Abstract
Whereas local microglial cells of the CNS rapidly respond to injury, little is known about the functional role of resident macrophages of the peripheral nervous system in nerve pathology. Using bone marrow chimeric rats, we recently identified individual resident endoneurial macrophages that rapidly became activated after nerve injury. However, the extent of local macrophage activation and its quantitative contribution to the total macrophage response is unknown. We now have created chimeric mice by transplanting bone marrow from green fluorescent protein (GFP)-transgenic mice into irradiated wild-type mice, allowing easy differentiation and quantification of hematogenous and resident endoneurial macrophages. After sciatic nerve crush injury, both GFP− and GFP+ resident macrophages, the latter having undergone physiological turnover from the blood before injury, rapidly underwent morphological alterations and increased in number. Proliferating GFP− and GFP+ resident macrophages were abundant and peaked 3 days after injury. A major lesion-induced influx of hematogenous macrophages with a disproportionate increase of GFP+ macrophages was not observed until Day 4. Throughout all time points examined, GFP− resident macrophages were strikingly frequent, reaching maximum numbers 9.5-fold above baseline. There was also a notable proportion of GFP− resident endoneurial macrophages phagocytosing myelin and expressing major histocompatibility complex class II. Our results demonstrate for the first time that the rapid response of resident endoneurial macrophages to nerve injury is quantitatively important and that local macrophages contribute significantly to the total endoneurial macrophage pool during Wallerian degeneration.
Similar content being viewed by others
Introduction
Macrophages play a central role in the pathogenesis of peripheral nerve diseases and during regeneration from traumatic nerve lesions (Bruck, 1997; Griffin et al, 1993; Kiefer et al, 2001). During experimental Wallerian degeneration and peripheral nerve inflammation, large numbers of hematogenous macrophages enter the nerve. Similarly, human peripheral neuropathies are associated with endoneurial macrophage differentiation and accumulation in diseased peripheral nerves (Kiefer et al, 1998). On a cellular and molecular level, macrophages phagocytose myelin, express a plethora of regulatory cytokines, secrete free radicals, provide trophic support for axon growth, and potentially act as antigen-presenting cells.
In addition to infiltrating hematogenous macrophages, a population of local, resident macrophages has long been recognized in the peripheral nerve (Arvidson, 1977; Monaco et al, 1992; Oldfors, 1980; Vass et al, 1993). The functional role of resident endoneurial macrophages in vivo remained largely undefined because no histochemical markers were available to discriminate them from hematogenous macrophages. Studies using peripheral nerve explants revealed only a limited capacity of resident endoneurial macrophages to remove myelin debris (Beuche and Friede, 1984; Bonnekoh et al, 1989). Consequently, scientific attention was focusing on macrophages immigrating from the blood rather than resident macrophages. In contrast, local microglial cells of the CNS act as highly versatile cells rapidly responding to a wide variety of pathological stimuli (Kreutzberg, 1996; Raivich et al, 1999).
To identify and investigate resident endoneurial macrophages in peripheral nerve pathology, we recently created bone marrow chimeric rats by transplanting wild-type bone marrow into lethally irradiated TK-tsa transgenic recipients (Mueller et al, 2001). In such animals, resident endoneurial macrophages are unequivocally identified by the transgene and differentiated from blood-derived, transgene-negative macrophages. We found a striking, extremely rapid response of resident endoneurial macrophages to nerve injury that was characterized by early morphological changes, proliferation, and phagocytosis, similar to the microglial response in the CNS (Mueller et al, 2001). We therefore hypothesized that resident macrophage activation might be much more important than previously thought and might add significantly to the overall macrophage response to nerve injury.
Chimeras based on TK-tsa transgene chimerism are excellent tools to study individual cells, but bulk observations and quantitative studies are difficult to realize for technical reasons (Mueller et al, 2000, 2001);. It thus remained uncertain whether activated resident endoneurial macrophages were isolated phenomena after a nerve lesion or represent a basic principle of peripheral nerve pathology similar to microglial activation in the CNS. It is also unknown how endoneurial macrophage activation relates to the influx of hematogenous macrophages in a temporospatial manner and whether the number of local endoneurial macrophages contributes significantly to the total endoneurial macrophage pool during Wallerian degeneration. To resolve these questions, we turned to green fluorescent protein (GFP)-transgenic mice (Okabe et al, 1997) as bone marrow donors to create a new mouse chimera system that would allow for the easy differentiation and quantification of GFP+ hematogenous macrophages and their local GFP− counterparts.
Results
Bone Marrow Chimeric Mice
Studies of peripheral blood smears 3 months after bone marrow transplantation revealed that 90 to 95% of all leukocytes exhibited the green fluorescence of GFP (Fig. 1C). Very occasional animals with a lesser degree of chimerism were discarded before additional experiments. All chimeric animals were healthy without evidence of autoimmune inflammation, especially neuritis, and had normal body weight. Splenic tissue from chimeric animals exhibited brightly green follicles, whereas stromal cells in the red pulp and vessels were GFP− (Fig. 1, A and D). On the contrary, chimeric animals that were prepared the opposite way, ie by transplanting wild-type bone marrow into irradiated GFP-transgenic mice, exhibited a reciprocal pattern with green fluorescence in vessels and stromal cells of the red pulp and little fluorescence in the follicles (Fig. 1, B and E). In these animals, blood smears contained only rare GFP+ leukocytes. Leukocytes and spleen cells from nonchimeric GFP-transgenic mice were always GFP+ (Fig. 1F).
