliferation ability of RASFs with different treatments for 12 h. All data are presented as the mean ± S.E.M. for at three independent experiments. **P < 0.01, ***P < 0.001 compared to the pH 6.0 or the vector group.
Effect of ASIC1a on the proliferation of synovial tissue in vivo
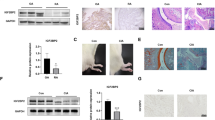
Experimental models of AA have been widely used to explore the pathogenesis of RA disease in preclinical studies. As shown in Fig. 6A, the pathological synovial hyperplasia was alleviated in rats with AA pretreated with the ASIC1a specific inhibitor (PcTX-1) compared to rats with AA that did not receive pretreatment. To be more specific, HE staining demonstrated pathological changes of synovial thickness, inflammatory cell infiltration, and cartilage erosion in rats with AA. And these changes could be reversed or alleviated by PcTX-1 in a dose-dependent manner. And the expression of ASIC1a was significantly increased in synovial membrane of rats with AA compared with control rats (Fig. 6B). Figure 6C, D shows that the expressions of PCNA and Ki67 were significantly decreased in rats with AA that were received PcTX-1 compared to rats with AA. All the above experimental results showed that ASIC1a promoted the proliferation of synovial tissue in vivo.
A Hematoxylin–eosin staining results of AA-induced rat synovium pathological changes after treatment with PcTx-1 (0.5, 1, and 2 μg/kg) and TA (1 mg/kg) (n = 8 per group). B Immunohistochemistry staining of ASIC1a expression in the control and AA-induced rat synovium (n = 8 per group). C, D Immunohistochemistry results of PCNA and Ki67 expression in AA rats synovium after treatment with PcTx-1 (0.5, 1, and 2 μg/kg) and TA (1 mg/kg). PcTx-1 was the specific inhibitor of ASIC1a and triamcinolone acetonide (TA) was a positive drug. All data are presented as the mean ± S.E.M., *P < 0.05, **P < 0.01, ***P < 0.001.
Effect of ASIC1a on the proliferation of RASFs through ERK/MAPK signaling
As shown in Fig. 7A, the protein expression of phosphorylated-c-Raf (p-c-Raf) in the pH 6.0 group was significantly increased compared with the control group, and could be reversed by ASIC1a-shRNA and the specific inhibitor of ASIC1a, PcTX-1. In contrast, ASIC1a overexpression significantly increased the protein levels of p-c-Raf (Fig. 7B). Likewise, acidification promoted the activation of phosphorylated-ERK1/2 (p-ERK1/2), which could be abrogated by ASIC1a-shRNA and the specific inhibitor of ASIC1a, PcTX-1 (Fig. 7C). According to Fig. 7D, OE-ASIC1a could significantly increase the protein level of p-ERK1/2. In Fig. 7E, compared with pH 6.0 group, the protein expression of PCNA was significantly decreased in RASFs with pretreatment of U0126 (the inhibitor of ERK1/2). In addition, the number and size of cell colonies were significantly decreased after inhibiting the activity of ERK with the treatment of U0126 (Fig. 7F). Similarly, as shown in Fig. 7G, CCK-8 assay analysis revealed that the cell proliferation rate was significantly decreased in the presence of U0126. Based on the above experimental results, it was curtained that ASIC1a promoted the proliferation of RASFs via the ERK/MAPK signaling.
A, B Western blotting analysis of p-c-Raf and Raf expressions in RASFs divided into control, pH 6.0, control shRNA, ASIC1a-shRNA, PcTx-1, vector, and OE-ASIC1a groups. C, D Western blotting analysis of p-ERK1/2 and ERK1/2 expressions in RASFs divided into control, pH 6.0, control shRNA, ASIC1a-shRNA, PcTx-1, vector, and OE-ASIC1a groups. E Western blotting analysis of PCNA expression levels in RASFs with pretreatment of acid (pH 6.0) and U0126 (10 μM) for 12 h. β-actin was served as an endogenous control and the results of gray density were analyzed by ImageJ software. F The colony formation assay was used to analyze the proliferation ability of RASFs with acid (pH 6.0) and U0126 (10 μM) for 12 h. G CCK-8 analysis of the cell proliferation rate of RASFs after treatment with acid (pH 6.0) and U0126 (10 μM) for 12 h. All data are presented as the mean ± S.E.M. for the three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
In the present study, we explored the effects of acid-activated ASIC1a on the proliferation of RASFs. The results demonstrate that ASIC1a was highly expressed in synovial tissues obtained from patients with RA and RASFs, which suggested that ASIC1a may be responsible for the pathological changes of RA. Interestingly, the activation of p-ERK1/2 could be reversed by PcTX-1 (the specific inhibitor of ASIC1a) and ASIC1a-shRNA. In addition, cell proliferation promoted by ERK1/2 could be abrogated by U0126 (the inhibitor of ERK). These results indicated that acid-activated ASIC1a promotes the proliferation of RASFs via the ERK signaling.
