Abstract
Acid-sensitive ion channel 1a (ASIC1a) is a member of the extracellular H+ activated cation channel family. Studies have shown that tissue acidification contributes to the formation of microvessels in rheumatoid arthritis (RA) synovial tissue, but its underlying mechanisms remain unclear. The purpose of this study was to investigate the role of tissue acidification in microvascular formation of arthritic synovial tissue and the effect of ASIC1a on vascular endothelial growth factor (VEGF) release from arthritic synovial tissue. Our results indicate that ASIC1a expression, VEGF expression, and microvessel density (MVD) are elevated in RA synovial tissue and adjuvant arthritis (AA) rat synovial tissue. When AA rats were treated with ASIC1a-specific blocker psalmotoxin-1 (PcTx-1), the expression of ASIC1a, VEGF expression, and MVD were all reduced. Acidification of RA synovial fibroblasts (RASF) can promote the release of VEGF. PcTx-1 and ASIC1a-short hairpin RNA can inhibit acid-induced release of VEGF. In addition, the ASIC1a overexpression vector can promote acid-induced VEGF release. This indicates that extracellular acidification induces the release of VEGF by RASF via ASIC1a. These findings suggest that blocking ASIC1a mediates the release of VEGF from synoviocytes may provide a potential therapeutic strategy for RA therapy.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease that affects ~1% of the world’s population. The patient’s clinical symptoms were persistent synovial inflammation, formation of vasospasm, and destruction of cartilage tissue [1, 2]. At present, research on RA mainly focuses on the proliferation of synovial tissue and inflammatory cell infiltration, while vasospasm is rarely studied as a pathological basis for synovial tissue hyperplasia and invasion [3, 4]. Our previous studies have shown that the use of human recombinant endostatin can significantly improve the degree of joint foot swelling in adjuvant arthritis (AA) rats, reduce the microvascular density of synovial tissue, and reduce the expression of vascular endothelial growth factor (VEGF) in tissues [5, 6]. RA synovial tissue is hypoxic, resulting in increased oxygen consumption and increased glycolysis, which causes lactic acid accumulation [7, 8]. Therefore, RA synovial tissue often exhibits acidosis similar to tumor tissue, and the patient’s intra-articular cavity PH can be reduced to below 6.0 [8,9,10]. The lower PH extracellular environment has been shown to be associated with the formation of vasospasm in synovial tissue of RA patients [11, 12]. It has been reported in the literature that acidosis can enhance the expression of VEGF gene and protein synthesis in cancer or other disease [13,14,15].
Acid-sensitive ion channels (ASICs) are a class of cation channels activated by acidification of the extracellular environment and are members of the epithelial sodium channel family, containing at least seven ASIC protein subunits (ASIC1a, ASIC1b, ASIC1b2, ASIC2a, ASIC2b, ASIC3, and ASIC4) [16]. At present, mammalian has identified four ASIC genes (ACCN1, ACCN2, ACCN3, and ACCN4), in which acid-sensitive ion channel 1a (ASIC1a) can simultaneously mediate NA+ and Ca2+ influx during extracellular acidification stimulation, ultimately leading to different cellular physiological responses [17, 18]. Our previous research found that the nonselective ASIC inhibitor amiloride can significantly improve foot swelling and joint damage in AA rats, and this process is related to Ca2+ influx mediated by ASIC1a activation [2, 19, 20]. It has been reported that the pH of joint synovial fluid in AA rats often decreases to about 6.0, and ASIC3 is expressed in rat synovial tissue [9, 21], but ASIC1a is not reported. It has been reported that the ischemia-anoxia reaction caused by inflammation can synergistically enhance the transport capacity of ASIC1a to Ca2+ [22,23,24]. Moreover, CO can mediate calcium influx through L-type calcium channels and activate VEGF gene under hypoxic conditions [25]. In summary, there is increasing evidence that ASIC1a plays a role in RA angiogenesis.
In our study, we first examined the expression levels of ASIC1a in RA synovial tissue and synovial tissue of AA rats. Second, we examined the expression levels of VEGF in the synovial tissue of RA synovial tissue and AA rat. The expression level of VEGF in human synovial fibroblasts stimulated by acidification at different PH 6.0 levels was explored. In addition, we regulated the expression and function of ASIC1a and assessed the effect of acidification stimulation on VEGF expression.
Materials and methods
Tissue collection, RA synovial fibroblast (RASF), and normal synovial fibroblast (NSF) culture
This study was approved by the Human Research Ethics Committee of the Anhui medical university (China). RA synovial tissue and normal synovial tissue were obtained from patients who underwent primary synovectomy and amputation from 2017 to 2019. Pathological examination identified RA synovial tissue and normal synovial tissue, which met the American RA classification criteria for rheumatology. Primary cells were extracted according to the reported methods. The cell was cultured in DMEM high glucose medium (Hyclone, Salt Lake City, UT, USA) supplemented with 20% fetal bovine serum (FBS; Gibco, St Louis, MO, USA). Cells were incubated at 37 °C, 0.5% CO2. All experiments were performed using synoviocyte cultures from 4th to 7th passages.
Animals
A total of 80 male Sprague-Dawley rats (SD rat) weighing 140–160 g were provided by the Animal Experimental Center of Anhui Medical University, Hefei, China. Rats are housed in an environment of 22 ± 2 °C, 12 h light and dark cycle, and free access to standard food and water. All animal experiments in this study were performed in strict accordance with the guidelines of the University Animal Care and Use Committee and were approved by the Ethics Committee of Anhui Medical University.
