Abstract
Mesenchymal chondrosarcomas are rare and highly aggressive sarcomas occurring in bone and soft tissue, with poor overall survival. Bcl-2 expression was previously shown to be upregulated in mesenchymal chondrosarcomas. We here report on a newly derived mesenchymal chondrosarcoma cell line, MCS170, in which we investigated treatment with the BH3 mimetic ABT-737 alone or in combination with conventional chemotherapy as a possible new therapeutic strategy. The presence of the characteristic HEY1-NCOA2 fusion was confirmed in the MCS170 cell line using FISH, RT-PCR, and sequencing. The MCS170 cell line was treated with ABT-737 alone or in combination with doxorubicin or cisplatin. Cell viability and proliferation was determined using WST-1 viability assays and the xCELLigence system. Expression of Bcl-2 family members was studied using immunohistochemistry. Apoptosis was determined using the caspase-glo 3/7 assay and western blot for PARP cleavage. The MCS170 cell line was sensitive to doxorubicin treatment with an IC50 of 0.09 μM after 72 h, but more resistant to cisplatin treatment with an IC50 of 4.5 μM after 72 h. Cells showed little sensitivity toward ABT-737 with an IC50 of 1.8 μM after 72 h. Combination treatments demonstrated ABT-737 synergism with cisplatin as well as doxorubicin as shown by induction of apoptosis and reduction in cell proliferation. Restoration of the apoptotic machinery by inhibition of Bcl-2 family members sensitizes MCS170 mesenchymal chondrosarcoma cells to conventional chemotherapy. This indicates that combining the inhibition of Bcl-2 family members with conventional chemotherapy can be a possible therapeutic strategy for patients with mesenchymal chondrosarcoma.
Similar content being viewed by others
Main
Chondrosarcomas are cartilage forming tumors accounting for 20% of all the primary bone malignancies.1 Chondrosarcoma consists of different subtypes with conventional chondrosarcoma being the most common (75%). More rare chondrosarcoma subtypes include dedifferentiated (10%),2 mesenchymal (3%),3 and clear cell chondrosarcoma (2%).4 Patients with chondrosarcoma are mainly treated by surgical removal of the tumor, because chemo and radiotherapy are generally ineffective.5
Mesenchymal chondrosarcoma is an aggressive, high-grade chondrosarcoma subtype with a reported 10-year survival rate between 27 and 67%.6, 7, 8 Recently, the survival of mesenchymal chondrosarcoma was determined using the Surveillance, Epidemiology, and End Results (SEER) database, showing that in a group of 205 patients, the 10-year survival was 43%.9 Genetically, it is characterized by a specific characteristic gene fusion between the bHLH domain of HEY1 (Hes-related family BHLH transcription factor with YRPW motif 1) and the C-terminal transcriptional activation domains of NCOA2 (nuclear receptor coactivator 2).10 Fusions involving NCOA2 have also been identified in other soft tissue tumor subtypes including spindle cell rhabdomyosarcoma (SRF-NCOA2 and TEAD1-NCOA2)11 and soft tissue angiofibroma (AHRR-NCOA2),12 as well as in acute myeloid leukemia (ETV6-NCOA213 and MYST3-NCOA214).
