Key Points
-
The same features are considered for diagnosis and classification of spondyloarthritis (SpA); for diagnosis, the probability of disease increases with the presence of more features; for classification, patients need to fulfill criteria
-
Limitations of the existing classification criteria prompted the Assessment of SpondyloArthritis international Society (ASAS) to develop new classification criteria for the full spectrum of axial and peripheral SpA
-
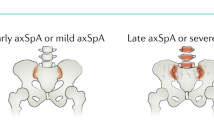
Patients with radiographically evident disease are not substantially different (in a clinical sense) from those without radiographic signs of SpA
-
The global prevalence of SpA is reported to be ∼1%, but this estimation is subject to many factors, including the prevalence of HLA-B27 and the methodology used
-
The reported prevalence of SpA might be expected to increase with better recognition of the disease; therefore, healthcare budgets might be affected
-
Timely diagnosis of SpA is important for educating patients about their disease and how to cope with it, and for providing information on lifestyle modifications, exercises and work participation
Abstract
In the past decade, major progress has been made in the recognition, classification and treatment of spondyloarthritis (SpA). Classification criteria have been developed for axial and peripheral SpA by the Assessment of SpondyloArthritis international Society (ASAS) as a response to new insight into the clinical picture and unmet needs. The ASAS criteria have contributed to a better understanding of the full spectrum of axial and peripheral SpA and of the potential for treatment. However, whether all patients fulfilling these criteria should be considered as having true SpA is a matter of debate. Furthermore, the implementation of the ASAS criteria might lead to an increase in the reported prevalence of SpA, as patients who were previously unidentified could now be classified as having the disease, which might have consequences for healthcare budgets. In this Review, the changes in the clinical picture and epidemiology of SpA are discussed in light of the ASAS classification criteria for SpA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Khan, M. A. Update on spondyloarthropathies. Ann. Intern. Med. 136, 896–907 (2002).
Rudwaleit, M. New approaches to diagnosis and classification of axial and peripheral spondyloarthritis. Curr. Opin. Rheumatol. 22, 375–380 (2010).
Rudwaleit, M. et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann. Rheum. Dis. 68, 777–783 (2009).
Rudwaleit, M. et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann. Rheum. Dis. 70, 25–31 (2011).
Rudwaleit, M., van der Heijde, D., Khan, M. A., Braun, J. & Sieper, J. How to diagnose axial spondyloarthritis early. Ann. Rheum. Dis. 63, 535–543 (2004).
van der Linden, S., Valkenburg, H. A. & Cats, A. Evaluation of diagnostic criteria for AS. A proposal for modification of the New York criteria. Arthritis Rheum. 27, 361–368 (1984).
Amor, B., Dougados, M. & Mijiyawa, M. Criteria of the classification of spondylarthropathies [French]. Rev. Rhum. Mal. Osteoartic. 57, 85–89 (1990).
Dougados, M. et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 34, 1218–1227 (1991).
Braun, J. et al. Use of dynamic magnetic resonance imaging with fast imaging in the detection of early and advanced sacroiliitis in spondylarthropathy patients. Arthritis Rheum. 37, 1039–1045 (1994).
Oostveen, J., Prevo, R., den Boer, J. & van de Laar, M. Early detection of sacroiliitis on magnetic resonance imaging and subsequent development of sacroiliitis on plain radiography. A prospective, longitudinal study. J. Rheumatol. 26, 1953–1958 (1999).
Pedersen, S. J., Weber, U. & Ostergaard, M. The diagnostic utility of MRI in spondyloarthritis. Best Pract. Res. Clin. Rheumatol. 26, 751–766 (2012).
Baeten, D., Breban, M., Lories, R., Schett, G. & Sieper, J. Are spondylarthritides related but distinct conditions or a single disease with a heterogeneous phenotype? Arthritis Rheum. 65, 12–20 (2013).
Weber, U. et al. The diagnostic utility of magnetic resonance imaging in spondylarthritis: an international multicenter evaluation of one hundred eighty-seven subjects. Arthritis Rheum. 62, 3048–3058 (2010).
Aydin, S. Z. et al. Validation of the ASAS criteria and definition of a positive MRI of the sacroiliac joint in an inception cohort of axial spondyloarthritis followed up for 8 years. Ann. Rheum. Dis. 71, 56–60 (2012).
