Abstract
The complex balance between the pro-activating and regulatory influences of the complement system can affect the pathogenesis of immune complex-mediated glomerulonephritis (ICGN). Key complement regulatory proteins include decay accelerating factor (DAF) and CD59, which inhibit C3 activation and C5b-9 generation, respectively. Both are glycosylphosphatidylinositol-linked cell membrane proteins, which are widely distributed in humans and mice. Chronic serum sickness induced by daily immunization with horse spleen apoferritin over 6 weeks was used to induce ICGN in DAF-, CD59- and DAF/CD59-deficient mice, with wild-type littermate mice serving as controls. Both DAF and DAF/CD59-deficient mice had an increased incidence of GN relative to wild-type controls associated with significantly increased glomerular C3 deposition. Disease expression in CD59-deficient mice was no different than wild-type controls. DAF- and DAF/CD59-deficient mice also had increased monocyte chemoattractant protein-1 mRNA expression and glomerular infiltration with CD45+ leukocytes. Our findings suggest that activation of C3 is strongly associated with experimental ICGN while downstream formation of C5b-9 is of lesser pathogenic importance in this model.
Similar content being viewed by others
Main
The complement system is an important part of innate immunity. The classical, alternative and lectin complement pathways have unique and shared components. Although the activating influences on each pathway is different, the end result of cleavage and activation of C3 and C5, and the non-enzymatic assembly of the C5b-9 membrane attack complex are equivalent.1 Given the potency of the complement system, its activation has the potential to cause unwanted inflammation on host tissue. Therefore, natural inhibitors are essential and are present throughout the three cascades.1, 2 Decay accelerating factor (DAF, CD55) and CD59 are widely expressed glycosylphosphatidylinositol (GPI)-anchored membrane complement regulatory proteins. DAF prevents C3 and C5 activation through all complement pathways by inhibiting formation and accelerating decay of C3/C5 convertases.3, 4 CD59 is the only cellular inhibitor of C5b-9 formation, which it accomplishes by blocking the interaction of C8 with C9.5
Complement activation clearly occurs in immune complex (IC)-mediated diseases in humans, including in the glomerulus in lupus nephritis, membanoproliferative glomerulonephritis (GN), postinfectious GN and membranous nephropathy.6 Horse spleen apoferritin-induced chronic serum sickness (CSS) is a model of human proliferative GN, characterized by glomerular IC deposition, hypercellularity and matrix expansion.7, 8 IC-mediated GN in this model clearly has a dependence on complement activation.9, 10, 11, 12 To dissect the roles of early and late complement activation cascades in this disease model, we focused on their relevant complement regulators, DAF and CD59. CSS was induced in DAF- and CD59-deficient mice, as well as mice doubly deficient in both. DAF deficiency led to a significantly increased incidence of proliferative GN, while CD59 deficiency did not affect disease development, supporting the relevance of complement activation products C3a, C3b and/or C5a, but not C5b-9, in the pathogenesis of immune complex-mediated glomerulonephritis (ICGN).
Materials and methods
Protocols for Animal Work
Mice with targeted deletions of DAF1 (DAF KO) and CD59a (CD59 KO) were generated and kindly provided by Dr Wenchao Song (University of Pennsylvania).13, 14 These mice were originally on a mixed C57BL/6 and 129 background, and were backcrossed onto the Balb/c strain for at least 10 generations. Homozygous DAF KO and CD59 KO mice were generated by intercrossing heterozygous mice and screened by flow cytometry as described.13, 15 Double knockout (DKO) mice were generated by intercrossing DAF- and CD59-deficient mice. Both male and female mice at 10 to 12 weeks old were included in this study.
ICGN was induced in DAF KO (n=14), CD59 KO (n=10) and DAF-CD59 DKO (n=11) mice with a daily peritoneal injection of 4 mg of horse spleen apoferritin (Calzyme Laboratories, Inc. San Luis Obispo, CA, USA) for 6 weeks. Identically treated littermate wild-type Balb/c mice (n=10) were studied contemporaneously as controls. Serum and urine samples were collected after 3 and 6 weeks of apoferritin immunization. The study was terminated at 6 weeks, at which time all mice were sacrificed and the kidneys were harvested for further studies. These animal experiments were approved by the University of Chicago Animal Care and Use Committee.