Histologically mesenchymal chondrosarcoma is characterized by variable amounts of differentiated cartilage admixed with small round undifferentiated cells.3 In 1983, Huvos et al8 described that patients with mesenchymal chondrosarcoma in which the small cell component predominated responded better to the combination of chemo- and radiotherapy. In a more recent study, investigating the effect of chemotherapy in 113 mesenchymal chondrosarcoma patients, it was shown that treatment with chemotherapy reduces the risk of recurrence and improves overall survival in patients with localized disease.7 Also in previous smaller case reports, patients with mesenchymal chondrosarcoma treated with chemotherapy showed a better survival compared with untreated patients.6, 15, 16, 17, 18 However, in a recent systematic review of 18 studies including 107 patients, no correlation between anthracycline-based chemotherapy treatment and survival was found.19
Conventional chondrosarcomas are resistant to chemotherapy. Low-grade chondrosarcomas consist of a large amount of cartilage matrix and slowly dividing cells, which was for long thought to be the cause of their insensitivity to chemotherapy. However, high-grade chondrosarcomas have less matrix and more rapidly dividing cells, and are also resistant to chemotherapy. Expression of multidrug resistance pumps has been shown on chondrosarcoma cell lines and patient tissues;20, 21, 22 however, this does not prevent doxorubicin from accumulating in the cell nuclei, indicating other mechanisms are involved in chondrosarcoma chemoresistance.21
Our group has previously shown that Bcl-2 family members have a key role in chemoresistance in conventional and dedifferentiated chondrosarcoma.21, 23 Pro- and antiapoptotic proteins of the Bcl-2 family have important roles in initiation of the intrinsic routes of apoptosis, changing the balance from anti- to proapoptotic, and activating Bak and Bax to facilitate mitochondrial outer membrane permeabilization. Antiapoptotic proteins, such as Bcl-2, Bcl-xL, and Bcl-w, prevent apoptosis by binding to proapoptotic proteins, thereby impeding Bak and Bax activation.24 Treatment of different chondrosarcoma cell lines with an inhibitor of the Bcl-2 family, including Bcl-2, Bcl-xL, and Bcl-w (ABT-737) in combination with doxorubicin or cisplatin resulted in a synergistic effect, as was determined by viability and apoptosis assays.21
As we previously demonstrated high expression of Bcl-2 and Bcl-xL in a panel of 23 mesenchymal chondrosarcomas,23 we hypothesized that Bcl-2 family members are also important in mesenchymal chondrosarcoma. So far, however, no mesenchymal chondrosarcoma cell lines were available for functional studies. We here report a novel mesenchymal chondrosarcoma cell line, MCS170, carrying the characteristic HEY1-NCOA2 fusion product, in which we functionally evaluated the role of Bcl-2 family members in chemosensitivity.
MATERIALS AND METHODS
Compounds
The BH3 mimetic ABT-737 (S1002, Selleckchem, Munich, Germany) is an inhibitor of Bcl-2, Bcl-xL, and Bcl-w, and was dissolved in DMSO to a stock concentration of 20 mM. Doxorubicin (DXR) and cisplatin (CDDP) were obtained from the in-house hospital pharmacy in a 0.9% NaCl solution in a 1 mg/ml concentration. Z-vad-FMK (550379, BD biosciences, San Jose, CA, USA) was used as a general caspase inhibitor and dissolved in DMSO to a stock concentration of 20 mM.
Cell Culture
The MCS170 cell line was generated from a recurrent mesenchymal chondrosarcoma of the spine resected from a 33-year-old man at the department of Pathology, Brigham and Women’s Hospital, and Harvard Medical School located in Boston, USA, and was cultured in IMDM medium (Gibco, Invitrogen Life Technologies, UK) supplemented with 15% heat-inactivated fetal calf serum (Gibco, Invitrogen Life Technologies, UK) and 1% penicillin–streptomycin (100 U/ml) (Gibco, Invitrogen Life Technologies, UK). Cells were grown at 37 °C in a humidified incubator with 95% air and 5% CO2. Cells were confirmed bimonthly for absence of mycoplasm infection, and cell line identity was confirmed before and after the experiments by STR profiling using the GenePrint10 System from Promega (Promega Benelux BV, Leiden, The Netherlands).
Molecular Analysis
The presence of the characteristic HEY1-NCOA2 fusion between exon 4 of HEY1 and exon 13 of NCOA2 was confirmed on RNA isolated using TRIzol (Invitrogen, Carlsbad, CA, USA) from the MCS170 cell line and a previously published mesenchymal chondrosarcoma patient sample as a positive control according to the methods described previously.10 Products were purified using MinElute 96 UF PCR purification kit (Qiagen, Venlo, The Netherlands) according to the manufacturer’s instructions and Sanger sequenced by Macrogen (macrogen.com).
Fluorescent in situ hybridization was performed as described previously25 using labeled BAC clones RP11-152C15 and RP11-888F10.10 MCS170 cells were screened for mutations in 50 frequently mutated cancer-related genes using the Ion AmpliSeq Cancer Hotspot Panel v2 (Life Technologies, Thermo Fisher Scientific, USA, catalog number 4475346) according to the manufacturer’s instructions.