Induction of adjuvant arthritis (AA) rat model
Intradermal immunization with Freund’s complete adjuvant in the left hind metatarsal footpad of SD rats promoted AA activation. The rats were divided into two groups: non-arthritic (n = 10) and arthritic rats (n = 70). Then, we divided the arthritic group into five subgroups: untreated AA group, psalmotoxin-1 (PcTx-1) 0.5 μg/kg-treated group, PcTx-1 1 μg/kg-treated group, PcTx-1 2 μg/kg-treated group, and triamcinolone acetonide (TA) 1 mg/kg-treated group. AA model was performed by injecting 0.1 ml aliquot of complete adjuvant (Biolead, Beijing, China) on the intraplantar surface subcutaneously. On the 10th day after immunization, PcTx-1 and TA were injected into the bilateral joint cavity once every 2 days and continued until day 26. The control group did not receive medical treatment.
Hematoxylin and eosin (HE) staining
The rats were anesthetized and sacrificed on day 28 after the initial immunization. Bilateral ankle joints were harvested and fixed in 4% paraformaldehyde for 48 h, decalcified in 10% ethylenediamine tetraacetic acid (EDTA), and embedded in paraffin. Serial sections (4 μm) were cut and stained with HE (Beyotime) and examined microscopically, as described previously.
Deregulation of ASIC1a in RASF by short hairpin RNA (shRNA)
Transfection was performed in a six-well plate. Synovial fibroblasts were maintained in 1 ml of complete medium and treated with 0.4 mM ASIC1a-specific shRNA lentiviral particles (Genechem, Shanghai, China) overnight; transduced with control shRNA lentiviral particles three hole. Then, the medium in each well was replaced with 1 ml of complete medium (without Polybrene) to select stable clones expressing shRNA by 3 mg/ml puromycin dihydrochloride. One week later, stable colonies were amplified for further study.
Overexpression of ASIC1a in RASF
Transfection was performed in a six-well plate. Synovial fibroblasts were maintained in 1 ml of complete medium and treated with 0.4 mM ASIC1a gene-lentiviral particles (Genechem, Shanghai, China) overnight; three wells transduced with empty lentiviral particles. Then, the medium in each well was replaced with 1 ml of complete medium (without Polybrene) to select stable clones expressing the target gene by 3 mg/ml puromycin dihydrochloride. One week later, stable colonies were amplified for further study.
Membrane protein extract
A total of 5 × 07 RASF were washed with phosphate buffer, and the cells were scraped off using a cell scraper. The cells were repeatedly frozen and thawed in liquid nitrogen twice to disrupt the cells, and the membrane protein was extracted using the cell membrane protein and cytoplasmic protein extraction kit (Biotime, Shanghai, China). Membrane proteins will be used in further experiments as described below.
Western blotting
Cell culture total protein was extracted using a total protein extraction kit (Bestbio, Shanghai, China), protein samples were separated using a 10% SDS–polyacrylamide gel, and then transferred to a polyvinyl difluoride membrane (Millipore, USA). The membrane was then blocked with TBST (10 mM Tris, 150 mM NaCl, and 0.05% Tween 20 (pH 8.3)) containing 5% skim milk for 1 h at room temperature. Membranes were incubated overnight at 4 °C with anti-ASIC1a (Affinity, Cell Signal Transduction, USA), anti-ASIC2 (Abcam, Cambridge, UK), anti-ASIC3 (Abcam, Cambridge, UK), anti-ASIC4 (Abcam, Cambridge, UK), anti-Na+/K+-ATPase (Abcam, Cambridge, UK), anti-VEGF (Bioss, Beijing, China), and anti-β-actin (Zsbio, Beijing, China) antibodies. Membranes were washed in TBST and incubated with the corresponding secondary antibody (1:10000) for 2 h at room temperature. The signal was observed with a chemiluminescence imager (Bio-RAD, USA) according to the manufacturer’s instructions. Images were quantified using the software Image lab (Bio-RAD, USA).
Immunostaining analysis
Human synovial tissue, RA (n = 42) and normal (n = 3), was fixed with 4% paraformaldehyde, in turn, and decalcified with 10% EDTA and embedded in paraffin. The human synovial tissue paraffin section and the above rat knee joint paraffin section were deparaffinized with xylene and hydrated with a gradient ethanol. The sections were incubated in 3% H2O2 for 5 min, washed with PBS, and the sections were blocked with 10% goat serum for 15 min at room temperature, followed by anti-ASIC1a (Proteintech Group, Wuhan, China), anti-VEGF (Bioss, Beijing, China), respectively. Anti-CD34 (Bioss, Beijing, China) antibodies were incubated for 1 h. The primary antibody was replaced with PBS containing 1% bovine serum albumin (BSA) as a negative control. Immunological reactivity was visualized using streptavidin/peroxidase method (Zhongshan Jinqiao Biotechnology Co., Ltd, Beijing, China) and diaminobenzidine as chromogen. Finally, the sections were counterstained with hematoxylin. Capture images using the 3DHISTECH Pannoramic SCAN slide scanning system.