Measurements of BUN and Urinary Albumin
Blood Urea Nitrogen (BUN) concentrations were detected with a Beckman Autoanalyzer (Beckman Coulter, Fullerton, CA, USA). Urinary creatinine concentrations were measured using a commercially obtained kit according to manufacturer's instructions (Stanbio Creatinine Procedure No. 0400, Stanbio Laboratory, Boerne, TX, USA). Urinary albumin concentrations were measured with a mouse albumin ELISA kit (Bethyl Laboratories, Montgomery, TX, USA) as described previously.11 Urinary albumin excretion was expressed as the ratio of urinary albumin to creatinine concentrations (μg/mg).
Measurements from Tissue
For light microscopy, kidney tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Four μm sections stained with periodic acid Schiff were examined by a renal pathologist (MH), who was blinded to the grouping of each section. For each slide, the presence of GN was determined based on the development of diffuse hypercellularity and matrix expansion in the mesangial area.
For immunofluorescence (IF) microscopy, tissues were snap frozen in 2-methylbutane in a container on dry ice. Four micrometer cryostat sections were processed for direct IF. Sections were fixed in ether–ethanol and stained with fluorescein isothiocyanate-conjugated antibodies (Abs) to mouse C3, IgG, IgA (Cappel Pharmaceuticals Inc., Aurora, OH, USA), and IgM (Sigma, Saint Loius, MO, USA). The staining intensity was semi-quantitatively scored from 0 to 4 by an observer blinded to the origin of the specimens as detailed previously.11
For immunohistochemistry, 4% paraformaldehyde-fixed and paraffin-embedded kidney sections were used. Slides were heated for 10 min in a microwave oven in pH 6.0 citrate buffer to retrieve antigen. Endogenous peroxidases and biotin were blocked with 0.3% H2O2 and Avidin/Biotin Blocking Kit (Vector Laboratories, Burlingame, CA, USA). Normal mouse serum (10% vol/vol) was also used as a separate blocking step. The slides were then incubated with rat anti-mouse CD45 (Cedarlane Laboratories, Ontario, Canada), followed by mouse anti-rat IgG2b (BD Biosciences Pharmingen, San Jose, CA, USA) and streptavidin-peroxidase (Sigma). Specifically bound Abs were detected by DAB Substrate Kit (Vector). To quantify renal leukocyte infiltration in each mouse kidney section, at least 20 glomeruli or 20 high-power fields (hpf, × 400) were examined in a blinded manner as described previously.16
In some instances, staining for proliferating cell nuclear antigen (PCNA) was performed in slides stained with anti-mouse CD45 as described above. Following DAB development, the peroxidases and biotin in the first staining were blocked with 0.3% H2O2 and Avidin/Biotin Blocking Kit. Then the slides were incubated with rabbit anti-PCNA (Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by biotin-conjugated anti rabbit IgG (Vector) and streptavidin-peroxidase (Sigma). Specific PCNA staining was then detected with the VIP Substrate Kit (Vector), followed by Methyl-green counter-staining (Vector). By this protocol, PCNA and CD45 staining were indicated by purple nuclear staining and brown cellular staining, respectively. PCNA+ cells were enumerated from at least 20 glomeruli per section in a blinded manner as described above.
Quantitative RT-PCR
Total RNA from renal cortex was extracted and cDNA produced as described previously.17 Quantitative RT-PCR (qRT-PCR) was performed with the QuantiTect SYBR Green RT-PCR Kit (Qiagen Inc., Valencia, CA, USA). Expression data were normalized to 18S RNA measured contemporaneously from the same samples. The primers used are provided in Table 1.
Anti-apoferritin ELISA
The development of anti-apoferritin Abs was confirmed by ELISA as previously described.11 Briefly, polystyrene plates were coated with 5 μg/ml of apoferritin. After blocking with 1% BSA, plates were incubated with serial dilutions of serum samples or anti-horse ferritin Ab (Jackson ImmunoResearch Labs, West Grove, PA, USA), followed by horseradish peroxidase-conjugated anti-mouse IgG (Kierkegaard and Perry Laboratories) and o-phenylenediamine peroxidase substrate (Sigma-Aldrich, Saint Loius, MO, USA). The data were presented as arbitrary units relative to a standard curve generated with anti-horse ferritin Abs.