Cell Viability Assay
When cells reached 75–80% confluence, they were washed with PBS, trypsinized, and counted using a Burker Turk counting chamber. MCS170 cells were plated in 96-well plates (10 000 cells/well) and allowed to adhere overnight. Cells were incubated with concentrations of ABT-737, doxorubicin, or cisplatin ranging between 0.1 μM and 100 μM for 24, 48, and 72 h after which a WST-1 assay was performed according to the manufacturer’s instructions (Roche diagnostics GmbH, Penzberg, Germany). Combination assays were performed 72 h after simultaneous addition of ABT-737 (0, 125, 500 nM or 1 μM) and doxorubicin (0, 50, 125 nM or 1 μM), or cisplatin (0, 500 nM, 1 or 5 μM). Results of the WST-1 assay were measured at 450 nm using a spectrophotometer (Victor3V, 1420 multilabel counter, Perkin Elmer, Netherlands). All viability assays were performed in triplicate and at least three times.
Cell Proliferation Measurement
The RTCA xCELLigence system (Roche Applied Sciences, Almere, The Netherlands) was used to measure cell proliferation in real time. MCS170 cells were plated at a density of 15 000 cells/well, 20 h before addition of either ABT-737 (1 μM), doxorubicin (125 nM or 1 μM), or cisplatin (5 μM), or the combination between ABT-737 with doxorubicin or cisplatin. After 96 h, the measurement was stopped and the results were analyzed using RTCA software. Experiments were performed in duplicate and repeated at least three times.
Immunohistochemistry
Protein expression of Bcl-2 (Dako, 124), Bcl-xL (cell signaling clone 54H6), and Bcl-w (Abcam 6C1) was determined using immunohistochemistry on paraffin-embedded cell pellets of MCS170 cells. Tonsil was used as a positive control for Bcl-2, kidney for Bcl-xl, and cerebellum for Bcl-w. Immunohistochemistry was performed according to the standard laboratory methods, using citrate (pH 6) as antigen retrieval method, as previously described.26
Western Blot
PARP (clone 46D11, Cell Signaling Technology, Leiden, The Netherlands) cleavage was determined after 24 h of compound treatment. Cells were treated with 1 μM ABT-737, 125 nM doxorubicin, 5 μM cisplatin, or a combination of ABT-737 and doxorubicin, or cisplatin. As a loading control, α-tubulin (clone DM1A, Cell Signaling Technology, Leiden, The Netherlands) expression was determined. Cells were harvested using hot SDS buffer (1% SDS, 10 mM Tris/EDTA with complete inhibitor and phosSTOP). Of each sample, 20 μg protein was separated by using SDS-PAGE and transferred to PVDF membranes. Blocking was performed using 5% BSA in PBS/0.1% Tween solution. Membranes were incubated with primary antibody overnight at 4 °C, followed by HRP-labeled secondary antibody for 30 min at room temperature. Blots were developed using ECL solution (Pierce ECL Western Blotting Substrate, Fisher Scientific, Landsmeer, The Netherlands).
Apoptosis Assay
Apoptosis was detected using the caspase-glo 3/7 assay (Promega, Madison, USA) according to the manufacturer’s instructions. In brief, 10 000 MCS170 cells were plated into white-walled 96-well plates (Corning B.V. Life Sciences, Amsterdam, The Netherlands) and the following day cells were treated with either 1 μM ABT-737, 125 nM or 1 μM doxorubicin, 5 μM cisplatin, or a combination of ABT-737 and doxorubicin or cisplatin for 24 h. Z-vad treatment was used as a control. Thereafter, the substrate provided with the kit was added in a 1:1 dilution and incubated for 30 min at room temperature. Luminescence was measured using a luminometer (Victor3V, 1420 multilabel counter, Perkin Elmer, Netherlands). In parallel, a viability assay was performed by using the method described in the viability assay section.