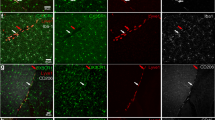
A to F, Splenic tissue from bone marrow chimeric mice and normal green fluorescent protein (GFP)-transgenic mice. Wild-type mice that received a GFP-transgenic bone marrow transplant demonstrate innumerable GFP+ hematogenous cells in spleen that are predominantly found in the follicles (A). Stromal cells in the red pulp (A) and endothelial cells (D) are GFP−. The blood smears of such animals reveal green fluorescence in leukocytes (C). In contrast, in chimeric mice that were prepared by transplanting wild-type bone marrow into GFP-transgenic mice, a reciprocal pattern is found (B and E). In normal GFP-transgenic mice, all cells exhibit green fluorescence (F). G to O, Transverse (G, L to O) and longitudinal sections (H to K) of normal and injured sciatic nerve from bone marrow chimeric mice created by transplanting bone marrow from GFP-transgenic mice into irradiated wild-type recipients. Immunohistochemistry for macrophages (CD68) shows red signals, whereas GFP exhibits green fluorescence. In normal nerves (G), both GFP− (*) and GFP+ (+) endoneurial macrophages were found as a result of physiological turnover. Twenty-four hours after sciatic nerve crush (H), only a few slightly activated GFP− (*) and GFP+ (+) endoneurial macrophages with thickened cell bodies could be observed. Two days after crush injury, very occasional endoneurial macrophages show morphological similarities with ramified microglial cells (I, arrow). Seven days after the lesion (J to L), GFP− (*) and GFP+ (+) endoneurial macrophages proximal to the lesion (J) were still in a resting state by morphological criteria. In the distal nerve segment (K and L), almost all macrophages were large, rounded cells with a phagocytic appearance. Despite the presence of many GFP+ macrophages (+), numerous activated GFP− resident macrophages (*) were still detected. Twenty-eight days after crush injury (M), activated GFP− resident endoneurial macrophages (*) could still be found together with many GFP+ macrophages (+). Some activated macrophages (*) could also be found in the sciatic nerve contralateral to the crushed nerve (N). Occasional GFP+ cells with a spindle-like morphology were detected as early as 4 days after crush injury that were negative for leukocyte markers (O, arrows). G to N counterstained with 4,6-diamidino-2-phenylindol (DAPI; blue). Scale bar: 200 μm (A and B); 150 μm (N); 100 μm (C); 75 μm (G); 50 μm (D-F); 40 μm (K and L); 30 μm (H to J, M); 20 μm (O).
We also examined sciatic nerves of chimeric mice already 2 weeks after transplantation. At this early time point, no GFP+ cells were detectable in the endoneurium, ruling out that bone marrow cells invade the peripheral nerve directly after bone marrow transplantation.
Peripheral Nerve Pathology after Crush Injury in Radiation Bone Marrow Chimeric Mice and Nonchimeric Controls
To assess possible differences of Wallerian degeneration between the two types of chimeric mice—nonchimeric GFP-transgenic mice and wild-type C57Bl6 mice—we compared myelin breakdown, axonal degeneration, and macrophage features in normal and injured nerves. Endoneurial cell counts, proliferating endoneurial cell nuclei, endoneurial macrophage numbers, and endoneurial macrophage morphology revealed no significant differences between the different groups examined. Endoneurial macrophage morphology and phagocytosis were also comparable. In summary, of the features of Wallerian degeneration after peripheral nerve injury that were analyzed, no statistically significant differences could be found between the two types of bone marrow chimeras: GFP transgenic mice and normal wild-type C57/Bl6 mice.
Macrophage Populations in the Normal Sciatic Nerve
Endoneurial macrophages were observed throughout the endoneurium (Fig. 1G). Quantitative studies revealed that they account for 6.3% of the total endoneurial cell population, well in line with previous reports in rats and mice (Mueller et al, 2001; Perry et al, 1987; Vass et al, 1993). Most of them were located in a longitudinal orientation between myelinated axons. Frequently, two or three fine processes arose at the ends of an elongated cell body stretching along the space between myelin sheets and occasionally seemed to be in contact with them. In addition, macrophages were found in a perivascular position.
Three months after bone marrow transplantation, numerous endoneurial macrophages were found that exhibited the green fluorescence of GFP whereas other macrophages did not (Fig. 1G). Quantification of GFP+ and GFP− endoneurial macrophages revealed that 55% of endoneurial macrophages were GFP+, indicating physiological turnover from the blood since bone marrow transplantation. The remaining 45% of macrophages were GFP−, ie long-term resident macrophages that had not undergone physiological turnover. These figures are in line with previous reports in rats (Mueller et al, 2001; Vass et al, 1993). There was no morphological difference between GFP+ and GFP− resident endoneurial macrophages, and we also could not observe a skewed distribution of GFP+ and GFP− macrophages within the endoneurium of normal sciatic nerves. Both macrophage populations equally expressed the macrophage markers F4/80, BM8, CD68, Iba-1 (Ito et al, 1998), and CD11b. Co-localization of the macrophage markers CD68 and Iba-1 showed that these markers detect the same cells. In addition, cross-reaction of Iba-1 with the neutrophile marker 7/4 and the lymphocyte marker CD3 was ruled out by co-localization studies. Whereas all resident endoneurial macrophages seemed to express major histocompatibility complex (MHC) class I antigens, only one fifth expressed MHC class II. Again, there was no difference between GFP+ and GFP− resident endoneurial macrophages.