Determination of vessel density
First, each slide was examined at a low magnification (40×), and the region having the highest container density (hot spot) was selected before observation under high magnification (200×). CD34 positive blood vessels were counted in three or five hotspots, respectively. Only continuous membranous staining was considered positive. Any large microvascular cell with a lumen or any single isolated endothelial cell is given a count. Blood vessels were counted in synovial membrane.
Immunofluorescence staining
RASF and NSF were cultured on adherent slides (Servcebio, Wuhan, China) and then fixed using 4% paraformaldehyde. After slides and rat knee sections were blocked with 1% BSA, primary antibodies against ASIC1a or CD34 (Proteintech Group, Wuhan, China) were stained overnight at 4 °C. After incubation with the fluorophore-coupled secondary antibody (Alexa Fluor® 488-labeled goat anti-rabbit IgG, Zsbio), DAPI was counterstained and images were captured in a laser confocal microscope (Zeiss). DAPI signals are detected in the nucleus and used to define nuclear and perinuclear regions.
Flow cytometry
RASF and NSF were collected and fixed with 4% paraformaldehyde. After blocking with 1% BSA, the primary antibody against ASIC1a (Proteintech Group, Wuhan, China) was stained overnight at 4 °C. After incubation with a fluorophore-conjugated secondary antibody (Alexa Fluor 488-labeled goat anti-rabbit IgG, Zsbio), analysis was performed using CytoFLEX (Beckman Coulter, USA). Data were analyzed using Cytexpert software (Beckman coulter, USA). The experiment was repeated for at least three times.
Enzyme-linked immunosorbent assay (ELISA)
The expression level of VEGF in the harvested cell culture supernatant was determined using a commercially available ELISA kit (Abcam, Cambridge, UK) according to the manufacturer’s instructions. All samples were tested in duplicate.
Statistics
The data were expressed as the mean SD with SPSS 17.0 software (SPSS Inc, Chicago, IL, USA). Statistical analysis was performed using the one-way ANOVA and unpaired Student’s t test for the comparison among the different treatment groups. Degrees of significance were defined by P < 0.05. The results shown were the representative of at least three separate experiments.
Result
ASIC1a is highly expressed in the synovium of human RA and in the synovium of AA rats
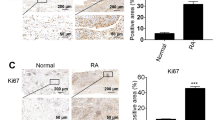
First, immunohistochemical results of human synovial tissue showed a significant up-regulation of ASIC1a expression in RASF compared with NSF (Fig. 1a). Then we used flow cytometry to identify the cells we used. The results showed that more than 99% of CD55 positive cells were identified as human synovial fibroblasts (Fig. 1b). The expression of ASIC1a in RASF and NSF was then investigated by flow cytometry, western blot, and immunostaining. Flow cytometry results showed that ASIC1a expression in RASF was significantly higher than NSF (Fig. 1c). Western blotting results showed that ASIC1a and ASIC3 protein expression was up-regulated in RASF compared with NSF, which was highly significant. ASICs 2 and 4 are not significantly improved (Fig. 1d). ASIC1a is the H+ cation channel acting on the membrane, so we studied the expression of ASIC1a on the RASF membrane. Western blot results showed that ASIC1a was highly expressed on the RASF membrane compared with NSF, consistent with previous flow cytometry results (Fig. 1e). Finally, the results of immunofluorescence staining of RASF and NSF also support the above results (Fig. 1f).
a Immunohistochemical visualization of ASIC1a in normal human synovial tissue and synovial tissue in RA patients (normal: n = 3, RA: n = 25) ***P < 0.001 versus normal group. b, c Detection of CD55 and ASIC1a in RASF by flow cytometry. d Western blotting analysis of ASIC1a and 2,3,4 protein expression in RASF. Histograms show semiquantitative analysis of gels from western blotting. e Western blotting analysis of ASIC1a protein expression in RASF membrane, histograms show semiquantitative analysis of gels from western blotting. Western blotting bands represent three independent experiments. Data are expressed as mean ± SD, *P < 0.05 versus NSF group, **P < 0.01 versus NSF group, ***P < 0.001 versus NSF group. f Representative photomicrographs of RASF were stained to detect ASIC1a (green fluorescence) and nucleus (DAPI, blue fluorescence). The scale bar represents 20 mm (To explain the references to colors in this legend, see the web version of this article.).
In summary, ASIC1a may be involved in the process of affecting arthritis.
Activation of ASIC1a can regulate the release of VEGF by RASF
In this study, we treated cells with a medium of pH = 6.0, and after a different period of time, ELISA, western blotting, and immunofluorescence were used to evaluate the synthesis and secretion of VEGF by RASF.
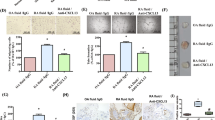
First, the ELISA results showed that the concentration of VEGF in the culture supernatant increased with the stimulation time, which had a cumulative effect (Fig. 2a). RASF could continue to release VEGF under acid stimulation. The results of western blot showed that the synthesis of VEGF by RASF reached the highest at 3 h, followed by a slight decrease, and it was confirmed that the acid could promote the synthesis of VEGF by RASF and release it (Fig. 2b). The results of immunofluorescence also support this view, and the intracellular VEGF concentration reached its highest at 3 h (Fig. 2c).
