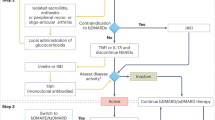
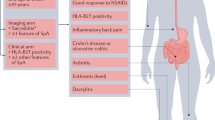
Abstract
Diagnosis and management of axial spondyloarthritis (axSpA) has vastly improved over the past two decades. With advances in the discernment of immunopathogenesis of this disease, new therapies have become available, which are associated with substantial improvement in symptoms, signs and quality of life. The four broad categories of approved treatment options are physical therapy and exercise (which have been known to be beneficial for millennia), NSAIDs (since the 1950s), TNF inhibitors (first FDA approval in 2003) and IL-17 inhibitors (first FDA approval in 2016). In addition, there have been a host of new developments in the axSpA field, including new treatment guidelines, the FDA approval of three biologic DMARDs to treat non-radiographic axSpA, the FDA and EMA approval of Janus kinase (JAK) inhibitors for ankylosing spondylitis, new data on the effect of biologic DMARDs on structural progression in ankylosing spondylitis, strategy trials on tapering or stopping TNF inhibitors in patients in remission, trials of treat-to-target strategy in axSpA, and several new molecules in phase III studies. This Review explores the developments in the management of axSpA.
Key points
-
The therapeutic armamentarium for axial spondyloarthritis is expanding after a gap of several years since TNF inhibitors were approved.
-
Two new classes of drugs (IL-17A and JAK inhibitors) with distinct mechanisms of action have now been approved, with more being studied.
-
Long-term suppression of inflammation could lead to retardation of radiographic progression.
-
Evidence-based guidelines from ACR–SAA–SPARTAN and ASAS–EULAR have many commonalities and few differences. They provide practical approaches towards the management of axial spondyloarthritis.
-
Important unmet needs in the management of this disease include new biomarkers for assessing disease activity, understanding the true impact of ‘treat-to-target’ strategy on long-term outcomes, personalized medicine to determine predictors of response, and comparative effectiveness between different classes of medications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Boonen, A. et al. Employment, work disability, and work days lost in patients with ankylosing spondylitis: a cross sectional study of Dutch patients. Ann. Rheum. Dis. 60, 353–358 (2001).
Rudwaleit, M. et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann. Rheum. Dis. 68, 777–783 (2009).
Ward, M. M. et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Rheumatol. 71, 1599–1613 (2019).
Ward, M. M. et al. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Rheumatol. 68, 282–298 (2016).
van der Heijde, D. et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann. Rheum. Dis. 76, 978–991 (2017).
Dagfinrud, H., Kvien, T. K. & Hagen, K. B. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database Syst. Rev. 2008, CD002822 (2008).
O’Dwyer, T., O’Shea, F. & Wilson, F. Exercise therapy for spondyloarthritis: a systematic review. Rheumatol. Int. 34, 887–902 (2014).
Levitova, A. et al. Clinical improvement and reduction in serum calprotectin levels after an intensive exercise programme for patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis. Arthritis Res. Ther. 18, 275 (2016).
Passalent, L. A., Soever, L. J., O’Shea, F. D. & Inman, R. D. Exercise in ankylosing spondylitis: discrepancies between recommendations and reality. J. Rheumatol. 37, 835–841 (2010).
Fabre, S. et al. Physical activity in patients with axial spondyloarthritis: a cross-sectional study of 203 patients. Rheumatol. Int. 36, 1711–1718 (2016).
Coulter, E. H., McDonald, M. T., Cameron, S., Siebert, S. & Paul, L. Physical activity and sedentary behaviour and their associations with clinical measures in axial spondyloarthritis. Rheumatol. Int. 40, 375–381 (2020).
Kasapoglu Aksoy, M., Birtane, M., Tas¸tekin, N. & Ekuklu, G. The effectiveness of structured group education on ankylosing spondylitis patients. J. Clin. Rheumatol. 23, 138–143 (2017).