Macrophage Morphology and Quantitative Changes of Endoneurial Macrophages during Wallerian Degeneration
Macrophage morphology and quantitative changes were assessed from 24 hours to 28 days after crush injury focusing on the distal segment. Twenty-four hours after crush injury, no apparent endoneurial changes were observed on cross-sections of the distal nerve segment (Fig. 2A), whereas many cells were now densely positioned around the epineurium. On longitudinal sections, occasional GFP− and GFP+ macrophages with minimal morphological signs of activation could be observed (Fig. 1H). Two days after a crush lesion, endoneurial macrophages showed a more prominent cell body, and on cross-sections, a few macrophages appeared bloated like phagocytic macrophages. Some macrophages that were observed on longitudinal sections 2 days after crush injury exhibited a ramified morphology resembling microglia (Fig. 1I). No morphological differences between GFP− and GFP+ macrophages were observed. At all later time points, most macrophages were rounded and appeared like phagocytic cells (Figs. 1, and 2, K to M), whereas macrophages proximal to the lesion remained in a resting state (Fig. 2J). In addition, occasional macrophages that remained small and elongated and resembled macrophages very early after crush injury were observed. Again, no morphological differences between the GFP− and the GFP+ macrophage population were observed at these time points. Occasional activated macrophages were also observed in the contralateral, uninjured sciatic nerve (Fig. 1N). It is interesting that as early as 4 days after crush injury and at all following time points, GFP+ cells with a spindle-shaped morphology that did not seem to express immunohistochemical markers for T-cell, granulocytes, and macrophages were found (Fig. 1 O). In addition these GFP+ cells were negative for the Schwann cell marker S100 at all time points examined (Day 4, Day 7, Day 14, Day 28). These spindle-shaped GFP+ cells did not appear in the contralateral sciatic nerve or in the uninjured nerves of bone marrow chimeric control mice. Activated macrophages that appeared in the uninjured sciatic nerve 7 days after contralateral sciatic nerve injury were another unexpected observation (Fig. 1G).
Changes of GFP− and GFP+ endoneurial macrophages at 1 (A), 2 (E), 3 (B), 7 (C and F), and 28 days (D) after a sciatic nerve crush lesion. Cross-sections of the sciatic nerve 5 mm distal to the lesion demonstrate the increase of macrophages over time and the notable presence of GFP− resident endoneurial macrophages at all time points examined. Longitudinal sections demonstrate the different macrophage dynamics in the proximal part, distal part, and the site of crush injury (E and F, arrow). Immunohistochemistry for macrophages (CD68, red signal) together with the green fluorescence of GFP. All sections were counterstained with DAPI (blue). Scale bar: 75 μm (A to D); 300 μm (E and F).
Quantitative studies (Fig. 3) revealed a steady increase of endoneurial macrophage numbers peaking at 14 days after crush lesion with a total macrophage count of 26.5 macrophages per 100 μm2 compared with 1.2 macrophages per 100 μm2 in control animals. Macrophage numbers decreased again to 17.8 per 100 μm2 on Day 28 (Fig. 3). When the numbers of GFP+- and GFP− macrophages were considered separately, both macrophage populations showed a comparable increase until Day 3. From Day 4 onward, there was still a continuous increase of GFP− endoneurial macrophages until Day 14, with peak levels of 4.9 GFP− macrophages per 100 μm2, corresponding to a 9.5-fold increase above baseline levels. However, the relative increase of GFP+ macrophages was now considerably higher, reaching 21.6 macrophages per 100 μm2 and corresponding to a 33.5-fold increase above baseline levels, indicating an additional lesion-associated influx of blood-derived macrophages. Consequently, the proportion of GFP+ and GFP− endoneurial macrophages progressively changed in favor of GFP+ macrophages. Whereas 45% of endoneurial macrophages were GFP− on Day 0 and 73% on Day 2, their proportion decreased to 18.6% on Day 14. On Day 28, a proportional decrease of both populations was found (Fig. 3).
Endoneurial macrophage numbers in control sciatic nerves and at different time points after crush injury 6 mm distal from the crush site. Total macrophage counts (black bars) were determined, and GFP− (hatched bars) and GFP+ (white bars) macrophage populations were differentiated. Data from at least three sections from three animals were collected. Statistical significance was tested for selected values by Student's t test. The increase of total macrophage numbers at Day 3 compared with controls was statistically significant (p < 0.05), whereas the shift in the ratio of GFP+ and GFP− macrophages at Day 3 compared with controls was not significant, pointing toward an approximately proportional increase of both types of macrophages. However, there was a significant shift in this ratio in favor of GFP+ macrophages compared with controls at Day 4 (p < 0.05), suggesting the additional influx of blood-derived macrophages.
Proliferation of Endoneurial Macrophage Populations
With the use of bromodeoxyuridine (BrdU) incorporation, proliferating cells were detected in the distal segment of the sciatic nerve after a crush lesion. Co-localization of BrdU+ cells with macrophage-specific antibodies and GFP allowed quantification of macrophage proliferation and the discrimination between GFP− and GFP+ proliferating macrophages (Fig. 4, A to C). Proliferating macrophages were first detectable 2 days after crush injury. Morphologically, these proliferating macrophages appeared more slender than other activated macrophages, in line with previous observations in rats (Mueller et al, 2001). No specific endoneurial distribution pattern of proliferating macrophages was obvious. Three days after crush injury, the greatest number of proliferating macrophages was found, and these macrophages were still observable after 4 and 7 days. This observation was confirmed in quantitative studies (Fig. 5). At all time points, both GFP− and GFP+ proliferating macrophages were observed. However, quantitative studies revealed that proliferation was slightly more prevalent in GFP−, long-term resident endoneurial macrophages throughout all time points (Fig. 5).
