Abstract
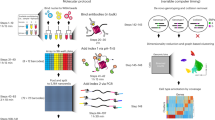
Interactions of proteins with DNA mediate many critical nuclear functions. Chromatin immunoprecipitation (ChIP) is a robust technique for studying protein–DNA interactions. Current ChIP assays, however, either require large cell numbers, which prevent their application to rare cell samples or small-tissue biopsies, or involve lengthy procedures. We describe here a 1-day micro ChIP (μChIP) protocol suitable for up to eight parallel histone and/or transcription factor immunoprecipitations from a single batch of 1,000 cells. μChIP technique is also suitable for monitoring the association of one protein with multiple genomic sites in 100 cells. Alterations in cross-linking and chromatin preparation steps also make μChIP applicable to ∼1-mm3 fresh- or frozen-tissue biopsies. From cell fixation to PCR-ready DNA, the procedure takes ∼8 h for 16 ChIPs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
O'Neill, L.P. & Turner, B.M. Histone H4 acetylation distinguishes coding regions of the human genome from heterochromatin in a differentiation-dependent but transcription-independent manner. EMBO J. 14, 3946–3957 (1995).
O'Neill, L.P. & Turner, B.M. Immunoprecipitation of chromatin. Methods Enzymol. 274, 189–197 (1996).
Collas, P. & Dahl, J.A. Chop it, ChIP it, check it: the current status of chromatin immunoprecipitation. Front. Biosci. 13, 929–943 (2008).
Bernstein, B.E. et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell 120, 169–181 (2005).
Boyer, L.A. et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122, 947–956 (2005).
Bernstein, B.E. et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326 (2006).
O'Neill, L.P., VerMilyea, M.D. & Turner, B.M. Epigenetic characterization of the early embryo with a chromatin immunoprecipitation protocol applicable to small cell populations. Nat. Genet. 38, 835–841 (2006).
Nelson, J.D., Denisenko, O., Sova, P. & Bomsztyk, K. Fast chromatin immunoprecipitation assay. Nucleic Acids Res. 34, e2 (2006).
Loh, Y.H. et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat. Genet. 38, 431–440 (2006).
Lee, T.I. et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell 125, 301–313 (2006).
Dahl, J.A. & Collas, P. Q2ChIP, a quick and quantitative chromatin immunoprecipitation assay unravels epigenetic dynamics of developmentally regulated genes in human carcinoma cells. Stem Cells 25, 1037–1046 (2007).
Guenther, M.G., Levine, S.S., Boyer, L.A., Jaenisch, R. & Young, R.A. A chromatin landmark and transcription initiation at most promoters in human cells. Cell 130, 77–88 (2007).
Acevedo, L.G. et al. Genome-scale ChIP-chip analysis using 10,000 human cells. Biotechniques 43, 791–797 (2007).
Attema, J.L. et al. Epigenetic characterization of hematopoietic stem cell differentiation using miniChIP and bisulfite sequencing analysis. Proc. Natl. Acad. Sci USA 104, 12371–12376 (2007).
Zhao, X.D. et al. Whole-genome mapping of histone H3 Lys4 and 27 trimethylations reveals distinct genomic compartments in human embryonic stem cells. Cell Stem Cell 1, 286–298 (2007).
O'Neill, L.P. & Turner, B.M. Immunoprecipitation of native chromatin: NChIP. Methods 31, 76–82 (2003).
Hudson, M.E. & Snyder, M. High-throughput methods of regulatory element discovery. Biotechniques 41, 673 675, 677 passim (2006).
Dunn, J.J., McCorkle, S.R., Everett, L. & Anderson, C.W. Paired-end genomic signature tags: a method for the functional analysis of genomes and epigenomes. Genet. Eng. (NY) 28, 159–173 (2007).
Aiba, K., Carter, M.G., Matoba, R. & Ko, M.S. Genomic approaches to early embryogenesis and stem cell biology. Semin. Reprod. Med. 24, 330–339 (2006).
Clark, D.J. & Shen, C.H. Mapping histone modifications by nucleosome immunoprecipitation. Methods Enzymol. 410, 416–430 (2006).
Négre, N., Lavrov, S., Hennetin, J., Bellis, M. & Cavalli, G. Mapping the distribution of chromatin proteins by ChIP on chip. Methods Enzymol. 410, 316–341 (2006).
Wu, J., Smith, L.T., Plass, C. & Huang, T.H. ChIP-chip comes of age for genome-wide functional analysis. Cancer Res. 66, 6899–6902 (2006).
Bulyk, M.L. DNA microarray technologies for measuring protein-DNA interactions. Curr. Opin. Biotechnol. 17, 422–430 (2006).
O'Geen, H., Nicolet, C.M., Blahnik, K., Green, R. & Farnham, P.J. Comparison of sample preparation methods for ChIP-chip assays. Biotechniques 41, 577–580 (2006).
Dahl, J.A. & Collas, P. MicroChIP—a rapid micro chromatin immunoprecipitation assay for small cell samples and biopsies. Nucleic Acids Res. 36, e15 (2008).