Statistical Analyses
All data are expressed as means±s.e.m. and were analyzed using Minitab software (State College, PA, USA). One-way analysis of variance followed by Tukey's pairwise comparisons was used for the measurements and χ2 analysis was used to analyze the incidence of disease among the groups. Potential correlations among variables were examined by Pearson product moment correlation coefficient.
Results
GN Occurs in DAF-Deficient Mice with CSS
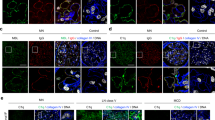
CSS was induced in DAF KO, CD59 KO, DAF-CD59 DKO mice, as well as littermate wild-type controls. After 6 weeks of immunizing with horse spleen apoferritin, all four groups of animals developed comparable levels of anti-apoferritin Abs (1.10±0.17, 0.68±0.17 0.69±0.08 and 0.79±0.17 U/ml in DAF KO, CD59 KO, DKO and wild-type groups, respectively). Along with the generation of circulating ICs in this model,11 all animals developed glomerular IC deposits consisting of IgG, IgM, IgA and C3, which were primarily in a mesangial distribution with extension to the peripheral capillary wall. DAF deficiency led to significantly increased C3 glomerular deposition (Figures 1a–d and 2). The semi-quantitative IF C3 scores were 3.1±0.1 and 3.2±0.2 in DAF KO and DKO groups, respectively, compared to 2.8±0.2 and 2.1±0.2 in CD59 KO and wild-type groups, respectively (P< 0.01, DAF KO and DKO vs wild-type control). Deficiency of DAF and/or CD59 did not affect IC deposition in the kidney as shown by comparable staining intensity for IgG, IgA and IgM in all groups (Figure 2).
Complement activation and histopathological features of ICGN. Representative IF micrographs show C3 activation in glomeruli of wild-type (a) and CD59 KO (b) mice with ICGN, that was significantly increased in DAF KO (c) and DKO (d) animals. DAF KO and DKO mice also developed mesangial proliferative GN (f) compared to the normal morphology in wild-type and CD59 KO mouse kidneys (e). Magnification, × 400.
The mice lacking DAF complement regulatory activity developed proliferative GN characterized by mesangial hypercellularity and extracellular matrix expansion (Figure 1f), not seen in mice with intact DAF (Figure 1e). Overall, 5 of 14 (35.7%) DAF KO and 5 of 11 (45.5%) DKO mice developed GN, respectively, compared with 0 of 10 CD59 KO and 1 of 10 wild-type mice (P=0.047). Thus, 40% of DAF-deficient mice developed GN compared to 5% of mice with intact DAF, irrespective of the presence or absence of CD59 (P=0.007).
Along with the deposition of ICs and complement activation products, mice with CSS also developed albuminuria after 3 weeks of apoferritin immunization which tended to increase at the 6 week time period (Figure 3). Consistent with past studies in this model, the albuminuria was relatively mild.10, 11 At each time point, DAF KO and DKO mice tended to have higher urinary albumin excretion, although the differences among the four groups did not reach statistical significance. All BUN levels obtained at 3 and 6 weeks of CSS were within the normal range (data not shown), indicating that the ICGN induced in this model did not appear to affect glomerular filtration.
Measurement of Inflammatory Cells and Mediators
Given the importance of inflammatory cells and their mediators in the development of ICGN, and the potential relevance of complement activation to both, their presence was sought in renal tissue. CD45+ leukocytes were present in glomeruli (Figure 4a) and the interstitium (Figure 4b) of mice with CSS. In DAF KO and DKO mice there were approximately double the numbers of glomerular CD45+ leukocytes compared to CD59 KO and wild-type mice with intact DAF (Figure 4c). In contrast, interstitial CD45+ leukocytes were equivalent among the groups (Figure 4d). Glomerular leukocyte numbers significantly correlated with GN (r=0.67, P<0.001) and C3 staining scores (r=0.43, P=0.003). The former is not unexpected, given that increased cellularity was used as criteria for GN, while the latter is consistent with the premise that activated complement at C3 and beyond mediates leukocyte infiltration.