Sudre, A., Figuereido, I. T., Lukas, C., Combe, B. & Morel, J. On the impact of a dedicated educational program for ankylosing spondylitis: effect on patient satisfaction, disease knowledge and spinal mobility, a pilot study. Jt. Bone Spine 79, 99–100 (2012).
Candelas, G. et al. Benefit of health education by a training nurse in patients with axial and/or peripheral psoriatic arthritis: a systematic literature review. Rheumatol. Int. 36, 1493–1506 (2016).
Lisowska, B., Kosson, D. & Domaracka, K. Lights and shadows of NSAIDs in bone healing: the role of prostaglandins in bone metabolism. Drug. Des. Devel. Ther. 12, 1753–1758 (2018).
Barkhuizen, A. et al. Celecoxib is efficacious and well tolerated in treating signs and symptoms of ankylosing spondylitis. J. Rheumatol. 33, 1805–1812 (2006).
Levinson, R. D. & Rosenbaum, J. T. Nonsteroidal anti-inflammatory drugs for prophylaxis of acute anterior uveitis. Ocul. Immunol. Inflamm. 18, 69–71 (2010).
Kroon, F. P. et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD010952.pub2 (2015).
Zochling, J., Bohl-Bühler, M. H., Baraliakos, X., Feldtkeller, E. & Braun, J. Nonsteroidal anti-inflammatory drug use in ankylosing spondylitis–a population-based survey. Clin. Rheumatol. 25, 794–800 (2006).
Liew, J. W. et al. Nonsteroidal antiinflammatory drug use and association with incident hypertension in ankylosing spondylitis. Arthritis Care Res. 72, 1645–1652 (2020).
Danve, A. & Raychaudhuri, S. P. Comorbidities in spondyloarthritis. Curr. Treat. Options Rheumatol. 3, 63–74 (2017).
Bhala, N. et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 382, 769–779 (2013).
Haroon, N. N., Paterson, J. M., Li, P., Inman, R. D. & Haroon, N. Patients with ankylosing spondylitis have increased cardiovascular and cerebrovascular mortality: a population-based study. Ann. Intern. Med. 163, 409–416 (2015).
Wanders, A. et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 52, 1756–1765 (2005).
Kroon, F., Landewé, R., Dougados, M. & van der Heijde, D. Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Ann. Rheum. Dis. 71, 1623–1629 (2012).
Sieper, J. et al. Effect of continuous versus on-demand treatment of ankylosing spondylitis with diclofenac over 2 years on radiographic progression of the spine: results from a randomised multicentre trial (ENRADAS). Ann. Rheum. Dis. 75, 1438–1443 (2016).
Karmacharya, P. et al. Effect of therapy on radiographic progression in axial spondyloarthritis: a systematic review and meta-analysis. Arthritis Rheumatol. 72, 733–749 (2020).
Clegg, D. O. et al. Comparison of sulfasalazine and placebo in the treatment of ankylosing spondylitis. A Department of Veterans Affairs Cooperative Study. Arthritis Rheum. 39, 2004–2012 (1996).
Haibel, H. et al. No efficacy of subcutaneous methotrexate in active ankylosing spondylitis: a 16-week open-label trial. Ann. Rheum. Dis. 66, 419–421 (2007).
Clegg, D. O., Reda, D. J. & Abdellatif, M. Comparison of sulfasalazine and placebo for the treatment of axial and peripheral articular manifestations of the seronegative spondylarthropathies: a Department of Veterans Affairs cooperative study. Arthritis Rheum. 42, 2325–2329 (1999).
Sepriano, A. et al. Effect of comedication with conventional synthetic disease-modifying antirheumatic drugs on retention of tumor necrosis factor inhibitors in patients with spondyloarthritis: a prospective cohort study. Arthritis Rheumatol. 68, 2671–2679 (2016).