Statistical Analysis
To evaluate synergy between treatment combinations, the Bliss independence model was used. In this model, the assumption is made that each drug acts independently of the other on the cells.27 This is used to predict the combined response (C) for the two single compounds (A and B) by the formula: C=A+B−A*B.28
RESULTS
MCS170 as a Model to Study Mesenchymal Chondrosarcoma Behavior In vitro
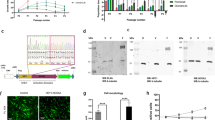
MCS170 cells morphologically show an elongated shape and they predominantly grow in groups of cells (Figure 1a). Using FISH, we confirmed the presence of the HEY1-NCOA2 intrachromosomal inversion in all cells similar to the positive control (Figure 1b), colocalization of one set of red and green probes was observed, whereas the other set of red and green probes were at limited distance. Expression of the HEY1-NCOA2 fusion product was confirmed using RT-PCR, and Sanger sequencing revealed that exon 4 of HEY1 was fused to exon 13 of NCOA2 (Figure 1c). The expression of the HEY1-NCOA2 fusion product was variable between different passage numbers of the cell line. No mutations were detected in any of the 50 oncogenes and tumor suppressor genes, including TP53, IDH1, and IDH2, in the Ion AmpliSeq Cancer Hotspot Panel v2 (Life Technologies). Cell identity as determined by the Cell ID GenePrint 10 system (Promega) is available on request.
Characteristics of the MCS170 cell line. (a) Morphology of MCS170 cells in culture demonstrated elongated cytoplasm. Cells are growing predominantly in groups. Picture was taken at passage 31 with a 10 times objective. (b) Interphase FISH using BAC clones RP11-152C15 and RP11-888F10 on the MCS170 cells, and a mesenchymal chondrosarcoma positive control sample (c) Sequence showing the fusion of exon 4 of HEY1 to exon 13 of NCOA2 in MCS170 cells.
MCS170 Cells are Sensitive for Doxorubicin, whereas Resistant for Cisplatin
To assess chemosensitivity, we treated MCS170 cells with the conventional chemotherapeutics doxorubicin or cisplatin. Cells were sensitive to doxorubicin treatment, especially after longer incubation periods, with an IC50 of 4.5 μM after 24 h of treatment that decreased to an IC50 of 0.08 μM after 72 h of exposure (Figure 2a and Table 1). Cells were resistant to cisplatin with an IC50 of 60 μM after 24 h and 4.5 μM after 72 h (Figure 2b).
Bcl-2 Family Members are Not Essential for Survival of MCS170 Cells
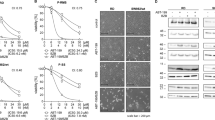
As previous studies indicated that high expression of Bcl-2 and Bcl-xL is a hallmark of mesenchymal chondrosarcoma,23 we determined the protein expression of Bcl-2 family members in the MCS170 cell line. Bcl-2 was expressed in MCS170 (Figure 3a) cells, although variable staining intensities were observed between different cells. Bcl-xl was highly expressed in MCS170 cells (Figure 3c), however, only very weak Bcl-w expression was observed (Figure 3e). To assess whether these Bcl-2 family members are essential for mesenchymal chondrosarcoma cells, these were inhibited in MCS170 cells using the BH3 mimetic ABT-737. After 72 h of treatment, an IC50 of 1.8 μM was observed (Figure 3g), showing that cells are not sensitive for Bcl-2 family member inhibition.
Bcl-2 and Bcl-xl are expressed in mesenchymal chondrosarcoma. (a–f) Expression of Bcl-2 (a and b) and Bcl-xl (c and d), but only low expression of Bcl-w (e and f) was found in paraffin-embedded MCS170 cells (a, c and e), compared with expression in control tissues of tonsil (b), kidney (d), and cerebellum (f and g). MCS170 cells show little sensitivity toward ABT-737 treatment after 24, 48, or 72 h of exposure.