Weber, U. et al. Development and validation of a magnetic resonance imaging reference criterion for defining a positive sacroiliac joint magnetic resonance imaging finding in spondyloarthritis. Arthritis Care Res. (Hoboken) 65, 977–985 (2013).
van den Berg, R. et al. ASAS modification of the Berlin algorithm for diagnosing axial spondyloarthritis: results from the SPondyloArthritis Caught Early (SPACE)-cohort and from the Assessment of SpondyloArthritis international Society (ASAS)-cohort. Ann. Rheum. Dis. 72, 1646–1653 (2013).
van den Berg, R. et al. Percentage of patients with spondyloarthritis in patients referred because of chronic back pain and performance of classification criteria: experience from the Spondyloarthritis Caught Early (SPACE) cohort. Rheumatology (Oxford) 52, 1492–1499 (2013).
Moltó, A. et al. Evaluation of the validity of the different arms of the ASAS set of criteria for axial spondyloarthritis and description of the different imaging abnormalities suggestive of spondyloarthritis: data from the DESIR cohort. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2013-204262.
van der Heijde, D. et al. Spinal inflammation in the absence of sacroiliac joint inflammation on magnetic resonance imaging in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 66, 667–673 (2014).
Rudwaleit, M. et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann. Rheum. Dis. 68, 1520–1527 (2009).
Rudwaleit, M. et al. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum. 60, 717–727 (2009).
Kiltz, U. et al. The degree of spinal inflammation is similar in patients with axial spondyloarthritis who report high or low levels of disease activity: a cohort study. Ann. Rheum. Dis. 71, 1207–1211 (2012).
Kiltz, U. et al. Do patients with non-radiographic axial spondylarthritis differ from patients with AS? Arthritis Care Res. (Hoboken) 64, 1415–1422 (2012).
Ciurea, A. et al. Tumor necrosis factor α inhibition in radiographic and nonradiographic axial spondyloarthritis: results from a large observational cohort. Arthritis Rheum. 65, 3096–3106 (2013).
Callhoff, J., Sieper, J., Weiss, A., Zink, A. & Listing, J. Efficacy of TNFα blockers in patients with AS and non-radiographic axial spondyloarthritis: a meta-analysis. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2014-205322.
Landewé, R., Dougados, M., Mielants, H., van der Tempel, H. & van der Heijde, D. Physical function in AS is independently determined by both disease activity and radiographic damage of the spine. Ann. Rheum. Dis. 68, 863–867 (2009).
Koumakis, E. et al. Heel pain in spondyloarthritis: results of a cross-sectional study of 275 patients. Clin. Exp. Rheumatol. 30, 487–491 (2012).
de Carvalho, H. M. et al. Gender characterization in a large series of Brazilian patients with spondyloarthritis. Clin. Rheumatol. 31, 687–695 (2012).
Dougados, M. et al. A randomised, multicentre, double-blind, placebo-controlled trial of etanercept in adults with refractory heel enthesitis in spondyloarthritis: the HEEL trial. Ann. Rheum. Dis. 69, 1430–1435 (2010).
Paramarta, J. E. et al. Efficacy and safety of adalimumab for the treatment of peripheral arthritis in spondyloarthritis patients without AS or psoriatic arthritis. Ann. Rheum. Dis. 72, 1793–1799 (2013).
Stolwijk, C., Boonen, A., Van Tubergen, A. & Reveille, J. D. Epidemiology of spondyloarthritis. Rheum. Dis. Clin. North Am. 38, 441–476 (2012).
Feldtkeller, E., Khan, M. A., van der Heijde, D., Van der Linden, S. & Braun, J. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with AS. Rheumatol. Int. 23, 61–66 (2003).
Sampaio-Barros, P. D., Conde, R. A., Bonfiglioli, R., Bértolo, M. B. & Samara, A. M. Characterization and outcome of uveitis in 350 patients with spondyloarthropathies. Rheumatol. Int. 26, 1143–1146 (2006).
Marzo-Ortega, H. et al. Baseline and 1-year magnetic resonance imaging of the sacroiliac joint and lumbar spine in very early inflammatory back pain. Relationship between symptoms, HLA-B27 and disease extent and persistence. Ann. Rheum. Dis. 68, 1721–1727 (2009).
Chung, H. Y., Machado, P., van der Heijde, D., D'Agostino, M. A. & Dougados, M. HLA-B27 positive patients differ from HLA-B27 negative patients in clinical presentation and imaging: results from the DESIR cohort of patients with recent onset axial spondyloarthritis. Ann. Rheum. Dis. 70, 1930–1936 (2011).
Akkoc, N. Are spondyloarthropathies as common as rheumatoid arthritis worldwide? A review. Curr. Rheumatol. Rep. 10, 371–378 (2008).