Recently, it has been demonstrated that hypoxia in the synovial tissues, and the metabolism dominated by anaerobic glycolysis give rise to the accumulation of lactic acid, which accelerates the drop in the pH of the synovial fluid [31, 32]. ASICs are voltage-independent proton-gated cation channels. Of seven ASICs subunits, ASIC1a, which is sensitive to pH value of 6.2 mediating the permeation of both Na+ and Ca2+ ions into the cells, is the most likely ASICs involved in pathophysiological conditions [33, 34]. CD55 protein is present in the synovial lining and has been identified as a surface marker for synovial fibroblasts [35]. We also have proved the coexpression of ASIC1a and CD55, which indicated that ASIC1a was highly expressed in the intimal lining of synovium [36]. The differential expression of ASIC1a between NSFs and RASFs suggested that it highly regulated the cellular process of RASFs. Prior investigators showed that acidification promotes the proliferation of other cells such as adenocarcinoma cell and colon cancer cell lines [37, 38]. Synovial hyperplasia is a prominent pathological feature of RA. It is reported that RASFs are key effector cells in the development of RA and are considered as promising targets for treating RA [39]. Our results showed that ASIC1a significantly increased the expressions of PCNA and Ki67 in the synovial membrane or RASFs. Meanwhile, AA experimental model was established to explore the effect of ASIC1a on synovial pathological changes in vivo. The results of HE and immunohistochemistry showed that synovial hyperplasia were ameliorated in rats with AA that received a PcTX-1 joint injection. Our previous studies have confirmed that PcTX-1 could reverse the effect of extracellular acidification on the expression of ASIC1a and inhibit the activity of ASIC1a [40, 41]. Although PcTX-1 has a significant reversed effect in rats with AA, it does not present a completely block ASIC1a. In the future work, ASIC1a knockout mice will be used to further confirm its mechanism on synovial hyperplasia and invasion as well as its effects on healthy joints.
The ERK/MAPK signaling pathway, which is the most thoroughly studied MAPK pathway, can be activated by elevation of Ca2+ and other signals [42]. In the current study, the phosphorylation of ERK1/2 could promote cells proliferation which was in line with the aforementioned studies. ERK/MAPK signal has been also known to control motility and invasiveness of cellular process. As reported in a study, sonic hedge signaling pathway increased the level of p-ERK1/2 to regulate proliferation and migration of fibroblast-like synoviocytes [43]. In addition, Niu et al. have demonstrated that ASIC1a can promote synovial invasion [44]. It suggests that ASIC1a may also play a role in promoting synovial invasion by activating ERK signal in part. In a recent study, it has been demonstrated that activated ASIC1a can promote the migration and adhesion of osteoclast and is involved in osteoclastogenesis, which are responsible for osteoporotic bone loss [45]. Therefore, whether ASIC1a affect RASFs spread of arthritis to unaffected joints and whether overproliferated RASFs can interact with cells (chondrocytes, osteoclasts, osteoblasts, etc.) to affect bone loss or renewal still need a further study.
In conclusion, the present study demonstrated that ASIC1a may partially promote RASFs proliferation and aggravated synovial hyperplasia via ERK/MAPK signaling. This study suggested that inhibiting the function of ASIC1a could be selected as a potential therapeutic strategy to prevent progressive synovial hyperplasia and provided a new mechanism explanation for ASIC1a served as an adjuvant treatment for RA.
Data availability
The data sets used during the current study are available from the corresponding author on reasonable request.
References
Liu H, Zhu Y, Gao Y, Qi D, Zhao L, Zhao L, et al. NR1D1 modulates synovial inflammation and bone destruction in rheumatoid arthritis. Cell Death Dis. 2020;11:129.
Neumann E, Lefèvre S, Zimmermann B, Gay S, Müller-Ladner U. Rheumatoid arthritis progression mediated by activated synovial fibroblasts. Trends Mol Med. 2010;16:458–68.
Noss E, Brenner M. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol Rev. 2008;223:252–70.
Liu Y, Pan YF, Xue YQ, Fang LK, Guo XH, Guo X, et al. uPAR promotes tumor-like biologic behaviors of fibroblast-like synoviocytes through PI3K/Akt signaling pathway in patients with rheumatoid arthritis. Cell Mol Immunol. 2018;15:171–81.
Mor A, Abramson SB, Pillinger MH. The fibroblast-like synovial cell in rheumatoid arthritis: a key player in inflammation and joint destruction. Clin Immunol. 2005;115:118–28.