Nelson, J.D., Denisenko, O. & Bomsztyk, K. Protocol for the fast chromatin immunoprecipitation (ChIP) method. Nat. Protoc. 1, 179–185 (2006).
Flanagin, S., Nelson, J.D., Castner, D.G., Denisenko, O. & Bomsztyk, K. Microplate-based chromatin immunoprecipitation method, Matrix ChIP: a platform to study signaling of complex genomic events. Nucleic Acids Res. 36, e17 (2008).
Mikkelsen, T.S. et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448, 553–560 (2007).
Kiermer, V. Embryos and biopsies on the ChIP-ing forecast. Nat. Methods 3, 583 (2006).
Lee, T.I., Johnstone, S.E. & Young, R.A. Chromatin immunoprecipitation and microarray-based analysis of protein location. Nat. Protoc. 1, 729–748 (2006).
Spencer, V.A., Sun, J.M., Li, L. & Davie, J.R. Chromatin immunoprecipitation: a tool for studying histone acetylation and transcription factor binding. Methods 31, 67–75 (2003).
Barski, A. et al. High-resolution profiling of histone methylations in the human genome. Cell 129, 823–837 (2007).
Orlando, V. Mapping chromosomal proteins in vivo by formaldehyde-crosslinked-chromatin immunoprecipitation. Trends Biochem. Sci. 25, 99–104 (2000).
Zeng, P.Y., Vakoc, C.R., Chen, Z.C., Blobel, G.A. & Berger, S.L. In vivo dual cross-linking for identification of indirect DNA-associated proteins by chromatin immunoprecipitation. Biotechniques 41, 694 696, 698 (2006).
Roh, T.Y., Cuddapah, S., Cui, K. & Zhao, K. The genomic landscape of histone modifications in human T cells. Proc. Natl. Acad. Sci USA 103, 15782–15787 (2006).
Håkelien, A.M., Landsverk, H.B., Robl, J.M., Skålhegg, B.S. & Collas, P. Reprogramming fibroblasts to express T-cell functions using cell extracts. Nat. Biotechnol. 20, 460–466 (2002).
Landsverk, H.B. et al. Reprogrammed gene expression in a somatic cell-free extract. EMBO Rep. 3, 384–389 (2002).
Vandesompele, J., De, P.A. & Speleman, F. Elimination of primer-dimer artifacts and genomic coamplification using a two-step SYBR green I real-time RT-PCR. Anal. Biochem. 303, 95–98 (2002).
Ito, K. et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N. Engl. J. Med. 352, 1967–1976 (2005).
Pollicino, T. et al. Hepatitis B virus replication is regulated by the acetylation status of hepatitis B virus cccDNA-bound H3 and H4 histones. Gastroenterology 130, 823–837 (2006).
Zuccato, C. et al. Widespread disruption of repressor element-1 silencing transcription factor/neuron-restrictive silencer factor occupancy at its target genes in Huntington's disease. J. Neurosci. 27, 6972–6983 (2007).
Ge, W. et al. Coupling of cell migration with neurogenesis by proneural bHLH factors. Proc. Natl. Acad. Sci USA 103, 1319–1324 (2006).
Huang, Y., Doherty, J.J. & Dingledine, R. Altered histone acetylation at glutamate receptor 2 and brain-derived neurotrophic factor genes is an early event triggered by status epilepticus. J. Neurosci. 22, 8422–8428 (2002).
Le, T.N. et al. Dlx homeobox genes promote cortical interneuron migration from the basal forebrain by direct repression of the semaphorin receptor neuropilin-2. J. Biol. Chem. 282, 19071–19081 (2007).
Acknowledgements
This work is supported by the FUGE, YFF, STAMCELLE and STORFORSK programs of the Research Council of Norway and by the Norwegian Cancer Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Philippe Collas is a consultant for Diagenode SA, Liege, Belgium
Supplementary information
Supplementary Figure 1
Real-time PCR profiles after analysis of DNA from a 100,000-cell ChIP and of a 100-cell ChIP (PDF 385 kb)
Rights and permissions
About this article
Cite this article
Dahl, J., Collas, P. A rapid micro chromatin immunoprecipitation assay (ChIP). Nat Protoc 3, 1032–1045 (2008). https://doi.org/10.1038/nprot.2008.68
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2008.68
This article is cited by
-
Spurious transcription causing innate immune responses is prevented by 5-hydroxymethylcytosine
Nature Genetics (2023)
-
iPromoter-Seqvec: identifying promoters using bidirectional long short-term memory and sequence-embedded features
BMC Genomics (2022)
-
Cooperative assembly of p97 complexes involved in replication termination
Nature Communications (2022)
-
Inactivation of Sirt6 ameliorates muscular dystrophy in mdx mice by releasing suppression of utrophin expression
Nature Communications (2022)
-
Laboratory methods to decipher epigenetic signatures: a comparative review
Cellular & Molecular Biology Letters (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.