Nissen, M. J. et al. The effect of comedication with a conventional synthetic disease-modifying antirheumatic drug on drug retention and clinical effectiveness of anti-tumor necrosis factor therapy in patients with axial spondyloarthritis. Arthritis Rheumatol. 68, 2141–2150 (2016).
Lie, E. et al. The effect of comedication with conventional synthetic disease modifying antirheumatic drugs on TNF inhibitor drug survival in patients with ankylosing spondylitis and undifferentiated spondyloarthritis: results from a nationwide prospective study. Ann. Rheum. Dis. 74, 970–978 (2015).
Ben Abdelghani, K. et al. AB0637 efficacy of comedication of conventional synthetic DMARDs with TNF blockers in patients with axial spondyloarthritis. Ann. Rheum. Dis. 79, 1613–1614 (2020).
Tracey, K. J. & Cerami, A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu. Rev. Med. 45, 491–503 (1994).
Braun, J. et al. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum. 38, 499–505 (1995).
Davis, J. C. Jr. et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum. 48, 3230–3236 (2003).
van der Heijde, D. et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum. 52, 582–591 (2005).
van der Heijde, D. et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 54, 2136–2146 (2006).
Inman, R. D. et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum. 58, 3402–3412 (2008).
Landewé, R. et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann. Rheum. Dis. 73, 39–47 (2014).
Maxwell, L. J. et al. TNF-alpha inhibitors for ankylosing spondylitis. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD005468.pub2 (2015).
Cruz-Machado, A. R. et al. Effect of biologic disease-modifying anti-rheumatic drugs targeting remission in axial spondyloarthritis: systematic review and meta-analysis. Rheumatology 59, 3158–3171 (2020).
Deodhar, A. et al. A fifty-two-week, randomized, placebo-controlled trial of certolizumab pegol in nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 71, 1101–1111 (2019).
Vastesaeger, N. et al. Predicting the outcome of ankylosing spondylitis therapy. Ann. Rheum. Dis. 70, 973–981 (2011).
Maneiro, J. R., Souto, A., Salgado, E., Mera, A. & Gomez-Reino, J. J. Predictors of response to TNF antagonists in patients with ankylosing spondylitis and psoriatic arthritis: systematic review and meta-analysis. RMD Open 1, e000017 (2015).
Baraliakos, X. et al. Predictors of clinical remission under anti-tumor necrosis factor treatment in patients with ankylosing spondylitis: pooled analysis from large randomized clinical trials. J. Rheumatol. 42, 1418–1426 (2015).
Macfarlane, G. J., Pathan, E., Jones, G. T. & Dean, L. E. Predicting response to anti-TNFα therapy among patients with axial spondyloarthritis (axSpA): results from BSRBR-AS. Rheumatology 59, 2481–2490 (2020).
Deodhar, A. & Yu, D. Switching tumor necrosis factor inhibitors in the treatment of axial spondyloarthritis. Semin. Arthritis Rheum. 47, 343–350 (2017).
Deodhar, A. et al. Unmet needs in ankylosing spondylitis patients receiving tumour necrosis factor inhibitor therapy; results from a large multinational real-world study. BMC Rheumatol. 4, 19 (2020).
Heiberg, M. S. et al. The comparative one-year performance of anti-tumor necrosis factor alpha drugs in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: results from a longitudinal, observational, multicenter study. Arthritis Rheum. 59, 234–240 (2008).
Wroński, J. & Fiedor, P. The safety profile of tumor necrosis factor inhibitors in ankylosing spondylitis: are TNF inhibitors safer than we thought? J. Clin. Pharmacol. 59, 445–462 (2019).
Manica, S. R. et al. Effectiveness of switching between TNF inhibitors in patients with axial spondyloarthritis: is the reason to switch relevant? Arthritis Res. Ther. 22, 195 (2020).
Burton, P. R. et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat. Genet. 39, 1329–1337 (2007).