A to C, Proliferation of GFP− (*) and GFP+ (+) endoneurial macrophages 3 days after sciatic nerve injury. Semithin serial sections of methyl methacrylate-embedded tissue were used to co-localize the macrophage specific antigen Iba-1 (A; red signal), GFP (B; green signal), and incorporated BrdU (C). The tyramide amplification of the signal in C leads to a slight diffusion of the nuclear BrdU signal despite its nuclear localization. Nuclear counterstaining with DAPI (blue signal). D, Fluorescent in situ tailing (green signal) was used to detect apoptotic cells (arrows) 2 days after crush injury. Nuclear counterstaining with DAPI (blue signal). Co-localization with macrophage markers was never detectable (not shown). E and F, Myelin phagocytosis by GFP− resident endoneurial macrophages 7 days after injury on longitudinal (E, arrows) and transverse sections (F, *, inlay with arrow). Co-localization of the macrophage marker CD 68 (blue signal), myelin basic protein (red signal), and GFP (green signal). G, All GFP+ (green signal) and GFP− macrophages (Iba-1, red signal) were expressing MHC-I (blue signal), shown here 14 days after injury. H and I, In addition, a subpopulation of endoneurial macrophages (Iba-1, red signal) of either type was positive for MHC-II (blue signal) shown here 4 (H, transverse section) and 7 days (I, longitudinal section) after injury. MHC II–positive resident endoneurial macrophages are marked with arrows. Scale bar: 30 μm (A to D); 25 μm (E); 75 μm (F and G); 30 μm (H and I).
Phagocytosis and MHC Expression by Endoneurial Macrophages
The phagocytic capacity of endoneurial macrophages of either population and their ability to express MHC antigens was investigated by co-localization of macrophage antigens Iba-1 and CD68 with the myelin constituent myelin basic protein (MBP; Fig. 4, E and F) and MHC-I (Fig. 4G) and MHC-II antigens (Fig. 4, H and I) on frozen sections by double fluorescence immunohistochemistry. All endoneurial macrophages of either population expressed MHC class I in normal nerves and at all time points after nerve injury. MHC class II was expressed only by a subpopulation of macrophages, making up for one fifth of all macrophages. The amount of MHC class II expressing cells was comparable between GFP+ and GFP− macrophages and did not change after crush injury.
Phagocytosis of myelin detected by ingested MBP was observed in GFP+ and GFP− macrophages as early as 2 days after crush injury. The number of actively phagocytic macrophages strongly increased during the course of Wallerian degeneration (Fig. 4, H and I). At all time points, except at 24 hours after crush injury, resident GFP− phagocytic macrophages were found.
Apoptosis
To dissect the mechanisms of the decrease in macrophage counts of GFP+ and GFP− populations on Day 28, we determined rates of apoptosis of identified macrophages by an in situ tailing technique (Fig. 4D) and immunohistochemistry for activated caspase 3. At no time point could unequivocal apoptosis of endoneurial macrophages of either population be detected. A small number of apoptotic Schwann cells was observed 2 and 3 days after crush injury by co-localization of apoptotic nuclei and the Schwann cell marker S100 (data not shown). Occasional apoptotic cells were also found around epineurial vessels. There was no correlation between epineurial apoptosis and the time after crush injury. In contrast, numerous cells positive for DNA strand breaks detected by in situ tailing and activated caspase-3 immunohistochemistry were found in liver tissue from mice that were treated with anti-fas antibody, which served as positive controls for these assays.
Discussion
Because conventional histochemical markers are unable to distinguish resident from hematogenous macrophages in peripheral nerve in vivo, we recently had used bone marrow chimeric rats to distinguish resident endoneurial macrophages from blood-derived macrophages after a nerve lesion. Chimerism had been based on the presence of the functionally silent TK-tsa transgene in recipient but not bone marrow donor rats (Mueller et al, 2001). However, sophisticated histochemical techniques and co-localization experiments on serial semithin sections (Mueller et al, 2000) had been necessary to characterize individual cells. An overall view, as well as quantitative data, had been very difficult to obtain. In this study, we used a novel mouse chimera system based on the transplantation of bone marrow from GFP-transgenic mice into wild-type recipients. GFP fluorescence is readily detected on frozen and MMA-embedded sections, and fluorescent cells are easily viewed and quantified already at low-power magnification. Similar systems have recently been used for cell tracking in the CNS and stem cell research (Blau et al, 2001; Priller et al, 2001). In normal peripheral nerves, earlier studies in chimeric rats had revealed that resident endoneurial macrophages undergo a slow and incomplete physiological turnover that was calculated to amount to 60% after 3 months (Vass et al, 1993). These results were confirmed in our present work in mice, pointing toward a phenomenon conserved between different species. Resident endoneurial macrophages in themselves are potentially heterogeneous, consisting of a long-term resident population without turnover from the blood and a more mobile local macrophage population undergoing physiological turnover. As in previous studies, we used chimeric mice 3 months after bone marrow transplantation to allow for mature chimerism. This was necessary to avoid any direct influence of the procedure upon the cellular response to nerve injury. Indeed, we found no differences between chimeric mice and controls that might point toward skewing of the results by irradiation and bone marrow transplantation. Because approximately half of all resident macrophages underwent physiological turnover in the interval after bone marrow transplantation, three different macrophage populations can theoretically be recognized in the lesioned peripheral nerve in our model: (a) hematogenous macrophages attracted by the lesion, (b) long-term resident macrophages, and (c) resident macrophages that recently had undergone physiological turnover from the blood. Long-term resident endoneurial macrophages were unequivocally identified in this system and were differentiated from hematogenous macrophages by the absence of transgenic GFP expression. However, resident endoneurial macrophages recently immigrated by physiological turnover carry the GFP transgene despite residing in the nerve before injury. They thus cannot be differentiated from lesion-induced infiltrating macrophages on the basis of GFP fluorescence. GFP+ macrophages are not necessarily lesion-attracted infiltrating macrophages but may be derived from resident endoneurial macrophages that had undergone turnover before nerve injury.