Inhibition of Bcl-2 Family Members Sensitizes Toward Conventional Chemotherapy
To evaluate a functional role for Bcl-2 family members in the response to chemotherapy, MSC170 cells were incubated for 72 h with ABT-737 and either doxorubicin, or cisplatin. Compared with single treatment, a reduction in viability was observed when ABT-737 was combined with doxorubicin or cisplatin for 72 h. (Figures 4a and b). To assess synergy, the excess over Bliss score was calculated. An excess over Bliss score of >12% (indicating synergy) was observed when 1 μM ABT-737 was used in combination with 125 nM doxorubicin, or when 0.5 or 1 μM ABT-737 was combined with 5 μM cisplatin pointing toward a small synergistic effect (Table 2). Real-time measurement of proliferation using the xCELLigence real-time cell proliferation assay demonstrated an early significant reduction in cell index when 1 μM ABT-737 was combined with 1 μM doxorubicin, as compared with single treatment. Consistent with the viability assay, combining 1 μM ABT-737 with 125 nM doxorubicin or 5 μM cisplatin resulted in a reduction in cell viability at 72 h of incubation and persisted until 96 h (Figures 4c and d).
Combination treatment of ABT-737 and doxorubicin, or cisplatin in MCS170 cells. (a and b) Viability after treatment of MCS170 cells for 72 h with ABT-737 and doxorubicin (a), or ABT-737 and cisplatin (b). Combination treatment shows a larger reduction in viability compared with single treatment after 72 h. (c and d) Real-time proliferation assay with the xCELLigence system of MCS170 cells treated with ABT-737 and doxorubicin, (c) or ABT-737 and cisplatin (d). The combination of 1 μM ABT-737 and 1 μM doxorubicin is most effective, and shows a faster reduction in cell index compared with single treatment. The combinations of 1 μM ABT-737 and 125 nM, or 5 μM cisplatin show a similar reduction in cell index and are more effective compared with single treatments.
Combination of ABT-737 with Doxorubicin or Cisplatin Increases Apoptosis
To assess if treatment with ABT-737 and combination with chemotherapy resulted in apoptosis, caspase 3/7 activity was measured after 24 h of treatment (Figure 5a). Combination treatment of ABT-737 with doxorubicin or cisplatin for 24 h substantially increased caspase 3/7-dependent apoptosis. This effect was inhibited by the pan caspase inhibitor Z-vad-FMK, confirming that the effect was caspase-dependent (Figure 5a). Consistent with results obtained using xCELLigence, combining 1 μM ABT-737 with 1 μM doxorubicin resulted in a large reduction of cell viability, after 24 h (Figure 5b). However, this effect could not completely be restored by adding z-vad, indicating that additional mechanisms that reduce viability, besides caspase-dependent apoptosis, may be operable. PARP cleavage was also measured using western blot (Figure 5c), confirming that combination treatment results in activation of apoptosis.
Apoptosis activation after combining ABT-737 and doxorubicin, or cisplatin in MCS170 cells. (a and b) Caspase 3/7 activity (a) and viability (b) measured after 24 h of treatment with doxorubicin, cisplatin, or ABT-737, and the combination showing highest caspase 3/7 activity and lowest viability in MCS170 cells treated with 1 μM ABT-737 combined with 1 μM doxorubicin. Pan caspase inhibitor Z-vad-FMK inhibits all the caspase 3/7 activity and restores part of the viability reduction observed after treatment with 1 μM ABT-737 and 1 μM doxorubicin. (c) Western blot image showing PARP cleavage in cells treated with 1 μM ABT-737 and 125 nM doxorubicin, or 5 μM cisplatin.
DISCUSSION
We here present the first mesenchymal chondrosarcoma cell line; MCS170, in which we could demonstrate that inhibition of Bcl-2 family members can restore chemosensitivity. Mesenchymal chondrosarcoma is an aggressive chondrosarcoma subtype with a poor prognosis. There is an urgent need for alternative treatment strategies, as the current prognosis is very poor with reported 10-year survival rates between 27 and 67%.6, 7, 8, 9
The generation of the MCS170 cell line as a model for mesenchymal chondrosarcoma will give the opportunity to perform more preclinical research to identify possible therapeutic candidates to treat patients with mesenchymal chondrosarcoma, as well as to study the functional role of the HEY1-NCOA2 fusion. The presence of the HEY1-NCOA2 fusion gene was confirmed at the DNA level and at the RNA level. As expected in translocation driven sarcomas, a low mutational burden was found, as no additional mutations were found in 50 oncogenes or tumor suppressor genes frequently mutated in cancer.