a Elisa for cell culture supernatants VEGF, **P < 0.01 versus 0 h group. b Western blotting analysis of VEGF protein expression in RASF. Histograms show semiquantitative analysis of gels from western blotting. Western blotting bands represent three independent experiments. Data are expressed as mean ± SD, **P < 0.01 versus 0 h group, ***P < 0.001 versus 0 h group. c Representative photomicrographs of RASF were stained to detect VEGF (green fluorescence) and nucleus (DAPI, blue fluorescence). The scale bar represents 20 mm. d ASIC1a protein expression levels were decreased in RASF by shRNA treatment. The silencing of ASIC1a in RASF was analyzed by western blotting. Histogram showing the semiquantitative analyses of the gels from western blotting. Western blot bands are representative of three independent experiments. Data were expressed as the mean ± SD, **P < 0.01 versus RASF group. e, f Elisa for cell culture supernatants VEGF after ASIC1a silencing or blocking, **P < 0.01 versus RASF group. g Markedly increased levels of the ASIC1a protein were detected by western blotting in RASF transfectants. Histogram showing the semiquantitative analyses of the gels from western blotting. Western blot bands are representative of three independent experiments. Data were expressed as the mean ± SD, ***P < 0.001 versus RASF group. h Elisa for cell culture supernatants VEGF after ASIC1a is overexpressed, **P < 0.01 versus RASF group.
The above results indicate that acidification stimulation can promote the synthesis of RASF and release VEGF.
ASIC1a silencing reduces VEGF released by RASF
In the previous study, we demonstrated that ASIC1a is highly expressed in the synovial tissue of RA joints. Therefore, we used shRNA to silence the ASIC1a gene and then analyzed the effect of reducing the expression of ASIC1a protein on the release of VEGF by RASF. The expression level of ASIC1a protein in RASF-shASIC1a was significantly reduced by western blotting analysis compared with control transfected cells (Fig. 2d).
After shASIC1a transfection, the ability of RASF to release VEGF under acidification stimulation was evaluated. The results showed that the RASF release of the RASF-shASIC1a cell group was reduced compared with the control group (Fig. 2e). We then inhibited ASIC1a protein activity by using PcTx-1 and assessed the release of VEGF under the same conditions. The results showed that the VEGF release was also reduced in the PcTx-1 group compared with the control group (Fig. 2f).
These results indicate that the ability of RASF to release VEGF under acid stimulation is directly related to ASIC1a.
Overexpression of ASIC1a increases RASF release of VEGF
To assess the effect of RASF overexpression of ASIC1a on VEGF release, we then transfected RASF with the ASIC1a expression vector. As shown, a significant increase in ASIC1a protein levels was detected by western blotting in RASF transfectants compared with control transfectants (Fig. 2g).
We then evaluated the ability of RASF-ASIC1a to release VEGF under acidification. The results showed that the level of VEGF release was significantly increased in the RASF-ASIC1a group compared with the control group (Fig. 2h). This means that the overexpression of ASIC1a enhances the ability of RASF to release VEGF.
Establishment of AA rat model and RA synovial tissue identification
First, we performed staining on synovial tissue sections of RA patients and normal subjects, and compared the pathological conditions. It can be seen from the figure that the synovial tissue of RA can be obviously hyperplasia, and the basal layer of the synovial membrane is thickened from the original 2–3 layers to multiple layers, in which obvious vascular enlargement is observed, and vasospasm is formed by multiple vascular sacs. There is a significant inflammatory cell infiltration at the synovial tissue border (Fig. 3a).
a Histological changes of the RA synovium. b The first is a representative image of paw edema in adjuvant arthritis rats, the second is the histological changes of the hind paws in the AA rat model, and the last is the immunohistochemical of CD34 in hind foot sections of the AA rat (n = 6). c Immunohistochemical visualization of MVD labeled with CD34 in AA synovium, ***P < 0.001 versus normal group. d Histological changes of hind paw in AA rat model after PcTx-1 and TA treatment. e Immunohistochemistry of ASIC1a in hind foot segments of AA rats after PcTx-1 treatment (n = 6). f Immunohistochemical visualization of ASIC1a in AA synovium, ***P < 0.001 versus normal group and ###P < 0.001 versus AA group.
The AA rat model we constructed showed a significant swelling on the opposite side of the drug-administered side on day 14, suggesting that our AA rat arthritis model was successful. In the pathological examination, it can be seen that the synovial tissue of AA rats is significantly proliferated compared with normal rats, the basal layer of synovial membrane is thickened, and it is eroded to surrounding cartilage tissue. Significant inflammatory cell infiltration was observed at the synovial border and significant vasospasm was seen. And interestingly, we used CD34 to specifically label vascular endothelial cells, and found that the number of microvessels in the synovial tissue of AA rats was significantly increased compared with normal synovium (Fig. 3b, c). This indicates that the AA rat model we constructed is basically consistent with the pathological changes of human RA. Considering the pathogenesis of the contralateral joint of the AA rat, we believe that the model is representative.
Then we used PcTx-1 and TA to treat AA rats. It was found by HE staining that PcTx-1 can significantly inhibit the development of arthritis in AA rats and reduce cartilage damage and inflammatory infiltration of AA rats in a drug concentration-dependent manner (Fig. 3d). This indicates that blocking ASIC1a can have a therapeutic effect on AA rats. Furthermore, we studied the expression of ASIC1a in the synovial tissue of AA rats before and after administration by immunohistochemistry. The results showed that the expression of ASIC1a in the synovial tissue of AA rat model was significantly higher than that in normal rats (Fig. 3e, f). After PcTx-1 treatment, the severity of disease in AA rats decreased, and the expression of ASIC1a decreased accordingly.