The immunohistochemical marker profile of resting endoneurial macrophages bears similarities with perivascular macrophages of the CNS but is slightly different from resident microglial cells. Microglia usually do not express MHC antigens in the normal rodent brain (Kreutzberg, 1996; Raivich et al, 1999). There was no difference in the marker profile between GFP+ and GFP− resident macrophages that might suggest a difference between the cells similar to that between perivascular macrophages and microglial cells of the brain.
After sciatic nerve injury, both GFP+ and GFP− resident endoneurial macrophages rapidly became activated distal to the lesion, retracted their processes, and rounded up. Quantitative studies distal to the lesion revealed an approximately proportional increase of both GFP+ and GFP− macrophages during the first days, correlating with the presence of numerous proliferating macrophages of either type that peaked 3 days after injury. These data reveal a remarkable similarity with lesion-induced microglial proliferation in the brain (Graeber et al, 1988; Raivich et al, 1998). After facial nerve injury, microglial proliferation in the facial nucleus exhibits a time course similar to macrophage proliferation in the sciatic nerve, and microglial numbers increase by about the same degree as resident macrophages do in our system. Similar findings were obtained from brain stab injury experiments (Amat et al, 1996). The proportional increase of GFP− and GFP+ endoneurial macrophages until Day 3 after injury and the detection of proliferating macrophages of either type argue that the early increase of GFP+ macrophages is largely derived from resident GFP+ endoneurial macrophages rather than from lesion-attracted infiltrating hematogenous macrophages. However, the number of proliferating GFP+ macrophages is lower than that of GFP− endoneurial macrophages and does not increase with the invasion of hematogenous macrophages, thus possibly pointing toward a reduced versatility of macrophages recently immigrated from the blood.
From Day 4 on—and peaking at 14 days—a disproportionate increase of GFP+ macrophages occurred, pointing toward a massive influx of lesion-attracted hematogenous macrophages. This finding is consistent with previous data on total macrophage counts (Taskinen and Roytta, 2000). Nevertheless, GFP− resident endoneurial macrophages remained strikingly frequent, composing nearly 20% of the total endoneurial macrophage population still on Day 14. Furthermore, their absolute numbers increased to nearly 10-fold above baseline on Day 14, and proliferating GFP− resident macrophages were continuously observed. Because lesion-attracted infiltrating macrophages are considered to be highly differentiated cells without proliferating capacity, GFP+ proliferating macrophages observed at the various time points are likely to be derived from resident GFP+ macrophages and not from infiltrating hematogenous macrophages. In our system, the proportion of resident endoneurial macrophages is thus underestimated when only GFP− endoneurial macrophages are considered. Because GFP+ resident endoneurial macrophages compose approximately 50% of the entire resident macrophage population before injury, the proportion of endoneurial macrophages derived from resident macrophages 14 days after injury may be even higher.
After Day 14, the numbers of both GFP+ and GFP− endoneurial macrophages decreased proportionately, suggesting similar mechanisms of elimination. We found no significant apoptosis of macrophages despite adequate technical controls. These data are in slight contrast to previous work in which small numbers of apoptotic macrophages after sciatic nerve transection were described (Kuhlmann et al, 2001). It is conceivable that the choice of the model, crush injury versus nerve transection, might account for the difference. Recent transplantation studies demonstrated the ability of endoneurial macrophages to leave the peripheral nerve and migrate to the spleen (Kuhlmann et al, 2001). Thus, migration rather than apoptosis may account for the reduction in endoneurial macrophage numbers late after nerve injury.