CD45+ leukocyte infiltration in kidneys of mice with ICGN. Leukocytes were present both in glomeruli (a) and the interstitium (b) of all mice (shown are representative micrographs from a DAF KO mouse). DAF KO and DKO mice had increased CD45+ cells in glomeruli but not the interstitium compared to CD59 KO and wild-type mice (c). *P<0.05, DAF vs wild-type and CD59 KO groups. #P<0.05, DKO vs wild-type. Magnification (a and b), × 400.
To provide insight into how complement activation might affect inflammatory cell recruitment in this model, we measured mRNA expression of RANTES, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-2, and interleukin-1β, which are cytokines/chemokines shown to be relevant in ICGN.16, 18, 19 In addition, receptors for the complement anaphylatoxins C3a and C5a were evaluated. Among these, only MCP-1 was significantly upregulated in the DAF-deficient mouse groups relative to CD59 KO and wild-type mice (Figure 5). MCP-1 mRNA correlated significantly with the numbers of glomerular (but not interstitial) leukocytes (r=0.562, P<0.001), supporting it may play a role in their recruitment to glomeruli in CSS.
In addition to extrinsic cells infiltrating glomeruli, intrinsic glomerular cells can be stimulated to proliferate in disease situations. To examine this, staining was performed for PCNA as a marker for cells undergoing DNA replication. In all groups of animals with CSS, there were cells within glomeruli which stained positively for PCNA; these were typically within mesangial regions and were invariably negative for CD45 (Figure 6a). In DAF and DKO mice with CSS, there were significantly increased numbers of PCNA+ glomerular cells (Figure 6b). Thus, CSS in DAF-deficient mice led to increased infiltration with extrinsic CD45+ cells and proliferation of intrinsic cells as marked by the presence of PCNA.
Glomerular PCNA+ cells in kidneys of mice with ICGN. Shown is a representative micrograph from a DAF KO mouse with ICGN (a). CD45+ leukocytes are indicated by arrows and PCNA+ intrinsic glomerular cell by the arrowhead. Magnification, × 1000. DAF KO and DKO mice with ICGN had increased PCNA+ cells relative to wild-type and CD59 KO mice (b). *P< 0.05, vs wild-type and CD59 KO groups.
Discussion
Even though the complement system tends to be selective, regulation of its activation is important to prevent bystander injury to host cells, particularly in those instances when ICs deposit in tissues. Relevant in this regard are DAF and CD59 which regulate C3/C5 convertases and formation of C5b-9, respectively.2 In this study in which ICs deposited in glomeruli, mice deficient in DAF developed GN which was not seen in DAF-sufficient animals. In contrast, disease development was not affected by the presence or absence of CD59. These data suggest that inhibition of C3/C5 activation by DAF can protect against the development of experimental ICGN while generation of C5b-9 and its regulation by CD59 are less consequential in this model.
Complement mediators besides C5b-9 generated by C3/C5 activation are C3a, C3b and C5a, each with cognate receptors on inflammatory cells. The C3a and C5a anaphylatoxins are potent chemoattractants, promoting leukocyte infiltration into affected tissues. Of the receptors for C3b products, ranging from CR1-4, the β2 integrin CR3 (CD11b/CD18) on inflammatory cells appears the most relevant to GN models.20 Stimulation through any or all of these receptors may be relevant in explaining the increase in glomerular leukocytes in DAF-deficient mice with CSS.