Wendling, D., Cedoz, J. P., Racadot, E. & Dumoulin, G. Serum IL-17, BMP-7, and bone turnover markers in patients with ankylosing spondylitis. Jt. Bone Spine 74, 304–305 (2007).
Appel, H. et al. Analysis of IL-17+ cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the Th17-mediated adaptive immune response. Arthritis Res. Ther. 13, R95 (2011).
Baeten, D. et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N. Engl. J. Med. 373, 2534–2548 (2015).
Kivitz, A. J. et al. Efficacy and safety of secukinumab 150 mg with and without loading regimen in ankylosing spondylitis: 104-week results from MEASURE 4 Study. Rheumatol. Ther. 5, 447–462 (2018).
Pavelka, K. et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res. Ther. 19, 285 (2017).
van der Heijde, D. et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet 392, 2441–2451 (2018).
Deodhar, A. et al. Efficacy and safety of ixekizumab in the treatment of radiographic axial spondyloarthritis: sixteen-week results from a phase III randomized, double-blind, placebo-controlled trial in patients with prior inadequate response to or intolerance of tumor necrosis factor inhibitors. Arthritis Rheumatol. 71, 599–611 (2019).
Deodhar, A. et al. Improvement of signs and symptoms of nonradiographic axial spondyloarthritis in patients treated with secukinumab: primary results of a randomized, placebo-controlled phase III study. Arthritis Rheumatol. 73, 110–120 (2020).
Deodhar, A. et al. Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet 395, 53–64 (2020).
Wei, J. C. et al. Efficacy and safety of brodalumab, an anti-IL17RA monoclonal antibody, in patients with axial spondyloarthritis: 16-week results from a randomised, placebo-controlled, phase 3 trial. Ann. Rheum. Dis. 80, 1014–1021 (2021).
O’Connor, W. Jr. et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat. Immunol. 10, 603–609 (2009).
Deodhar, A. et al. Long-term safety of secukinumab in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: integrated pooled clinical trial and post-marketing surveillance data. Arthritis Res. Ther. 21, 111 (2019).
van der Heijde, D. et al. Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis (SELECT-AXIS 1): a multicentre, randomised, double-blind, placebo-controlled, phase 2/3 trial. Lancet 394, 2108–2117 (2019).
Deodhar, A. et al. Tofacitinib for the treatment of ankylosing spondylitis: a phase III, randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 80, 1004–1013 (2021).
Deodhar, A. et al. Upadacitinib in active ankylosing spondylitis: 1-year results from the double-blind, placebo-controlled SELECT-AXIS 1 study and open-label extension. Arthritis Rheumatol. 74, 70–80 (2021).
FDA. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death (2022).
van der Heijde, D. et al. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum. 58, 1324–1331 (2008).
van der Heijde, D. et al. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum. 58, 3063–3070 (2008).
van der Heijde, D. et al. Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res. Ther. 11, R127 (2009).
Haroon, N. et al. The impact of tumor necrosis factor α inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum. 65, 2645–2654 (2013).
Molnar, C. et al. TNF blockers inhibit spinal radiographic progression in ankylosing spondylitis by reducing disease activity: results from the Swiss Clinical Quality Management cohort. Ann. Rheum. Dis. 77, 63–69 (2018).
Maas, F. et al. Reduction in spinal radiographic progression in ankylosing spondylitis patients receiving prolonged treatment with tumor necrosis factor inhibitors. Arthritis Care Res. 69, 1011–1019 (2017).
van der Heijde, D. et al. Limited radiographic progression and sustained reductions in MRI inflammation in patients with axial spondyloarthritis: 4-year imaging outcomes from the RAPID-axSpA phase III randomised trial. Ann. Rheum. Dis. 77, 699–705 (2018).
Braun, J. et al. Secukinumab shows sustained efficacy and low structural progression in ankylosing spondylitis: 4-year results from the MEASURE 1 study. Rheumatology 58, 859–868 (2019).