We also observed numerous GFP− and GFP+ resident endoneurial macrophages early after injury phagocytosing MBP and expressing MHC class II antigen. This finding confirmed and extended our previous observations in rats (Mueller et al, 2001). Because MHC expression is a prerequisite of antigen presentation, resident endoneurial macrophages may potentially act as antigen-presenting cells in an immune situation. Furthermore, we observed many MHC+ GFP− macrophages that retained a slim and elongated morphology even at advanced stages of Wallerian degeneration. Studies on resident liver macrophages demonstrated that activation by morphological criteria may not correspond with functional activation, including cytokine production. The latter may well occur in morphologically silent macrophages (Laskin et al, 2001). It will therefore be interesting to see whether similar concepts might apply to resident endoneurial macrophages. The capacity of endoneurial macrophages to phagocytose myelin was previously observed in explant cultures of sciatic nerves (Bonnekoh et al, 1989). However, efficient myelin removal was not achieved until peritoneal macrophages were allowed to enter the nerve. Peripheral macrophage depletion in vivo also leads to inefficient myelin phagocytosis during Wallerian degeneration (Beuche and Friede, 1986; Bruck et al, 1996). Others found only a minor influence of macrophage depletion on myelin breakdown (Perry et al, 1995), a finding that was attributed to a major role of Schwann cells in myelin degradation. Although these data were used to suggest only a minor role for resident endoneurial macrophages, one should remember that maximal damage was inflicted to the peripheral nerve in studies of complete nerve crush or transection. In the brain, traumatic injury and large ischemic lesions also cause a massive macrophage influx, obscuring the microglial reaction that is more easily observed after minor lesions (Kreutzberg, 1996). Our present data point toward a sizable local macrophage response to nerve injury that might well suffice to deal with minor or prolonged insults as seen in chronic neuropathies.
An interesting finding was the detection of GFP+ cells that were negative for macrophage markers. These cells had a spindle-like morphology, suggesting that hematogenous cells other than macrophages are involved in the degenerative and regenerative processes after peripheral nerve trauma. The co-localization with the Schwann cell marker S100 revealed no GFP+ Schwann cells until 28 days after nerve injury, which does not rule out that these cells differentiate into Schwann cells at a later time point. These cells are yet to be identified and characterized.
Taken together, our data indicate that resident endoneurial macrophages not only undergo an extremely rapid response to nerve injury but also represent a quantitatively significant subpopulation of macrophages in the lesioned sciatic nerve. The lesion-induced endoneurial macrophage response is thus not generated exclusively by macrophage influx but rather is the result of joint actions of resident and hematogenous macrophages. Many of the properties of resident endoneurial macrophages, including MHC expression, proliferation, and phagocytosis, and the temporal profile of their activation closely resemble properties of activated microglial cells. Their role in the cellular response to nerve injury thus may be of greater importance than previously thought.
Materials and Methods
Radiation Bone Marrow Chimeric Mice
All animal experiments were approved by the veterinary office at the Bezirksregierung, Münster, Germany. GFP transgenic mice on a C57/Bl6 genetic background were used (Okabe et al, 1997). Wild-type C57/Bl6 mice were obtained from Charles River (Sulzfeld, Germany). Bone marrow chimeric mice were created as described previously (Hickey and Kimura, 1988; Mueller et al, 2001). In brief, wild-type C57/Bl6 mice were lethally irradiated with 5 Gray. Subsequently, donor bone marrow cells were prepared from the long bones of GFP+ mice, and 2 × 107 cells were transplanted by injection into the tail vein. For control purposes, additional chimeric mice were created by transplanting wild-type bone marrow into GFP− transgenic mice. Unless otherwise stated, all experiments were performed in chimeric mice where wild-type C57/Bl6 mice served as recipients and GFP-transgenic mice as bone marrow donors. Mice were allowed to recover for 3 months to establish mature chimerism before additional experiments were performed.
For controlling the effectiveness of bone marrow chimerism, blood samples were collected from each animal before additional experiments. Blood smears were prepared, and the proportion of GFP+ leukocytes was determined by fluorescence microscopy. To rule out that GFP+ bone marrow cells invade the peripheral nerve directly after bone marrow transplantation, we examined sciatic nerves of bone marrow chimeric mice carrying GFP+ bone marrow as early as 2 weeks after bone marrow transplantation (n = 3).
Sciatic Nerve Injury and Tissue Processing
The right sciatic nerve was exposed under anesthesia with di-bromo-ethanol and crushed distal to the sciatic notch in a standardized way for 10 seconds with a fine forceps. Groups of three mice were allowed to survive for 1 day, 2 days, 3 days, 4 days, 7 days, 14 days, and 28 days after crush injury. There were 26 controls: 6 unoperated bone marrow chimeric mice that were examined 3 months (n = 3) and 2 weeks (n = 3) after bone marrow transplantation; 2 operated wild-type mice for each of the above time points; 3 operated GFP-transgenic mice investigated 3 days, 7 days, and 28 days after crush injury; and 3 operated chimeric mice created by transplanting wild-type bone marrow into GFP-transgenic recipients, investigated 3 days, 7 days, and 28 days after crush injury.
At the time that was predetermined for killing the mice, a 6% hydroxyethyl-starch solution (HAES steril; Fresenius, Bad Homburg, Germany) was perfused for 1 minute through the left ventricle while the mice were under deep ether anesthesia, followed by 2% phosphate-buffered paraformaldehyde at pH 7.4 for 10 minutes. Two hours earlier, all animals had received 75 mg/kg BrdU (Sigma, Deisenhofen, Germany) intraperitoneally to mark proliferating cells. Unoperated and operated sciatic nerves, spleen, thymus, and liver were removed and postfixed in the same fixative for an additional 3 hours at 4°C.