Preclinical studies on mesenchymal chondrosarcoma are rare and only few possible therapeutic options have been described on the basis of expression analysis in small sample groups. A small study investigating a panel of different proteins in three mesenchymal chondrosarcoma cases identified PKC-α, PDGFR, Bcl-2,29 and mTOR30 as potential therapeutic targets. In a larger study including 23 cases of mesenchymal chondrosarcoma, TGF-β, Bcl-2, and Bcl-xL were identified as possible therapeutic targets in mesenchymal chondrosarcoma.23 No mutations in hot spot regions of TP53 were identified in a panel of 33 mesenchymal chondrosarcomas; however, 61.3% showed nuclear overexpression of the p53 protein.31
In this study, we focused on evaluating the role of Bcl-2 family members and conventional chemotherapy in mesenchymal chondrosarcoma. The antiapoptotic proteins Bcl-2 and Bcl-xL were found to be highly expressed in chondrosarcoma, especially in the mesenchymal subtype, suggesting an important role for these proteins in mesenchymal chondrosarcoma chemoresistance and thereby a possible novel treatment strategy.23
The MCS170 cell line was sensitive for doxorubicin, especially when cells were treated for 72 h, but resistant for cisplatin. This is in line with a recently published retrospective study including 119 patients, showing that mesenchymal chondrosarcoma patients with localized disease have reduced risk of recurrence when treated with anthracycline-based chemotherapy, suggesting that it is beneficial to add adjuvant chemotherapy to the standard treatment regimen.7 However, a recent analysis of the available literature could not confirm these data.19 Also the study of Xu et al19 did not detect a difference in overall survival when patients with localized disease were compared with patients that showed metastasis.
Bcl-2 and especially Bcl-xL were found to be highly expressed in the MCS170 cell line, which is consistent with the known high expression of these Bcl-2 family members in mesenchymal chondrosarcoma primary tumors.23 To investigate the role of Bcl-2 family members in mesenchymal chondrosarcoma, the BH3 mimetic ABT-737 was used to inhibit the antiapoptotic proteins Bcl-2, Bcl-xL, and Bcl-w in MCS170. Although ABT-737 monotherapy was ineffective, combination of ABT-737 and doxorubicin resulted in a synergistic induction of apoptosis and reduction in viability, suggesting a role for Bcl-2 family members in resistance to chemotherapy in mesenchymal chondrosarcoma.
Induction of apoptosis is important for the effectiveness of many therapies, which can be prevented by high levels of antiapoptotic proteins or downregulation of proapoptotic proteins. By directly targeting the intrinsic apoptotic pathway using BH3 mimetic ABT-737, more proapoptotic proteins will be available to activate Bak and Bax, resulting in higher efficacy of other therapies that induce apoptosis.24
The combination of Bcl-2 family protein inhibitors and chemotherapeutics has activity in vitro in a wide variety of tumors,32 including conventional as well as dedifferentiated chondrosarcoma.21, 23 Now that the MCS170 cell line is available, we had the opportunity to show this experimentally in a mesenchymal chondrosarcoma model as well, indicating that patients with mesenchymal chondrosarcoma might also benefit from this treatment combination.
ABT-263 (Navitoclax) is an orally available derivative of ABT-737 with the same working mechanism, which makes it a more attractive compound compared with ABT-737. ABT-263 is currently under evaluation in clinical trials, however, owing to side effects caused by the high Bcl-xL expression on platelets, only low doses could be administered.33 Here, we demonstrate that treatment with BH3 mimetics can sensitize mesenchymal chondrosarcoma for conventional chemotherapy, whereas single-agent administration is not effective. This raises the possiblity to administer these agents in combination, increasing the activity, while lowering toxicity in mesenchymal chondrosarcoma.
Mesenchymal chondrosarcoma is a rare aggressive chondrosarcoma subtype with a poor prognosis. In this study, we describe the first mesenchymal chondrosarcoma cell line; MCS170, containing the characteristic HEY1-NCOA2 fusion, that can be used as a model to identify new therapeutic targets. As mesenchymal chondrosarcoma is known to have high Bcl-2 and Bcl-xl expression,23 we used this cell line to functionally investigate the role of Bcl-2 family members in chemosensitivity. We demonstrate that inhibition of Bcl-2 family members restores the apoptotic machinery in mesenchymal chondrosarcoma, rendering the cells sensitive to conventional chemotherapy, especially doxorubicin.