ASIC1a can affect angiogenesis in RASF
In this section, we first studied the expression of VEGF in RA synovial tissue. It can be seen from the figure that the expression of VEGF in RA synovial tissue is significantly higher than that in normal people (Fig. 4a). Correspondingly, we used CD34-labeled vascular endothelial cells to study the number of microvessels in synovial tissue of RA patients. The results showed that the microvessel density (MVD) in RA synovial tissue was significantly increased and clustered compared with normal subjects and consistent with the clinical description of vasospasm (Fig. 4b, c).
a Immunohistochemistry and Immunohistochemical visualization of VEGF in RA synovium (normal: n = 3, RA: n = 25), ***P < 0.001 versus normal group. b, c Immunohistochemistry of CD34 in RA synovium (normal: n = 3, RA: n = 25) and immunohistochemical visualization of MVD labeled with CD34 in AA synovium, ***P < 0.001 versus normal group. d, f Immunohistochemical and immunohistochemical visualization of VEGF in hind foot joints of AA rats after PcTx-1 treatment (n = 6), ***P < 0.001 versus normal group and ###P < 0.001 versus AA group. e, g Immunohistochemistry of CD34 in hind foot joints of AA rats after PcTx-1 treatment and immunohistochemical visualization of MVD labeled with CD34 (normal: n = 3, RA: n = 25), ***P < 0.001 versus normal group and ###P < 0.001 versus AA group. h Representative photomicrographs of hind foot joints of AA rats were stained to detect the vascular wall labeled with CD34 (green fluorescence) and nucleus (DAPI, blue fluorescence) after PcTx-1 treatment. The scale bar represents 20 mm.
Previously, we used recombinant human endostatin (rh-End) to treat arthritis in AA rats. The results showed that rh-End can reduce the expression of MVD and VEGF in synovial tissue of AA rats, protect cartilage and bone, and inhibit the development of RA [6].
This time we investigated the expression of VEGF and MVD in the synovium of AA rats after PcTx-1 treatment. The results of immunohistochemistry showed that the expression of VEGF in the synovial tissue of AA rats was significantly increased compared with the normal group. When treated with PcTx-1, the concentration of VEGF into PcTx-1 in the synovium of AA rats was decreased in a concentration-dependent manner (Fig. 4d, f). The CD34 marker showed a significant increase in MVD in the synovial tissue of AA rats, and the vascular distribution was concentrated in the synovial hyperplasia, including the synovial tissue of the eroded cartilage. When treated with different doses of PcTx-1, MVD was significantly reduced, and erosion of cartilage and bone by synovial tissue was reduced (Fig. 4e, g). In addition to MVD, we also investigated changes in vessel wall thickness after the use of PCTX-1. It is well known that the formation of vasospasm will increase the local MVD, and the original blood vessels appear lesions, the main feature is the thickening of the blood vessel wall. Our immunofluorescence results showed that the vascular wall of the synovial tissue of AA rats was significantly thickened. Compared with the normal group, the blood vessel wall of AA rats was thickened from the original monolayer to 2–3 layers, and obvious associated blood vessels appeared. Different doses of PcTx-1 can inhibit the development of vascular disease, including preventing the thickening of blood vessel walls or inhibiting the formation of new blood vessels (Fig. 4h).
In conclusion, ASIC1a can prevent the development of vasospasm by regulating the secretion of VEGF in arthritis.
Discussion
In this study, we investigated the role of ASIC1a in inducing VEGF release in synovial angiogenesis induced by acidosis (Fig. 5). The results indicate that acidosis induces the release of VEGF by RASF through ASIC1a and participates in the process of angiogenesis. ASIC1a has high expression in both RA synovium and AA rat synovium, and arthritis in AA rats is alleviated after inhibition of ASIC1a, joint destruction is reduced, both MVD and VEGF expression is decreased. In addition, ASIC1a has been shown to be involved in acid-induced RASF release of VEGF. Treatment of RASF with an acidified medium can increase the expression level of VEGF in the culture supernatant. The ability of cells to release VEGF in acidified medium can be reduced after prior treatment with PcTx-1 or shASIC1a. Transfection of RASF with the ASIC1a expression vector increases the ability of cells to release VEGF in acidified medium. We used to think that acidosis and ASIC1a promote the development of RA in the late stage mainly by inducing chondrocyte death. This study suggests that acidosis and ASIC1a are involved in the development of RA in early inflammation. And they promote disease progression to the middle and late stages of RA in a way that affects microangiogenesis.
Changes in extracellular pH homeostasis are a common feature of most inflammatory tissues as well as tumor tissues [26, 27]. In RA, acidosis caused by extracellular pH homeostasis is associated with the induction of chondrocyte apoptosis and autophagy and other processes leading to cartilage tissue degradation, which is very similar to damage caused by acidosis [28,29,30]. However, there is evidence that acidosis promotes tumor development, migration, and invasion [31,32,33]. Only acid has been reported to mediate the pain response of healthy joints via ASIC3, since ASIC3 is a reported acid-sensitive pain receptor, but it is not known that ASIC is involved in the RA synovial inflammatory response [21, 34, 35]. Our previous study used rh-End to treat arthritis in AA rats and found that its treatment is associated with ASIC1a [6]. Therefore, we studied the relationship between acidosis and vascular hyperplasia of RA and explored its molecular mechanism. It has been shown that acidosis can affect the development of RA disease by regulating the release of VEGF by ASIC1a.