In addition to their presence on leukocytes, the receptors for C3a and C5a are present in the kidney. Both are located on proximal tubular epithelial cells,21, 22, 23 while in the glomerulus, C3a and C5a receptors appear specific to podocytes and mesangial cells, respectively.16, 21, 24, 25 Systemic administration of lipopolysaccharide can markedly increase mesangial C5aR expression, which is dependent on urokinase receptor activation.26 Early in the course of murine lupus nephritis, both C3a and C5a receptor are significantly upregulated, while later in the course of disease, their specific inhibition16, 19 or deletion27 lessened glomerular disease manifestations. Although not proven directly, it seems most likely mesangial C5a receptor was most relevant in our studies, in spite of its not being upregulated in renal cortex. Of the many effects that can occur through C5a receptor activation, induction of MCP-1 production, blocking apoptosis and promoting entry into the cell cycle are arguably the most important to our findings here.26, 28, 29, 30, 31
As the activating proteins in the complement systems of humans and mice are largely comparable, their study in mice can provide useful insights into human pathophysiology.32 The complement regulators in mice are also largely conserved in humans with some notable differences. In mice, there are two forms of DAF (DAF1 and DAF2) encoded on chromosome 133 and CD59 (CD59a and CD59b) encoded on chromosome 2;34 DAF1 and CD59a have 85% nucleotide identity to their respective alternate forms. DAF1 is a GPI-anchored protein which is expressed widely in mouse tissues, while DAF2 is a type I membrane protein that is highly expressed only in mouse spleens and testis.3, 35 Similarly, the GPI-linked CD59a is widely expressed in mouse tissues, while CD59b is restricted to the mouse testis.34 DAF1 and CD59a appear comparable to the single human DAF and CD59 proteins both in structure and distribution.
The relevance of DAF and CD59 to glomerular diseases has been inferred from their distribution in human pathological specimens and through the use of short-term rodent studies with neutralizing Abs. For instance, there is increased and wider expression of DAF and CD59 in human IC glomerular diseases such as lupus nephritis and membranous nephropathy.36, 37, 38 This has been taken as evidence for a protective response initiated by glomerular cells under complement ‘attack’, for which there also is support from in vitro studies.39 Neutralization of the function of DAF delays recovery from puromycin-induced podocyte injury, consistent with its localization on the apical portion of this glomerular cell in rats.40 CD59 appears to be present on the three intrinsic glomerular cells in humans and rodents41, 42 where it effectively limits C5b-9 formation.43, 44 Since complement activation occurs in sequence, effective regulation earlier in the pathways may mean CD59 is dispensable in normal circumstances; however, if the tempo of complement activation overwhelms C3/C5 inhibitors, CD59 is key to prevent cellular injury from C5b-9 formation.45, 46
The recent generation and study of DAF and CD59 KO mice has allowed further understanding of their roles in disease models such as nephrotoxic serum nephritis.13, 14 While this is a glomerular disease model most analogous to human anti-glomerular basement membrane GN, the reactivities of the heterologous Abs clearly extend to glomerular cells.47 The disease features of this model were exacerbated in mice lacking DAF,14, 48 CD59,49 or both,50 including podocyte ultrastructural changes and proteinuria, likely due to C5b-9-mediated podocyte injury.50
In addition to the aforementioned duplicated forms of DAF and CD59 not present in humans, rodents have the unique complement regulator p65/5I2 antigen,51, 52 which is now termed Crry (CR1-related gene y) because of its relatedness to human CR1.53 While Crry has functional and structural similarities to human CR1, it is much more widely distributed than CR154 and in many sites may serve the purpose of DAF and/or MCP in humans.55 This is clearly the case in erythrocytes, which undergo C5b-9-mediated hemolysis due to DAF and CD59 deficiencies in human paroxysmal nocturnal hemoglobinuria,56 while Crry is the key complement regulator on mouse erythrocytes.13 In the rodent glomerulus, Crry appears to be predominantly mesangial cell-associated,44, 55 where it can limit complement activation.57 The role for Crry in mouse models of disease such as CSS has experimental obstacles given the embryonic lethality of Crry deficiency.58
Besides the glomerular cell-bound complement regulators DAF, Crry and CD59, complement factor H clearly is important to prevent spontaneous complement activation in glomeruli. Its deficiency or absence from type I genetic mutations or gene targeting can lead to spontaneous development of MPGN type II in mice, pigs and humans.59, 60, 61 Factor H is also important in the CSS model of ICGN, as it leads to C3b inactivation in the glomerular capillary wall, a key factor to dampen inflammation in this model.10, 62 Taking all available information together, it appears that DAF and CFH are relevant to C3 activation in glomerular capillary wall cellular and non-cellular sites, respectively.