For frozen sections, tissues were embedded in Tissue-tek (Sakura Finetek, Zoeterwoude, The Netherlands) and snap-frozen in isopentane cooled in liquid nitrogen. Seven-micrometer-thick cryosections were made on a Leica CM3050 cryotome (Leica, Bensheim, Germany) and mounted on coated slides (Superfrost Plus; Langenbrinck, Emmendingen, Germany). From crushed sciatic nerves, both longitudinal and transverse sections were prepared. All transverse sections were taken approximately 5 mm distal to the crush site. In addition, sciatic nerve tissue was embedded in methyl methacrylate as described previously (Mueller et al, 2001; Muller et al, 2000). Series of 0.5-μm transverse serial sections were cut on a Reichert-Jung ultracut ultramicrotome, transferred onto coated glass slides, and dried at 35°C for 2 hours.
Immunohistochemical Procedures
Fluorescence immunohistochemistry on frozen sections was performed using standard protocols described previously (Mueller et al, 2000). In brief, primary antibodies were diluted, incubated, and detected as summarized in Table 1. Biotinylated secondary antibodies were used followed by a fluorescent signal detection with A594-streptavidin (Molecular Probes, Leiden, Netherlands) (Panchuk-Voloshina et al, 1999). Triple stainings to detect GFP, macrophage markers, and an additional antigen were performed with tyramide amplification followed by detection with A350-streptavidin (Molecular Probes) (Mueller et al, 2000). Methyl methacrylate–embedded sections were also stained as described previously (Mueller et al, 2000). In brief, sections were deplasticized in acetone, and antigens were retrieved using microwave irradiation for 15 to 30 minutes dependent on the primary antibody (Table 1). Fluorescence immunohistochemistry was performed the same way as for frozen tissue, with an additional amplification step using tyramide as indicated in Table 1. Nuclear counterstaining was achieved on both frozen and methylmethacrylate semithin sections by using a fluorescence saving mounting medium containing 4,6-diamidino-2-phenylindol (Vector Laboratories, Burlingame, California).
Detection of Apoptotic Cells
Apoptotic cells were detected by in situ tailing (Roche, Mannheim, Germany) and by an antibody directed against activated caspase-3 (BD Biosciences, Hamburg, Germany) on methyl methacrylate–embedded tissue. In brief, incubation of de-embedded sections with terminal deoxynucleotide transferase and a nucleotide mixture containing fluorescein labeled UTP for 60 minutes at 37°C labeled DNA strand breaks in apoptotic cells. Specificity of detection of apoptotic cells was controlled at the same time points by the detection of activated caspase-3, which correlated well with the staining of the in situ tailing. Liver tissue from mice that had received antibodies directed against the fas-ligand to induce apoptosis served as positive controls. Liver tissue from healthy mice served as negative controls.
Image Analysis
Sections were examined under a Leica DM fluorescence microscope, and images were digitized and transferred to a PC using a Diagnostic Instruments SPOT II camera system (Visitron, München, Germany). Merging of fluorescent signals was done by SPOT II software. For quantification of different macrophage populations, cell nuclei, GFP+ cells, proliferating cells, and macrophages were counted using Analysis 3.1 Software (SIS, Münster, Germany). Analysis 3.1 was also used to determine the endoneurial area under study. Serial sections of methyl methacrylate–embedded sciatic nerve allowed for the detection and characterization of proliferating macrophages by co-localizing the macrophage antigens Iba-1 and CD68, BrdU, and S100. Only proliferating cells that were positive for a macrophage marker and negative for the Schwann cell marker S100 were accepted as proliferating macrophages and were counted.
Statistics
Statistical analysis was performed by using Student's t test or Mann-Whitney U test, when appropriate. P < 0.05 was considered statistically significant.
References
Amat JA, Ishiguro H, Nakamura K, and Norton WT (1996). Phenotypic diversity and kinetics of proliferating microglia and astrocytes following cortical stab wounds. Glia 16: 368–382.
Arvidson B (1977). Cellular uptake of exogenous horseradish peroxidase in mouse peripheral nerve. Acta Neuropathol (Berl) 37: 35–41.
Beuche W and Friede RL (1984). The role of non-resident cells in Wallerian degeneration. J Neurocytol 13: 767–796.
Beuche W and Friede RL (1986). Myelin phagocytosis in Wallerian degeneration of peripheral nerves depends on silica-sensitive, bg/bg-negative and Fc-positive monocytes. Brain Res 378: 97–106.
Blau HM, Brazelton TR, and Weimann JM (2001). The evolving concept of a stem cell: Entity or function? Cell 105: 829–841.
Bonnekoh PG, Scheidt P, and Friede RL (1989). Myelin phagocytosis by peritoneal macrophages in organ cultures of mouse peripheral nerve. A new model for studying myelin phagocytosis in vitro. J Neuropathol Exp Neurol 48: 140–153.
Bruck W (1997). The role of macrophages in Wallerian degeneration. Brain Pathol 7: 741–752.
Bruck W, Huitinga I, and Dijkstra CD (1996). Liposome-mediated monocyte depletion during wallerian degeneration defines the role of hematogenous phagocytes in myelin removal. J Neurosci Res 46: 477–484.
Graeber MB, Tetzlaff W, Streit WJ, and Kreutzberg GW (1988). Microglial cells but not astrocytes undergo mitosis following rat facial nerve axotomy. Neurosci Lett 85: 317–321.
Griffin JW, George R, and Ho T (1993). Macrophage systems in peripheral nerves. A review. J Neuropathol Exp Neurol 52: 553–560.
Hickey WF and Kimura H (1988). Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science 239: 290–292.
Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, and Kohsaka S (1998). Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res 57: 1–9.