ASICs are a family of proton-gated cation channels in which ASIC1a is highly sensitive to changes in extracellular PH [36,37,38]. It was first reported to be abundantly expressed in the nervous system, and in ischemia-induced brain damage, sustained activation of ASIC1a can result in a large amount of cationic influx such as Ca2+, Na+, and Zn(2+) [39, 40]. But there are not many reports in other organizations. There is evidence that inflammatory factors such as IL-1β and TNF-α upregulate the expression of ASIC1a in chondrocytes through the NF-kB pathway, thereby enhancing the apoptosis of chondrocytes induced by acidosis [9]. This aspect illustrates the enhancement of ASIC1a function under inflammatory response, and on the other hand, ASIC1a may become an important link in the downstream of inflammatory response [41, 42]. Based on the above, we examined the expression of ASIC1a in synovial tissue and normal human synovial tissue of RA patients, and demonstrated that the RA inflammatory environment not only affects cartilage, but also increases the sensitivity of synovial tissue to acid. This also explains why the injection of normal saline into the normal joint cavity can only mediate the pain response through ASIC3 without producing more severe joint pathological changes through ASIC1a [21]. In the study of ASIC1a, Ca2+ influx is often seen as a channel open sign. Studies have shown that inflammation often enhance calcium overload caused by ASIC1a, while Ca2+ can mediate downstream complex signaling pathways [42]. In our recent research, it was demonstrated that the ASIC1a-Ca(2+)-NFAT axis plays an important role in RA. NFAT4 activated by ASIC1a affects the development of RA inflammation by regulating the secretion of RANTES [43]. Although we did not include VEGF in the cytokine list we screened in this study, NFAT has long been reported to be involved in embryo development and angiogenesis [44, 45]. In our study, ASIC1a in RASF promoted VEGF release after acidification activation, while ASIC release was reduced when silencing or inhibiting ASIC1a. In contrast, when ASIC1a is overexpressed, VEGF release can be significantly increased under the same treatment. Combined with the analysis of our previous research results, we have reason to predict that the Ca(2+)-NFAT axis may play a key role in this process, but more detailed arguments need further study.
It is worth mentioning that for the first time, we have shown that ASIC1a is one of the causes of VEGF secretion, which is a mitogen that can promote the growth of endothelial cells with high specificity [46]. Studies have generally suggested that hypoxia is the main cause of VEGF release during the inflammatory response [47]. Interestingly, hypoxia can not only lead to the up-regulation of ASIC1a’s membrane positioning, but also enhance the function of ASIC1a [48]. And in studies of cerebral ischemia, Pctx-1 pretreatment can reduce neuronal damage caused by hypoxia [49]. This shows that hypoxia does not only provide conditions for the activation of ASIC1a, but also has important synergy with ASIC1a. In other words, intervention in ASIC1a in RA may simultaneously reduce pathological changes caused by hypoxia. Our study of articular microvasculature in AA rats provides some evidence for this view, but further details still need to be validated. And not just hypoxia, we mentioned earlier that IL-1β and TNF-α are also associated with ASIC1a. Based on the above, we predict that ASIC1a may become an important link in the connection of various RA-promoting diseases, that is, ASIC1a may be a new target for RA treatment.
In conclusion, the activated ASIC1a in RA is involved in regulating the release of VEGF from cells and ultimately promotes the progression of disease progression. Although we found that changes in pH homeostasis induced angiogenesis in RA and initially explored its pathways, other potential factors affecting RA angiogenesis still need to be explored. In addition, we did not delve into the downstream mechanism of ASIC1a in the process of VEGF release. Therefore, the molecular mechanism of acidosis induced VEGF release remains to be further studied.
References
Dai B, Zhu F, Chen Y, Zhou R, Wang Z, Xie Y, et al. ASIC1a promotes acid-induced autophagy in rat articular chondrocytes through the AMPK/FoxO3a pathway. Int J Mol Sci. 2017;18:E2125.
Chen Y, Zhu CJ, Zhu F, Dai BB, Song SJ, Wang ZQ, et al. Necrostatin-1 ameliorates adjuvant arthritis rat articular chondrocyte injury via inhibiting ASIC1a-mediated necroptosis. Biochem Biophys Res Commun. 2018;504:843–50.
Smeets TJ, Kraan MC, Galjaard S, Youssef PP, Smith MD, Tak PP. Analysis of the cell infiltrate and expression of matrix metalloproteinases and granzyme B in paired synovial biopsy specimens from the cartilage-pannus junction in patients with RA. Ann Rheum Dis. 2001;60:561–5.
Huh YH, Lee G, Lee KB, Koh JT, Chun JS, Ryu JH. HIF-2alpha-induced chemokines stimulate motility of fibroblast-like synoviocytes and chondrocytes into the cartilage-pannus interface in experimental rheumatoid arthritis mouse models. Arthritis Res Ther. 2015;17:302.
Yue L, Shen YX, Feng LJ, Chen FH, Yao HW, Liu LH, et al. Blockage of the formation of new blood vessels by recombinant human endostatin contributes to the regression of rat adjuvant arthritis. Eur J Pharmacol. 2007;567:166–70.