In conclusion, our current study suggests that DAF-regulated complement activation at the C3 level is relevant to the development of experimental ICGN. In contrast, CD59-inhibited C5b-9 formation may not be a pathogenic factor in this model. The mechanisms behind this protective effect can be attributed to limiting direct and indirect effects of the complement activation products, C3a, C5a, C3b on intrinsic glomerular cells and extrinsic leukocytes.
References
Walport MJ . Advances in immunology: complement (first of two parts). N Engl J Med 2001;344:1058–1066.
Liszewski MK, Atkinson JP . The complement system. In: Paul WE (eds). Fundamental Immunology, vol 3. Raven Press: New York, 1993, pp 917–939.
Harris CL, Rushmere NK, Morgan BP . Molecular and functional analysis of mouse decay accelerating factor (CD55). Biochem J 1999;341 (Pt 3):821–829.
Nicholson-Weller A, Wang C . Structure and function of decay accelerating factor CD55. J Lab Clin Med 1994;123:485–491.
Davies A, Lachmann PJ . Membrane defence against complement lysis: The structure and biological properties of CD59. Immunol Res 1993;12:258–275.
Quigg RJ . Complement and autoimmune glomerular diseases. Curr Dir Autoimmun 2004;7:165–180.
Stilmant MM, Couser WG, Cotran RS . Experimental glomerulonephritis in the mouse associated with mesangial deposition of autologous ferritin immune complexes. Lab Invest 1975;32:746–756.
Iskandar SS, Gifford DR, Emancipator SN . Immune complex acute necrotizing glomerulonephritis with progression to diffuse glomerulosclerosis. A murine model. Lab Invest 1988;59:772–779.
Falk RJ, Jennette JC . Immune complex induced glomerular lesions in C5 sufficient and deficient mice. Kidney Int 1986;30:678–686.
Alexander JJ, Pickering MC, Haas M, et al. Complement factor H limits immune complex deposition and prevents inflammation and scarring in glomeruli of mice with chronic serum sickness. J Am Soc Nephrol 2005;16:52–57.
Quigg RJ, Lim A, Haas M, et al. Immune complex glomerulonephritis in C4- and C3-deficient mice. Kidney Int 1998;53:320–330.
Welch TR, Frenzke M, Witte D, et al. C5a is important in the tubulointerstitial component of experimental immune complex glomerulonephritis. Clin Exp Immunol 2002;13:43–48.
Miwa T, Zhou L, Hilliard B, et al. Crry, but not CD59 and DAF, is indispensable for murine erythrocyte protection in vivo from spontaneous complement attack. Blood 2002;99:3707–3716.
Sogabe H, Nangaku M, Ishibashi Y, et al. Increased susceptibility of decay-accelerating factor deficient mice to anti-glomerular basement membrane glomerulonephritis. J Immunol 2001;167:2791–2797.
Miwa T, Sun X, Ohta R, et al. Characterization of glycosylphosphatidylinositol-anchored decay accelerating factor (GPI-DAF) and transmembrane DAF gene expression in wild-type and GPI-DAF gene knockout mice using polyclonal and monoclonal antibodies with dual or single specificity. Immunology 2001;104:207–214.
Bao L, Osawe I, Haas M, et al. Signaling through up-regulated C3a receptor is key to the development of experimental lupus nephritis. J Immunol 2005;175:1602–1610.
Bao L, Zhou J, Holers VM, et al. Excessive matrix accumulation in the kidneys of MRL/lpr lupus mice is dependent on complement activation. J Am Soc Nephrol 2003;14:2516–2525.
Anders HJ, Vielhauer V, Schlondorff D . Chemokines and chemokine receptors are involved in the resolution or progression of renal disease. Kidney Int 2003;63:401–415.
Bao L, Osawe I, Puri T, et al. C5a promotes development of experimental lupus nephritis which can be blocked with a specific receptor antagonist. Eur J Immunol 2005;35:3012–3020.
Tang T, Rosenkranz A, Assmann KM, et al. A role for Mac-1 (CDIIb/CD18) in immune complex-stimulated neutrophil function in vivo: Mac-1 deficiency abrogates sustained Fcgamma receptor-dependent neutrophil adhesion and complement-dependent proteinuria in acute glomerulonephritis. J Exp Med 1997;186:1853–1863.