Reveille, J. D., Witter, J. P. & Weisman, M. H. Prevalence of axial spondylarthritis in the United States: estimates from a cross-sectional survey. Arthritis Care Res. (Hoboken) 64, 905–910 (2012).
Hukuda, S. et al. Spondyloarthropathies in Japan: nationwide questionnaire survey performed by the Japan Ankylosing Spondylitis Society. J. Rheumatol. 28, 554–559 (2001).
Boyer, G. S. et al. Prevalence of spondyloarthropathies in Alaskan Eskimos. J. Rheumatol. 21, 2292–2297 (1994).
Alexeeva, L. et al. Prevalence of spondyloarthropathies and HLA-B27 in the native population of Chukotka, Russia. J. Rheumatol. 21, 2298–2300 (1994).
Muñoz-Fernández, S. et al. Early spondyloarthritis: results from the pilot registry ESPIDEP. Clin. Exp. Rheumatol. 28, 498–503 (2010).
Bhatia, K., Prasad, M. L., Barnish, G. & Koki, G. Antigen and haplotype frequencies at three human leucocyte antigen loci (HLA-A, -B, -C) in the Pawaia of Papua New Guinea. Am. J. Phys. Anthropol. 75, 329–340 (1988).
Gofton, J. P., Chalmers, A., Price, G. E. & Reeve, C. E. HL-A and AS in, B. C. Indians. J. Rheumatol. 11, 572–573 (1984).
Benevolenskaia, L. I., Erdes, S., Krylov, M. & Chekalina, N. A. The epidemiology of spondyloarthropathies among the native inhabitants of Chukotka. 2. The prevalence of HLA-B27 in the population and among spondyloarthropathy patients [Russian]. Ter Arkh 66, 41–44 (1994).
Gran, J. T., Mellby, A. S. & Husby, G. The prevalence of HLA-B27 in Northern Norway. Scand. J. Rheumatol. 13, 173–176 (1984).
Gran, J. T., Husby, G. & Hordvik, M. Prevalence of AS in males and females in a young middle-aged population of Tromso, northern Norway. Ann. Rheum. Dis. 44, 359–367 (1985).
Johnsen, K., Gran, J. T., Dale, K. & Husby, G. The prevalence of AS among Norwegian Samis (Lapps). J. Rheumatol. 19, 1591–1594 (1992).
Khan, M. A. HLA-B27 and its subtypes in world populations. Curr. Opin. Rheumatol. 7, 263–269 (1995).
Mustafa, K. N., Hammoudeh, M. & Khan, M. A. HLA-B27 Prevalence in Arab populations and among patients with AS. J. Rheumatol. 39, 1675–1677 (2012).
Costantino, F. et al. Prevalence of spondyloarthritis in reference to HLA-B27 in the French population: results of the GAZEL cohort. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2013-204436.
Geirsson, A. J., Eyjolfsdottir, H., Bjornsdottir, G., Kristjansson, K. & Gudbjornsson, B. Prevalence and clinical characteristics of AS in Iceland—a nationwide study. Clin. Exp. Rheumatol. 28, 333–340 (2010).
Carbone, L. D. et al. Ankylosing spondylitis in Rochester, Minnesota, 1935–1989 Is the epidemiology changing? Arthritis Rheum. 35, 1476–1482 (1992).
Bakland, G., Nossent, H. C. & Gran, J. T. Incidence and prevalence of AS in Northern Norway. Arthritis Rheum. 53, 850–855 (2005).
Dean, L. E. et al. Global prevalence of AS. Rheumatology (Oxford) 53, 650–657 (2014).
Bakland, G., Alsing, R., Singh, K. & Nossent, J. C. Assessment of SpondyloArthritis International Society criteria for axial spondyloarthritis in chronic back pain patients with a high prevalence of HLA-B27. Arthritis Care Res. (Hoboken) 65, 448–453 (2013).
Strand, V. et al. Prevalence of axial spondyloarthritis in United States rheumatology practices: Assessment of SpondyloArthritis International Society criteria versus rheumatology expert clinical diagnosis. Arthritis Care Res. (Hoboken) 65, 1299–1306 (2013).
Van Tubergen, A. & Weber, U. Diagnosis and classification in spondyloarthritis: identifying a chameleon. Nat. Rev. Rheumatol. 8, 253–261 (2012).
Savolainen, E., Kaipiainen-Seppanen, O., Kröger, L. & Luosujärvi, R. Total incidence and distribution of inflammatory joint diseases in a defined population: results from the Kuopio 2000 arthritis survey. J. Rheumatol. 30, 2460–2468 (2003).