Hu W, Xia LJ, Chen FH, Wu FR, Tang J, Chen CZ, et al. Recombinant human endostatin inhibits adjuvant arthritis by down-regulating VEGF expression and suppression of TNF-alpha, IL-1beta production. Inflamm Res. 2012;61:827–35.
Rong C, Chen FH, Jiang S, Hu W, Wu FR, Chen TY, et al. Inhibition of acid-sensing ion channels by amiloride protects rat articular chondrocytes from acid-induced apoptosis via a mitochondrial-mediated pathway. Cell Biol Int. 2012;36:635–41.
Li X, Wu FR, Xu RS, Hu W, Jiang DL, Ji C, et al. Acid-sensing ion channel 1a-mediated calcium influx regulates apoptosis of endplate chondrocytes in intervertebral discs. Expert Opin Ther Targets. 2014;18:1–14.
Zhou RP, Dai BB, Xie YY, Wu XS, Wang ZS, Li Y, et al. Interleukin-1beta and tumor necrosis factor-alpha augment acidosis-induced rat articular chondrocyte apoptosis via nuclear factor-kappaB-dependent upregulation of ASIC1a channel. Biochim Biophys Acta Mol Basis Dis. 2018;1864:162–77.
Zhou RP, Wu XS, Wang ZS, Xie YY, Ge JF, Chen FH. Novel insights into acid-sensing ion channels: implications for degenerative diseases. Aging Dis. 2016;7:491–501.
Jing Z, Xu H, Chen X, Zhong Q, Huang J, Zhang Y, et al. The proton-sensing G-protein coupled receptor GPR4 promotes angiogenesis in head and neck cancer. PLoS ONE. 2016;11:e0152789.
Dewhirst MW, Richardson R, Cardenas-Navia I, Cao Y. The relationship between the tumor physiologic microenvironment and angiogenesis. Hematol Oncol Clin North Am. 2004;18:973–90.
Leske DA, Wu J, Mookadam M, Chen Y, Fautsch MP, Holmes JM, et al. The relationship of retinal VEGF and retinal IGF-1 mRNA with neovascularization in an acidosis-induced model of retinopathy of prematurity. Curr Eye Res. 2006;31:163–9.
Shi Q, Le X, Wang B, Abbruzzese JL, Xiong Q, He Y, et al. Regulation of vascular endothelial growth factor expression by acidosis in human cancer cells. Oncogene. 2001;20:3751–6.
Fukumura D, Xu L, Chen Y, Gohongi T, Seed B, Jain RK. Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res. 2001;61:6020–4.
Dusenkova S, Ru F, Surdenikova L, Nassenstein C, Hatok J, Dusenka R, et al. The expression profile of acid-sensing ion channel (ASIC) subunits ASIC1a, ASIC1b, ASIC2a, ASIC2b, and ASIC3 in the esophageal vagal afferent nerve subtypes. Am J Physiol Gastrointest Liver Physiol. 2014;307:G922–30.
Grunder S, Chen X. Structure, function, and pharmacology of acid-sensing ion channels (ASICs): focus on ASIC1a. Int J Physiol Pathophysiol Pharmacol. 2010;2:73–94.
van Bemmelen MX, Huser D, Gautschi I, Schild L. The human acid-sensing ion channel ASIC1a: evidence for a homotetrameric assembly state at the cell surface. PLoS ONE. 2015;10:e0135191.
Wu X, Ren G, Zhou R, Ge J, Chen FH. The role of Ca(2+) in acid-sensing ion channel 1a-mediated chondrocyte pyroptosis in rat adjuvant arthritis. Lab Invest. 2019;99:499–513.
Zhou R, Wu X, Wang Z, Ge J, Chen F. Interleukin-6 enhances acid-induced apoptosis via upregulating acid-sensing ion channel 1a expression and function in rat articular chondrocytes. Int Immunopharmacol. 2015;29:748–60.
Sugimura N, Ikeuchi M, Izumi M, Kawano T, Aso K, Kato T, et al. Repeated intra-articular injections of acidic saline produce long-lasting joint pain and widespread hyperalgesia. Eur J Pain. 2015;19:629–38.
Zhang Y, Zhang T, Wu C, Xia Q, Xu D. ASIC1a mediates the drug resistance of human hepatocellular carcinoma via the Ca(2+)/PI3-kinase/AKT signaling pathway. Lab Invest. 2017;97:53–69.
Jernigan NL, Herbert LM, Walker BR, Resta TC. Chronic hypoxia upregulates pulmonary arterial ASIC1: a novel mechanism of enhanced store-operated Ca2+ entry and receptor-dependent vasoconstriction. Am J Physiol Cell Physiol. 2012;302:C931–40.
Duan B, Wang YZ, Yang T, Chu XP, Yu Y, Huang Y, et al. Extracellular spermine exacerbates ischemic neuronal injury through sensitization of ASIC1a channels to extracellular acidosis. J Neurosci. 2011;31:2101–12.
Choi YK, Kim JH, Lee DK, Lee KS, Won MH, Jeoung D, et al. Carbon monoxide potentiation of L-Type Ca(2+) channel activity increases HIF-1alpha-independent VEGF expression via an AMPKalpha/SIRT1-mediated PGC-1alpha/ERRalpha axis. Antioxid Redox Signal. 2017;27:21–36.
Riemann A, Reime S, Thews O. Tumor acidosis and hypoxia differently modulate the inflammatory program: measurements in vitro and in vivo. Neoplasia. 2017;19:1033–42.