References
Hogendoorn PCW, Bovee JVMG, Nielsen GP. Chondrosarcoma (grades I-III), including primary and secondary variants and periosteal chondrosarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. (eds). WHO Classification of Tumours of Soft Tissue and Bone. IARC: Lyon, France, 2013, pp 264–268..
Inwards C, Hogendoorn PCW. Dedifferentiated chondrosarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. (eds). WHO Classification of Tumours of Soft Tissue and Bone. IARC: Lyon, France, 2013, pp 269–270..
Nakashima Y, de Pinieux G, Ladanyi M. Mesenchymal chondrosarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. (eds). WHO Classification of Tumours of Soft Tissue and Bone. IARC: Lyon, France, 2013, pp 271–272..
McCarthy EF, Hogendoorn PCW. Clear cell chondrosarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. (eds). WHO classification of Tumours of Soft Tissue and Bone. IARC: Lyon, France, 2013, pp 273–274..
Gelderblom H, Hogendoorn PCW, Dijkstra SD et al. The clinical approach towards chondrosarcoma. Oncologist 2008;13:320–329.
Dantonello TM, Int-Veen C, Leuschner I et al. Mesenchymal chondrosarcoma of soft tissues and bone in children, adolescents, and young adults: experiences of the CWS and COSS study groups. Cancer 2008;112:2424–2431.
Frezza AM, Cesari M, Baumhoer D et al. Mesenchymal chondrosarcoma: prognostic factors and outcome in 113 patients. A European Musculoskeletal Oncology Society study. Eur J Cancer 2015;51:374–381.
Huvos AG, Rosen G, Dabska M et al. Mesenchymal chondrosarcoma. A clinicopathologic analysis of 35 patients with emphasis on treatment. Cancer 1983;51:1230–1237.
Schneiderman BA, Kliethermes SA, Nystrom LM . Survival in mesenchymal chondrosarcoma varies based on age and tumor location: a survival analysis of the SEER database. Clin Orthop Relat Res 2016; doi:10.1007/s11999-016-4779-2.
Wang L, Motoi T, Khanin R et al. Identification of a novel, recurrent HEY1-NCOA2 fusion in mesenchymal chondrosarcoma based on a genome-wide screen of exon-level expression data. Genes Chromosomes Cancer 2012;51:127–139.
Mosquera JM, Sboner A, Zhang L et al. Recurrent NCOA2 gene rearrangements in congenital/infantile spindle cell rhabdomyosarcoma. Genes Chromosomes Cancer 2013;52:538–550.
Jin Y, Moller E, Nord KH et al. Fusion of the AHRR and NCOA2 genes through a recurrent translocation t(5;8)(p15;q13) in soft tissue angiofibroma results in upregulation of aryl hydrocarbon receptor target genes. Genes Chromosomes Cancer 2012;51:510–520.
Strehl S, Nebral K, Konig M et al. ETV6-NCOA2: a novel fusion gene in acute leukemia associated with coexpression of T-lymphoid and myeloid markers and frequent NOTCH1 mutations. Clin Cancer Res 2008;14:977–983.
Carapeti M, Aguiar RC, Goldman JM et al. A novel fusion between MOZ and the nuclear receptor coactivator TIF2 in acute myeloid leukemia. Blood 1998;91:3127–3133.
Bishop MW, Somerville JM, Bahrami A et al. Mesenchymal chondrosarcoma in children and young adults: a single institution retrospective review. Sarcoma 2015;2015:608279.
Cesari M, Bertoni F, Bacchini P et al. Mesenchymal chondrosarcoma. An analysis of patients treated at a single institution. Tumori 2007;93:423–427.
Harwood AR, Krajbich JI, Fornasier VL . Mesenchymal chondrosarcoma: a report of 17 cases. Clin Orthop Relat Res 1981;158:144–148.
Shakked RJ, Geller DS, Gorlick R et al. Mesenchymal chondrosarcoma: clinicopathologic study of 20 cases. Arch Pathol Lab Med 2012;136:61–75.