Neumann E, Riepl B, Knedla A, Lefevre S, Tarner IH, Grifka J, et al. Cell culture and passaging alters gene expression pattern and proliferation rate in rheumatoid arthritis synovial fibroblasts. Arthritis Res Ther. 2010;12:R83.
Hu F, Mu R, Zhu J, Shi L, Li Y, Liu X, et al. Hypoxia and hypoxia-inducible factor-1alpha provoke toll-like receptor signalling-induced inflammation in rheumatoid arthritis. Ann Rheum Dis. 2014;73:928–36.
Tian Y, Bresenitz P, Reska A, El Moussaoui L, Beier CP, Grunder S, et al. Glioblastoma cancer stem cell lines express functional acid sensing ion channels ASIC1a and ASIC3. Sci Rep. 2017;7:13674.
Zhou R, Wu X, Wang Z, Ge J, Chen F. Interleukin-6 enhances acid-induced apoptosis via upregulating acid-sensing ion channel 1a expression and function in rat articular chondrocytes. Int Immunopharmacol. 2015;29:748–60.
Geborek P, Saxne T, Pettersson H, Wollheim F. Synovial fluid acidosis correlates with radiological joint destruction in rheumatoid arthritis knee joints. J Rheumatol. 1989;16:468–72.
Hu F, Yang S, Zhao D, Zhu S, Wang Y, Li J. Moderate extracellular acidification inhibits capsaicin-induced cell death through regulating calcium mobilization, NF-κB translocation and ROS production in synoviocytes. Biochem Biophys Res Commun. 2012;424:196–200.
Zhou R, Dai B, Xie Y, Wu X, Wang Z, Li Y, et al. Interleukin-1β and tumor necrosis factor-α augment acidosis-induced rat articular chondrocyte apoptosis via nuclear factor-kappaB-dependent upregulation of ASIC1a channel. Biochim Biophys Acta Mol Basis Dis. 2018;1864:162–77.
Goldie I, Nachemson A. Synovial pH in rheumatoid knee-joints. I. The effect of synovectomy. Acta Orthop Scand. 1969;40:634–41.
Quagliato L, Freire R, Nardi A. The role of acid-sensitive ion channels in panic disorder: a systematic review of animal studies and meta-analysis of human studies. Transl Psychiatry. 2018;8:185.
Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S. Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Investig. 2002;110:1185–90.
Chen Y, Zhu C, Zhu F, Dai B, Song S, Wang Z, et al. Necrostatin-1 ameliorates adjuvant arthritis rat articular chondrocyte injury via inhibiting ASIC1a-mediated necroptosis. Biochem Bio Res Commun. 2018;504:843–50.
Song S, Tao J, Li S, Qian X, Niu R, Wang C, et al. 17β-estradiol attenuates rat articular chondrocyte injury by targeting ASIC1a-mediated apoptosis. Mol Cell Endocrinol. 2020;505:110742.
Bianchi L, Driscoll M. Protons at the gate: DEG/ENaC ion channels help us feel and remember. Neuron. 2002;34:337–40.
Grunder S, Pusch M. Biophysical properties of acid-sensing ion channels (ASICs). Neuropharmacology. 2015;94:9–18.
Li X, Wu FR, Xu RS, Hu W, Jiang DL, Ji C, et al. Acid-sensing ion channel 1a-mediated calcium influx regulates apoptosis of endplate chondrocytes in intervertebral discs. Expert Opin Ther Targets. 2014;18:1–14.
Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–7.
Haynes WD, Atkinson WE, Morris WD, Sternberg JJ. Early breast cancer. J Ark Med Soc. 1987;83:353–4.
Wemmie JA, Taugher RJ, Kreple CJ. Acid-sensing ion channels in pain and disease. Nat Rev Neurosci. 2013;14:461–71.
Sun X, Zhao D, Li Y, Sun Y, Lei X, Zhang J, et al. Regulation of ASIC1 by Ca2+/calmodulin-dependent protein kinase II in human glioblastoma multiforme. Oncol Rep. 2013;30:2852–8.
Jin C, Yuan FL, Gu YL, Li X, Liu MF, Shen XM, et al. Over-expression of ASIC1a promotes proliferation via activation of the beta-catenin/LEF-TCF axis and is associated with disease outcome in liver cancer. Oncotarget. 2017;8:25977–88.
Wang Y, Sun Y, Zuo L, Wang Y, Huang Y. ASIC1a promotes high glucose and PDGF-induced hepatic stellate cell activation by inducing autophagy through CaMKKbeta/ERK signaling pathway. Toxicol Lett. 2019;300:1–9.
Zhou B, Wang Z, Zhao Y, Brautigan D, Zhang Z. The specificity of extracellular signal-regulated kinase 2 dephosphorylation by protein phosphatases. J Biol Chem. 2002;277:31818–25.
Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–44.