Braun MC, Reins RY, Li TB, et al. Renal expression of the C3a receptor and functional responses of primary human proximal tubular epithelial cells. J Immunol 2004;173:4190–4196.
Fayyazi A, Scheel O, Werfel T, et al. The C5a receptor is expressed in normal renal proximal tubular but not in normal pulmonary or hepatic epithelial cells. Immunology 2000;99:38–45.
Zahedi R, Braun M, Wetsel RA, et al. The C5a receptor is expressed by human renal proximal tubular epithelial cells. Clin Exp Immunol 2000;121:226–233.
Braun M, Davis III AE . Cultured human glomerular mesangial cells express the C5a receptor. Kidney Int 1998;54:1542–1549.
Wilmer WA, Kaumaya PT, Ember JA, et al. Receptors for the anaphylatoxin C5a (CD88) on human mesangial cells. J Immunol 1998;160:5646–5652.
Shushakova N, Tkachuk N, Dangers M, et al. Urokinase-induced activation of the gp130/Tyk2/Stat3 pathway mediates a pro-inflammatory effect in human mesangial cells via expression of the anaphylatoxin C5a receptor. J Cell Sci 2005;118:2743–2753.
Wenderfer SE, Ke B, Hollmann TJ, et al. C5a receptor deficiency attenuates T cell function and renal disease in MRLlpr mice. J Am Soc Nephrol 2005;16:3572–3582.
Perianayagam MC, Madias NE, Pereira BJ, et al. CREB transcription factor modulates Bcl2 transcription in response to C5a in HL-60-derived neutrophils. Eur J Clin Invest 2006;36:353–361.
Guo RF, Ward PA . Role of C5a in inflammatory responses. Annu Rev Immunol 2005;23:821–852.
Strey CW, Markiewski M, Mastellos D, et al. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J Exp Med 2003;198:913–923.
Laudes IJ, Chu JC, Huber-Lang M, et al. Expression and function of C5a receptor in mouse microvascular endothelial cells. J Immunol 2002;169:5962–5970.
Quigg RJ . Complement and the kidney. J Immunol 2003;171:3319–3324.
Spicer AP, Seldin MF, Gendler SJ . Molecular cloning and chromosomal localization of the mouse decay-accelerating factor genes. J Immunol 1995;155:3079–3091.
Powell MB, Marchbank KJ, Rushmere NK, et al. Molecular cloning, chromosomal localization, expression, and functional characterization of the mouse analogue of human CD59. J Immunol 1997;158:1692–1702.
Song WC, Deng C, Raszmann K, et al. Mouse decay-accelerating factor: selective and tissue-specific induction by estrogen of the gene encoding the glycosylphosphatidylinositol-anchored form. J Immunol 1996;157:4166–4172.
Cosio FG, Sedmak DD, Mahan JD, et al. Localization of decay accelerating factor in normal and diseased kidneys. Kidney Int 1989;36:100–107.
Abe K, Miyazaki M, Koji T, et al. Expression of decay accelerating factor mRNA and complement C3 mRNA in human diseased kidney. Kidney Int 1998;54:120–130.
Tamai H, Matsuo S, Fukatsu A, et al. Localization of 20-kD homologous restriction factor (HRF20) in diseased human glomeruli. An immunofluorescence study. Clin Exp Immunol 1991;84:256–262.
Cosio FG, Shibata T, Rovin BH, et al. Effects of complement activation products on the synthesis of decay accelerating factor and membrane cofactor protein by human mesangial cells. Kidney Int 1994;46:986–992.
Bao L, Spiller OB, St JP, et al. Decay-accelerating factor expression in the rat kidney is restricted to the apical surface of podocytes. Kidney Int 2002;62:2010–2021.
Ichida S, Yuzawa Y, Okada H, et al. Localization of the complement regulatory proteins in the normal human kidney. Kidney Int 1994;46:89–96.
Funabashi K, Okada N, Matsuo S, et al. Tissue distribution of complement regulatory membrane proteins in rats. Immunology 1994;81:444–451.
Quigg RJ, Holers VM, Morgan BP, et al. Crry and CD59 regulate complement in rat glomerular epithelial cells and are inhibited by the nephritogenic antibody of passive Heymann nephritis. J Immunol 1995;154:3437–3443.