Braun, J. et al. Spinal radiographic progression over 2 years in ankylosing spondylitis patients treated with secukinumab: a historical cohort comparison. Arthritis Res. Ther. 21, 142 (2019).
Baraliakos, X. et al. Comparison of the effects of secukinumab and adalimumab biosimilar on radiographic progression in patients with ankylosing spondylitis: design of a randomized, phase IIIb study (SURPASS). Clin. Drug Investig. 40, 269–278 (2020).
Yang, K., Oak, A. S. W. & Elewski, B. E. Use of IL-23 inhibitors for the treatment of plaque psoriasis and psoriatic arthritis: a comprehensive review. Am. J. Clin. Dermatol. 22, 173–192 (2021).
Deodhar, A. et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol. 71, 258–270 (2019).
Baeten, D. et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann. Rheum. Dis. 77, 1295–1302 (2018).
McGonagle, D. G., McInnes, I. B., Kirkham, B. W., Sherlock, J. & Moots, R. The role of IL-17A in axial spondyloarthritis and psoriatic arthritis: recent advances and controversies. Ann. Rheum. Dis. 78, 1167–1178 (2019).
Siebert, S., Millar, N. L. & McInnes, I. B. Why did IL-23p19 inhibition fail in AS: a tale of tissues, trials or translation? Ann. Rheum. Dis. 78, 1015–1018 (2019).
Smith, J. A. & Colbert, R. A. Review: the interleukin-23/interleukin-17 axis in spondyloarthritis pathogenesis: Th17 and beyond. Arthritis Rheumatol. 66, 231–241 (2014).
Landewé, R. et al. Efficacy and safety of continuing versus withdrawing adalimumab therapy in maintaining remission in patients with non-radiographic axial spondyloarthritis (ABILITY-3): a multicentre, randomised, double-blind study. Lancet 392, 134–144 (2018).
Van den Bosch, F. et al. OP0107 etanercept withdrawal and re-treatment in patients with inactive non-radiographic axial spondyloarthritis at 24 weeks: results of re-embark, an open-label, phase IV trial. Ann. Rheum. Dis. 79, 70–70 (2020).
Landewé, R. B. et al. Maintenance of clinical remission in early axial spondyloarthritis following certolizumab pegol dose reduction. Ann. Rheum. Dis. 79, 920–928 (2020).
Landewé, R. B. et al. Continuing versus withdrawing ixekizumab treatment in patients with axial spondyloarthritis who achieved remission: efficacy and safety results from a placebo-controlled, randomised withdrawal study (COAST-Y). Ann. Rheum. Dis. 80, 1022–1030 (2021).
Lawson, D. O. et al. Tumor necrosis factor inhibitor dose reduction for axial spondyloarthritis: a systematic review and meta-analysis of randomized controlled trials. Arthritis Care Res. 73, 861–872 (2021).
Danve, A. & Deodhar, A. Treat to target in axial spondyloarthritis: what are the issues? Curr. Rheumatol. Rep. 19, 22 (2017).
Molto, A. et al. Efficacy of a tight-control and treat-to-target strategy in axial spondyloarthritis: results of the open-label, pragmatic, cluster-randomised TICOSPA trial. Ann. Rheum. Dis. 80, 1436–1444 (2021).
Moltó, A. et al. THU0370 cluster-randomized pragmatic clinical trial evaluating the potential benefit of a tight-control and treat-to-target strategy in axial spondyloarthritis: the results of the TICOSPA trial. Ann. Rheum. Dis. 79, 417–417 (2020).
Liew, J. W. et al. Patient-reported disease activity in an axial spondyloarthritis cohort during the COVID-19 Pandemic. ACR Open Rheumatol. 2, 533–539 (2020).
Kang, J. H. & Lin, H. C. Obstructive sleep apnea and the risk of autoimmune diseases: a longitudinal population-based study. Sleep. Med. 13, 583–588 (2012).