Sazonova O, Blishchenko E, Tolmazova A, Khachin D, Leontiev K, Karelin A, et al. Stimulation of fibroblast proliferation by neokyotorphin requires Ca influx and activation of PKA, CaMK II and MAPK/ERK. FEBS J. 2007;274:474–84.
Brummer T, McInnes C. RAF kinase dimerization: implications for drug discovery and clinical outcomes. Oncogene. 2020;39:4155–69.
Fearon U, Hanlon M, Wade S, Fletcher J. Altered metabolic pathways regulate synovial inflammation in rheumatoid arthritis. Clin Exp Immunol. 2019;197:170–80.
Guo X, Chen G. Hypoxia-inducible factor is critical for pathogenesis and regulation of immune cell functions in rheumatoid arthritis. Front Immunol. 2020;11:1668.
Xiong Z, Zhu X, Chu X, Minami M, Hey J, Wei W, et al. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–98.
Yermolaieva O, Leonard A, Schnizler M, Abboud F, Welsh M. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci USA. 2004;101:6752–7.
Karpus O, Heutinck K, Wijnker P, Tak P, Hamann J. Triggering of the dsRNA sensors TLR3, MDA5, and RIG-I induces CD55 expression in synovial fibroblasts. PloS one. 2012;7:e35606.
Zhang Y, Qian X, Yang X, Niu R, Song S, Zhu F, et al. ASIC1a induces synovial inflammation via the Ca/NFATc3/ RANTES pathway. Theranostics. 2020;10:247–64.
Fitzgerald R, Omary M. Triadafilopoulos G acid modulation of HT29 cell growth and differentiation. An in vitro model for Barrett’s esophagus. J Cell Sci. 1997;110:663–71.
Sarosi G, Jaiswal K, Herndon E, Lopez-Guzman C, Spechler S, Souza R, et al. Acid increases MAPK-mediated proliferation in Barrett’s esophageal adenocarcinoma cells via intracellular acidification through a Cl-/HCO3- exchanger. Am J Physiol Gastrointest Liver Physiol. 2005;289:G991–7.
Zhang Q, Liu J, Zhang M, Wei S, Li R, Gao Y, et al. Apoptosis induction of fibroblast-like synoviocytes is an important molecular-mechanism for herbal medicine along with its active components in treating rheumatoid arthritis. Biomolecules. 2019;9:79540.
Dai B, Zhu F, Chen Y, Zhou R, Wang Z, Xie Y, et al. ASIC1a promotes acid-induced autophagy in rat articular chondrocytes through the AMPK/FoxO3a pathway. Int J Mol Sci. 2017;18:2125.
Gao W, Xu Y, Ge J, Chen F. Inhibition of acid‑sensing ion channel 1a attenuates acid‑induced activation of autophagy via a calcium signaling pathway in articular chondrocytes. Int J Mol Med. 2019;43:1778–88.
Lawrence M, Jivan A, Shao C, Duan L, Goad D, Zaganjor E, et al. The roles of MAPKs in disease. Cell Res. 2008;18:436–42.
Liu F, Feng X, Zhu S, Huang H, Chen Y, Pan Y, et al. Sonic hedgehog signaling pathway mediates proliferation and migration of fibroblast-like synoviocytes in rheumatoid arthritis MAPK/ERK signaling pathway. Front Immunol. 2018;9:2847.
Niu R, Hang X, Feng Y, Zhang Y, Qian X, Song S, et al. ASIC1a promotes synovial invasion of rheumatoid arthritis via Ca/Rac1 pathway. Int Immunopharmacol. 2020;79:106089.
Hirasawa Y, Kato Y, Fujita K. Transurethral enucleation with bipolar for benign prostatic hyperplasia: 2-year outcomes and the learning curve of a single surgeon’s experience of 603 consecutive patients. J Endourol. 2017;31:679–85.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant number 81873986).
Author information
Authors and Affiliations
Contributions
JT: Writing—review and editing. ZL: Data analysis. XQ: Performed study concept. YZ: Performed development methodology. YX: Review and revision of the paper. JS: Investigation and validation. XH: Supervision. SS: Investigation. XP: Supervision and editing. FC: provided technical and material support and editing. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tao, J., Lu, Z., Su, J. et al. ASIC1a promotes the proliferation of synovial fibroblasts via the ERK/MAPK pathway. Lab Invest 101, 1353–1362 (2021). https://doi.org/10.1038/s41374-021-00636-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-021-00636-z
This article is cited by
-
BNIP3 mediates the different adaptive responses of fibroblast-like synovial cells to hypoxia in patients with osteoarthritis and rheumatoid arthritis
Molecular Medicine (2022)
-
Inhibition of NUCB2 suppresses the proliferation, migration, and invasion of rheumatoid arthritis synovial fibroblasts from patients with rheumatoid arthritis in vitro
Journal of Orthopaedic Surgery and Research (2022)