De Angelis, R., Salaffi, F. & Grassi, W. Prevalence of spondyloarthropathies in an Italian population sample: a regional community-based study. Scand. J. Rheumatol. 36, 14–21 (2007).
Townes, J. M. et al. Reactive arthritis following culture-confirmed infections with bacterial enteric pathogens in Minnesota and Oregon: a population-based study. Ann. Rheum. Dis. 67, 1689–1696 (2008).
Söderlin, M. K., Borjesson, O., Kautiainen, H., Skogh, T. & Leirisalo-Repo, M. Annual incidence of inflammatory joint diseases in a population based study in southern Sweden. Ann. Rheum. Dis. 61, 911–915 (2002).
Saraux, A. et al. Prevalence of spondyloarthropathies in France: 2001. Ann. Rheum. Dis. 64, 1431–1435 (2005).
Van Hoeven, L., Luime, J., Han, H., Vergouwe, Y. & Weel, A. Identifying axial spondyloarthritis in Dutch primary care patients, ages 20–45 years, with chronic low back pain. Arthritis Care Res. (Hoboken) 66, 446–453 (2014).
Sieper, J. & van der Heijde, D. Review: Nonradiographic axial spondyloarthritis: new definition of an old disease? Arthritis Rheum. 65, 543–551 (2013).
Poddubnyy, D. et al. The frequency of non-radiographic axial spondyloarthritis in relation to symptom duration in patients referred because of chronic back pain: results from the Berlin early spondyloarthritis clinic. Ann. Rheum. Dis. 71, 1998–2001 (2012).
Bennett, A. N. et al. Severity of baseline magnetic resonance imaging-evident sacroiliitis and HLA-B27 status in early inflammatory back pain predict radiographically evident AS at eight years. Arthritis Rheum. 58, 3413–3418 (2008).
Poddubnyy, D. et al. Rates and predictors of radiographic sacroiliitis progression over 2 years in patients with axial spondyloarthritis. Ann. Rheum. Dis. 70, 1369–1374 (2011).
Gong, Y. et al. Ten years' experience with needle biopsy in the early diagnosis of sacroiliitis. Arthritis Rheum. 64, 1399–1406 (2012).
Braun, J. et al. Prevalence of spondylarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum. 41, 58–67 (1998).
Haglund, E. et al. Prevalence of spondyloarthritis and its subtypes in southern Sweden. Ann. Rheum. Dis. 70, 943–948 (2011).
Saraux, A. et al. Prevalence of rheumatoid arthritis and spondyloarthropathy in Brittany, France. Societe de Rhumatologie de l'Ouest. J. Rheumatol. 26, 2622–2627 (1999).
Smolen, J. S. et al. Treating spondyloarthritis, including AS and psoriatic arthritis, to target: recommendations of an international task force. Ann. Rheum. Dis. 73, 6–16 (2014).
Van der Burg, L. R., Ter Wee, M. M. & Boonen, A. Effect of biological therapy on work participation in patients with AS: a systematic review. Ann. Rheum. Dis. 71, 1924–1933 (2012).
Acknowledgements
The author thanks A. Boonen for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares that she has received speaker's fees from Abbvie, MSD, Pfizer and UCB, consultancy honoraria from Abbvie, Janssen–Cilag, MSD, Pfizer and UCB, and that she has an unrestricted research grant from Pfizer and Roche.
Rights and permissions
About this article
Cite this article
van Tubergen, A. The changing clinical picture and epidemiology of spondyloarthritis. Nat Rev Rheumatol 11, 110–118 (2015). https://doi.org/10.1038/nrrheum.2014.181
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2014.181
This article is cited by
-
Analyzing web searches for axial spondyloarthritis in Germany: a novel approach to exploring interests and unmet needs
Rheumatology International (2023)
-
Improving the design of RCTs in non-radiographic axial spondyloarthritis
Nature Reviews Rheumatology (2022)
-
Expert recommendations on early diagnosis and referral of axial spondyloarthritis in the Kingdom of Saudi Arabia
Clinical Rheumatology (2022)
-
Clinical Features and Drug Retention of TNF Inhibitors in Older Patients with Ankylosing Spondylitis: Results from the KOBIO Registry
BioDrugs (2022)
-
Neutrophil/lymphocyte and platelet/lymphocyte ratios as potential markers of disease activity in patients with Ankylosing spondylitis: a case-control study
Advances in Rheumatology (2020)