Quigg RJ, Morgan BP, Holers VM, et al. Complement regulation in the rat glomerulus: Crry and CD59 regulate complement in glomerular mesangial and endothelial cells. Kidney Int 1995;48:412–421.
Matsuo S, Nishikage H, Yoshida F, et al. Role of CD59 in experimental glomerulonephritis in rats. Kidney Int 1994;46:191–200.
Cunningham PN, Hack BK, Ren G, et al. Glomerular complement regulation is overwhelmed in passive Heymann nephritis. Kidney Int 2001;60:900–909.
Chugh S, Yuan H, Topham PS, et al. Aminopeptidase A: a nephritogenic target antigen of nephrotoxic serum. Kidney Int 2001;59:601–613.
Lin F, Emancipator SN, Salant DJ, et al. Decay-accelerating factor confers protection against complement-mediated podocyte injury in acute nephrotoxic nephritis. Lab Invest 2002;82:563–569.
Turnberg D, Botto M, Warren J, et al. CD59a deficiency exacerbates accelerated nephrotoxic nephritis in mice. J Am Soc Nephrol 2003;14:2271–2279.
Lin F, Salant DJ, Meyerson H, et al. Respective roles of decay-accelerating factor and CD59 in circumventing glomerular injury in acute nephrotoxic serum nephritis. J Immunol 2004;172:2636–2642.
Wong W, Fearon DT . p65: a C3b-binding protein on murine cells that shares antigenic determinants with the human C3b receptor (CR1) and is distinct from murine C3b receptor. J Immunol 1985;134:4048–4056.
Takizawa H, Okada N, Okada H . Complement inhibitor of rat cell membrane resembling mouse Crry/p65. J Immunol 1994;152:3032–3038.
Paul MS, Aegerter M, O'Brien SE, et al. The murine complement receptor gene family. I. Analysis of mCRY gene products and their homology to human CR1. J Immunol 1989;142:582–589.
Kim Y-U, Kinoshita T, Molina H, et al. Mouse complement regulatory protein Crry/p65 uses the specific mechanisms of both human decay-accelerating factor and membrane cofactor protein. J Exp Med 1995;181:151–159.
Li B, Sallee C, Dehoff M, et al. Mouse Crry/p65: characterization of monoclonal antibodies and the tissue distribution of a functional homologue of human MCP and DAF. J Immunol 1993;151:4295–4305.
Johnson RJ, Hillmen P . Paroxysmal nocturnal haemoglobinuria: Nature's gene therapy? Mol Pathol 2002;55:145–152.
Nishikage H, Baranyi L, Okada H, et al. Role of a complement regulatory protein in rat mesangial glomerulonephritis. J Am Soc Nephrol 1995;6:234–242.
Xu C, Mao D, Holers VM, et al. A critical role for murine complement regulator Crry in fetomaternal tolerance. Science 2000;287:498–501.
Ault BH, Schmidt BZ, Fowler NL, et al. Human factor H deficiency. Mutations in framework cysteine residues and block in H protein secretion and intracellular catabolism. J Biol Chem 1997;272:25168–25175.
Hegasy GA, Manuelian T, Hogasen K, et al. The molecular basis for hereditary porcine membranoproliferative glomerulonephritis type II: point mutations in the factor H coding sequence block protein secretion. Am J Pathol 2002;161:2027–2034.
Pickering MC, Cook HT, Warren J, et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet 2002;31:424–428.
Alexander JJ, Aneziokoro OGB, Chang A, et al. Distinct and separable roles of the complement system in factor H-deficient bone marrow chimeric mice with immune complex disease. J Am Soc Nephrol 2006;17:1354–1361.
Acknowledgements
Grant support: National Institutes of Health grant R01DK041873
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bao, L., Haas, M., Minto, A. et al. Decay-accelerating factor but not CD59 limits experimental immune-complex glomerulonephritis. Lab Invest 87, 357–364 (2007). https://doi.org/10.1038/labinvest.3700522
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.3700522
Keywords
This article is cited by
-
The Lupus-derived Anti-double-stranded DNA IgG Contributes to Myofibroblast-like Phenotype in Mesangial Cells
Journal of Clinical Immunology (2012)