Xu J, Li D, Xie L et al. Mesenchymal chondrosarcoma of bone and soft tissue: a systematic review of 107 patients in the past 20 years. PLoS One 2015;10:e0122216.
Terek RM, Schwartz GK, Devaney K et al. Chemotherapy and P-glycoprotein expression in chondrosarcoma. J Orthop Res 1998;16:585–590.
van Oosterwijk JG, Herpers B, Meijer D et al. Restoration of chemosensitivity for doxorubicin and cisplatin in chondrosarcoma in vitro: BCL-2 family members cause chemoresistance. Ann Oncol 2012;23:1617–1626.
Wyman JJ, Hornstein AM, Meitner PA et al. Multidrug resistance-1 and p-glycoprotein in human chondrosarcoma cell lines: expression correlates with decreased intracellular doxorubicin and in vitro chemoresistance. J Orthop Res 1999;17:935–940.
van Oosterwijk JG, Meijer D, van Ruler MA et al. Screening for potential targets for therapy in mesenchymal, clear cell, and dedifferentiated chondrosarcoma reveals Bcl-2 family members and TGFbeta as potential targets. Am J Pathol 2013;182:1347–1356.
Hata AN, Engelman JA, Faber AC . The BCL2 family: key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov 2015;5:475–487.
Pajor L, Szuhai K, Mehes G et al. Combined metaphase, interphase cytogenetic, and flow cytometric analysis of DNA content of pediatric acute lymphoblastic leukemia. Cytometry 1998;34:87–94.
Baranski Z, Booij TH, Cleton-Jansen AM et al. Aven-mediated checkpoint kinase control regulates proliferation and resistance to chemotherapy in conventional osteosarcoma. J Pathol 2015;236:348–359.
Greco WR, Bravo G, Parsons JC . The search for synergy: a critical review from a response surface perspective. Pharmacol Rev 1995;47:331–385.
Borisy AA, Elliott PJ, Hurst NW et al. Systematic discovery of multicomponent therapeutics. Proc Natl Acad Sci USA 2003;100:7977–7982.
Brown RE, Boyle JL . Mesenchymal chondrosarcoma: molecular characterization by a proteomic approach, with morphogenic and therapeutic implications. Ann Clin Lab Sci 2003;33:131–141.
Brown RE . Morphoproteomic portrait of the mTOR pathway in mesenchymal chondrosarcoma. Ann Clin Lab Sci 2004;34:397–399.
Park YK, Park HR, Chi SG et al. Overexpression of p53 and rare genetic mutation in mesenchymal chondrosarcoma. Oncol Rep 2000;7:1041–1047.
Cragg MS, Harris C, Strasser A et al. Unleashing the power of inhibitors of oncogenic kinases through BH3 mimetics. Nat Rev Cancer 2009;9:321–326.
Vela L, Marzo I . Bcl-2 family of proteins as drug targets for cancer chemotherapy: the long way of BH3 mimetics from bench to bedside. Curr Opin Pharmacol 2015;23:74–81.
Acknowledgements
We thank René Zwartbol, Pauline Wijers-Koster and Jolieke van Oosterwijk for technical assistance. The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 278742 (Eurosarc) and the Dutch Cancer Society (UL2010-4873).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JVMGB and YdJ received a small research grant from Servier. The remaining authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
de Jong, Y., van Maldegem, A., Marino-Enriquez, A. et al. Inhibition of Bcl-2 family members sensitizes mesenchymal chondrosarcoma to conventional chemotherapy: report on a novel mesenchymal chondrosarcoma cell line. Lab Invest 96, 1128–1137 (2016). https://doi.org/10.1038/labinvest.2016.91
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2016.91
This article is cited by
-
GABAB receptor regulates proliferation in the high-grade chondrosarcoma cell line OUMS-27 via apoptotic pathways
BMC Cancer (2018)
-
Bcl-xl as the most promising Bcl-2 family member in targeted treatment of chondrosarcoma
Oncogenesis (2018)
-
Targeting glutaminolysis in chondrosarcoma in context of the IDH1/2 mutation
British Journal of Cancer (2018)