Zhang HM, Liu MY, Lu JX, Zhu ML, Jin Q, Ping S, et al. Intracellular acidosis via activation of Akt-girdin signaling promotes post ischemic angiogenesis during hyperglycemia. Int J Cardiol. 2019;277:205–11.
Xie YY, Li Y, Zhou RP, Dai BB, Qian YJ, Wu XS, et al. Effects of autophagy on acid-sensing ion channel 1a-mediated apoptosis in rat articular chondrocytes. Mol Cell Biochem. 2018;443:181–91.
Zhou R, Zhu F, Wu X, Song S, Chen Y, Zhu C, et al. Effects of autophagy on apoptosis of articular chondrocytes in adjuvant arthritis rats. J Cell Mol Med. 2019;23:7879–84.
Wang YZ, Wang JJ, Huang Y, Liu F, Zeng WZ, Li Y, et al. Tissue acidosis induces neuronal necroptosis via ASIC1a channel independent of its ionic conduction. Elife. 2015;4:e05682.
Chen X, Sun X, Wang Z, Zhou X, Xu L, Li F, et al. Involvement of acid-sensing ion channel 1a in gastric carcinoma cell migration and invasion. Acta Biochim Biophys Sin. 2018;50:440–6.
Thews O, Riemann A. Tumor pH and metastasis: a malignant process beyond hypoxia. Cancer Metastasis Rev. 2019;38:113–29.
Ibrahim-Hashim A, Estrella V. Acidosis and cancer: from mechanism to neutralization. Cancer Metastasis Rev. 2019;38:149–55.
Gregory NS, Brito RG, Fusaro M, Sluka KA. ASIC3 is required for development of fatigue-induced hyperalgesia. Mol Neurobiol. 2016;53:1020–30.
Ikeuchi M, Kolker SJ, Sluka KA. Acid-sensing ion channel 3 expression in mouse knee joint afferents and effects of carrageenan-induced arthritis. J Pain. 2009;10:336–42.
Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci. 2003;26:477–83.
Xiong ZG, Pignataro G, Li M, Chang SY, Simon RP. Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases. Curr Opin Pharmacol. 2008;8:25–32.
Cai F, Hong X, Tang X, Liu NC, Wang F, Zhu L, et al. ASIC1a activation induces calcium-dependent apoptosis of BMSCs under conditions that mimic the acidic microenvironment of the degenerated intervertebral disc. Biosci Rep. 2019;39:BSR20192708.
Zhou RP, Leng TD, Yang T, Chen FH, Xiong ZG. Acute ethanol exposure promotes autophagy-lysosome pathway-dependent ASIC1a protein degradation and protects against acidosis-induced neurotoxicity. Mol Neurobiol. 2019;56:3326–40.
Zhou R, Leng T, Yang T, Chen F, Hu W, Xiong ZG. Beta-estradiol protects against acidosis-mediated and ischemic neuronal injury by promoting ASIC1a (acid-sensing ion channel 1a) protein degradation. Stroke. 2019;50:2902–11.
de Weille J, Bassilana F. Dependence of the acid-sensitive ion channel, ASIC1a, on extracellular Ca(2+) ions. Brain Res. 2001;900:277–81.
Mari Y, Katnik C, Cuevas J. ASIC1a channels are activated by endogenous protons during ischemia and contribute to synergistic potentiation of intracellular Ca(2+) overload during ischemia and acidosis. Cell Calcium. 2010;48:70–82.
Zhang YH, Qian XW, Yang XJ, Niu RW, Song SJ, Zhu F, et al. ASIC1a induces synovial inflammation via the Ca2+/NFATc3/RANTES pathway. Theranostics. 2020;10:247–64.
Zeini M, Hang CT, Lehrer-Graiwer J, Dao T, Zhou B, Chang CP. Spatial and temporal regulation of coronary vessel formation by calcineurin-NFAT signaling. Development. 2009;136:3335–45.
Muller MR, Sasaki Y, Stevanovic I, Lamperti ED, Ghosh S, Sharma S, et al. Requirement for balanced Ca/NFAT signaling in hematopoietic and embryonic development. Proc Natl Acad Sci USA. 2009;106:7034–9.
Hilmi C, Guyot M, Pages G. VEGF spliced variants: possible role of anti-angiogenesis therapy. J Nucleic Acids. 2012;2012:162692.
Briancon-Marjollet A, Pepin JL, Weiss JW, Levy P, Tamisier R. Intermittent hypoxia upregulates serum VEGF. Sleep Med. 2014;15:1425–6.
Herbert LM, Resta TC, Jernigan NL. RhoA increases ASIC1a plasma membrane localization and calcium influx in pulmonary arterial smooth muscle cells following chronic hypoxia. Am J Physiol Cell Physiol. 2018;314:C166–76.
Li MH, Leng TD, Feng XC, Yang T, Simon RP, Xiong ZG. Modulation of acid-sensing ion channel 1a by Intracellular pH and its role in ischemic stroke. J Biol Chem. 2016;291:18370–83.
Acknowledgements
This work was supported by the National Science Foundation of China (Grant Number 81873986).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qian, X., Zhang, Y., Tao, J. et al. Acidosis induces synovial fibroblasts to release vascular endothelial growth factor via acid-sensitive ion channel 1a. Lab Invest 101, 280–291 (2021). https://doi.org/10.1038/s41374-020-0423-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-020-0423-6