Son, S. M., Kim, D. S. & Lee, J. S. Fibromyalgia in axial spondyloarthritis: a meta-analysis. J. Clin. Rheumatol. 28, e222–e227 (2020).
Dougados, M. et al. Rate and predisposing factors for sacroiliac joint radiographic progression after a two-year follow-up period in recent-onset spondyloarthritis. Arthritis Rheumatol. 68, 1904–1913 (2016).
van der Heijde, D. et al. Dual neutralisation of interleukin-17A and interleukin-17F with bimekizumab in patients with active ankylosing spondylitis: results from a 48-week phase IIb, randomised, double-blind, placebo-controlled, dose-ranging study. Ann. Rheum. Dis. 79, 595–604 (2020).
Deodhar A. et al. Tofacitinib for the treatment of adult patients with ankylosing spondylitis: primary analysis of a phase 3, randomized, double-blind, placebo-controlled study [abstract]. Arthritis Rheumatol. https://acrabstracts.org/abstract/tofacitinib-for-the-treatment-of-adult-patients-with-ankylosing-spondylitis-primary-analysis-of-a-phase-3-randomized-double-blind-placebo-controlled-study/ (2020).
van der Heijde, D. et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active ankylosing spondylitis (TORTUGA): results from a randomised, placebo-controlled, phase 2 trial. Lancet 392, 2378–2387 (2018).
Sieper, J. et al. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann. Rheum. Dis. 72, 815–822 (2013).
Dougados, M. et al. Symptomatic efficacy of etanercept and its effects on objective signs of inflammation in early nonradiographic axial spondyloarthritis: a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 66, 2091–2102 (2014).
Sieper, J. et al. A randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 67, 2702–2712 (2015).
Haibel, H. et al. Treatment of active ankylosing spondylitis with pamidronate. Rheumatology 42, 1018–1020 (2003).
Acknowledgements
The authors would like to thank Kirsten McGorty PharmD (Yale New Haven Hospital) for the help with Tables 1 and 2 and Figs 2 and 3.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
A. Danve: research grants from Novartis, Lilly; advisory boards at Janssen, AbbVie and Amgen; member of the Spondyloarthritis Research and Treatment Network and the Assessment of Spondyloarthritis International Society. A. Deodhar: research grants from AbbVie, Celgene, Eli Lilly, Glaxo Smith Kline, Novartis, Pfizer and UCB; advisory boards and consulting at AbbVie, Amgen, Aurinia, Bristol Myers Squibb, Celgene, Eli Lilly, Glaxo Smith Kline, Janssen, MoonLake, Novartis, Pfizer and UCB; member of the Spondyloarthritis Research and Treatment Network, the Assessment of Spondyloarthritis International Society and the Scientific Advisory Board of Spondylitis Association of America.
Peer review
Peer review information
Nature Reviews Rheumatology thanks A. Molto, D. Poddubnyy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Danve, A., Deodhar, A. Treatment of axial spondyloarthritis: an update. Nat Rev Rheumatol 18, 205–216 (2022). https://doi.org/10.1038/s41584-022-00761-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-022-00761-z
This article is cited by
-
The efficacy of tofacitinib combined with bDMARDs in the treatment of ankylosing spondylitis patients with inadequate response to bDMARDs: a retrospective study
BMC Rheumatology (2024)
-
Drug persistence in patients with rheumatic and musculoskeletal diseases during a major economic crisis: results from a nationwide cross-sectional online survey
Rheumatology International (2024)
-
Dose Tapering and Discontinuation of Biologic DMARDs in Axial Spondyloarthritis: A Narrative Review (2023 SPARTAN Annual Meeting Proceedings)
Current Rheumatology Reports (2024)
-
The complement factor H-related protein-5 (CFHR5) exacerbates pathological bone formation in ankylosing spondylitis
Journal of Molecular Medicine (2024)
-
How to Monitor Disease Activity of Axial Spondyloarthritis in Clinical Practice
Current Rheumatology Reports (2024)