Kiefer R, Kieseier BC, Bruck W, Hartung HP, and Toyka KV (1998). Macrophage differentiation antigens in acute and chronic autoimmune polyneuropathies. Brain 121: 469–479 (published erratum appears in Brain (1998) 121:1190).
Kiefer R, Kieseier BC, Stoll G, and Hartung H (2001). The role of macrophages in immune-mediated damage to the peripheral nervous system. Prog Neurobiol 64: 109–127.
Kreutzberg GW (1996). Microglia: A sensor for pathological events in the CNS. Trends Neurosci 19: 312–318.
Kuhlmann T, Bitsch A, Stadelmann C, Siebert H, and Bruck W (2001). Macrophages are eliminated from the injured peripheral nerve via local apoptosis and circulation to regional lymph nodes and the spleen. J Neurosci 21: 3401–3408.
Laskin DL, Weinberger B, and Laskin JD (2001). Functional heterogeneity in liver and lung macrophages. J Leukoc Biol 70: 163–170.
Monaco S, Gehrmann J, Raivich G, and Kreutzberg GW (1992). MHC-positive, ramified macrophages in the normal and injured rat peripheral nervous system. J Neurocytol 21: 623–634.
Mueller M, Wacker K, Hickey WF, Ringelstein EB, and Kiefer R (2000). Co-Localization of multiple antigens and specific DNA: A novel method using methyl methacrylate-embedded semithin serial sections and catalyzed reporter deposition. Am J Pathol 157: 1829–1838.
Mueller M, Wacker K, Ringelstein EB, Hickey WF, Imai Y, and Kiefer R (2001). Rapid response of identified resident endoneurial macrophages to nerve injury. Am J Pathol 159: 2187–2197.
Muller DM, Pender MP, and Greer JM (2000). A neuropathological analysis of experimental autoimmune encephalomyelitis with predominant brain stem and cerebellar involvement and differences between active and passive induction. Acta Neuropathol (Berl) 100: 174–182.
Okabe M, Ikawa M, Kominami K, Nakanishi T, and Nishimune Y (1997). ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett 407: 313–319.
Oldfors A (1980). Macrophages in peripheral nerves. An ultrastructural and enzyme histochemical study on rats. Acta Neuropathol 49: 43–49.
Panchuk-Voloshina N, Haugland RP, Bishop-Stewart J, Bhalgat MK, Millard PJ, Mao F, and Leung WY (1999). Alexa dyes, a series of new fluorescent dyes that yield exceptionally bright, photostable conjugates. J Histochem Cytochem 47: 1179–1188.
Perry VH, Brown MC, and Gordon S (1987). The macrophage response to central and peripheral nerve injury. A possible role for macrophages in regeneration. J Exp Med 165: 1218–1223.
Perry VH, Tsao JW, Fearn S, and Brown MC (1995). Radiation-induced reductions in macrophage recruitment have only slight effects on myelin degeneration in sectioned peripheral nerves of mice. Eur J Neurosci 7: 271–280.
Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, Fernandez-Klett F, Prass K, Bechmann I, de Boer BA, Frotscher M, Kreutzberg GW, Persons DA, and Dirnagl U (2001). Targeting gene-modified hematopoietic cells to the central nervous system: Use of green fluorescent protein uncovers microglial engraftment. Nat Med 7: 1356–1361.
Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, and Kreutzberg GW (1999). Neuroglial activation repertoire in the injured brain: Graded response, molecular mechanisms and cues to physiological function. Brain Res Brain Res Rev 30: 77–105.
Raivich G, Haas S, Werner A, Klein MA, Kloss C, and Kreutzberg GW (1998). Regulation of MCSF receptors on microglia in the normal and injured mouse central nervous system: A quantitative immunofluorescence study using confocal laser microscopy. J Comp Neurol 395: 342–358.
Taskinen HS and Roytta M (2000). Increased expression of chemokines (MCP-1, MIP-1alpha, RANTES) after peripheral nerve transection. J Peripher Nerv Syst 5: 75–81.
Vass K, Hickey WF, Schmidt RE, and Lassmann H (1993). Bone marrow-derived elements in the peripheral nervous system. An immunohistochemical and ultrastructural investigation in chimeric rats. Lab Invest 69: 275–282.
Acknowledgements
We thank Antje Stöber and Margret Lindermann for excellent technical support and Dr. Imai, Tokyo, Japan, for providing us with the Iba-1 antibody.
This work was supported by the Deutsche Forschungsgemeinschaft (Ki 532/3-2 and -3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mueller, M., Leonhard, C., Wacker, K. et al. Macrophage Response to Peripheral Nerve Injury: The Quantitative Contribution of Resident and Hematogenous Macrophages. Lab Invest 83, 175–185 (2003). https://doi.org/10.1097/01.LAB.0000056993.28149.BF
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1097/01.LAB.0000056993.28149.BF
This article is cited by
-
Delay modulates the immune response to nerve repair
npj Regenerative Medicine (2023)
-
Effect of Azithromycin on Sciatic Nerve Injury in the Wistar Rats
Neurochemical Research (2023)
-
The primary macrophage chemokine, CCL2, is not necessary after a peripheral nerve injury for macrophage recruitment and activation or for conditioning lesion enhanced peripheral regeneration
Journal of Neuroinflammation (2022)
-
LXR agonist improves peripheral neuropathy and modifies PNS immune cells in aged mice
Journal of Neuroinflammation (2022)
-
The macrophage: a key player in the pathophysiology of peripheral neuropathies
Journal of Neuroinflammation (2022)