Abstract
Water has multiple glassy states, often called amorphous ices. Low-density (LDA) and high-density (HDA) amorphous ice are separated by a dramatic, first-order like phase transition. It has been argued that the LDA-HDA transformation connects to a first-order liquid-liquid phase transition (LLPT) above the glass transition temperature Tg. Direct experimental evidence of the LLPT is challenging to obtain, since the LLPT occurs at conditions where water rapidly crystallizes. In this work, we explore the implications of a LLPT on the pressure dependence of Tg(P) for LDA and HDA by performing computer simulations of two water models – one with a LLPT and one without. In the absence of a LLPT, Tg(P) for all glasses nearly coincide. When there is a LLPT, different glasses exhibit dramatically different Tg(P) which are directly linked with the LLPT. Available experimental data for Tg(P) are only consistent with the scenario including a LLPT.
Similar content being viewed by others
Introduction
The ubiquity and biological importance of water, as well as its numerous puzzling anomalous properties, have sparked a long history of water research. Over the last 40 years, there have been several frameworks proposed to explain the unusual behavior of liquid water1,2,3, most notably the liquid-liquid critical point (LLCP) hypothesis4,5, the stability limit conjecture6 and the singularity-free scenario7. Most relevant to this work is the LLCP hypothesis, which proposes that the unusual behavior of liquid water can be explained by the existence of two thermodynamically distinct phases – a low-density liquid (LDL) and high-density liquid (HDL) – below a critical temperature Tc. In this scenario, LDL and HDL are separated by a liquid-liquid phase transition (LLPT) ending at a LLCP. While there are many simulations of atomistic water models4,8,9,10,11,12,13,14,15,16,17 and other tetrahedrally coordinated liquids18,19,20,21 that support this possibility, direct experimental confirmation of this proposal has been prevented by the nucleation of crystalline ice. Recent experiments of water confined at the nanoscale (e.g., Ref.22), where crystallization can be suppressed, support the view that two liquids, LDL and HDL, exist at low temperatures. Although encouraging, it is not clear at present what role the confining surfaces play on water phase behavior (see, e.g., Ref.23).
Some of the strongest experimental evidence suggesting a liquid-liquid transition in water are obtained from glassy water. Specifically, experiments show the presence of two distinct glassy states, low-density (LDA) and high-density amorphous (HDA) ice, which can be reversibly interconverted by the application (or removal) of pressure24,25,26,27,28. Very recently, Winkel et al. observed the transformation from high- to low-density water by removal of pressure at 140 K and suggested it to be of first-order in the ultraviscous liquid domain just above Tg29. In the context of the LLCP hypothesis, LDA and HDA are envisioned as the glass counterparts of LDL and HDL, respectively. Unambiguous evidence that LDA and HDA are thermodynamically distinct and that they are thermodynamically continuously connected with LDL and HDL, respectively, would imply the existence of polymorphism in liquid water and strongly support the existence of a LLCP. Unfortunately, the rapid crystallization and non-equilibrium nature of glassy water complicates elucidating the relationship between the equilibrium liquid and LDA/HDA and hence complicates determining whether two liquid phases exist in water.
A possible way to overcome this challenge is to take advantage of the fact that, at the glass transition temperature Tg, the ergodic liquid is restored (if only briefly before crystallization occurs30,31,32). Consequently, the pressure dependence of Tg(P) provides valuable information on the thermodynamics of the liquid state. This is conceptually similar to using the metastable melting loci to extract information about the liquid state, an approach developed by Mishima and Stanley5. If there are thermodynamically distinct liquid states (which have a discontinuous change in dynamics at the LLPT) there should be a discontinuous change in the locus Tg(P) obtained from quenching the liquid under pressure. Additionally, pressure treated glassy states are expected to have dramatically different Tg(P)33. Hence, Tg(P) may offer a direct indicator of a LLPT. The primary obstacle to examining this approach experimentally is maintaining specific large pressure and avoiding crystallization during thermal studies of various glasses, although some researchers29,34,35,36 have begun to overcome these problems.
Motivated by these considerations, here we examine the behavior of the locus of Tg(P) for the amorphous ices using computer simulations of two models of water – one that has a highly accessible LLPT (the ST2 model)4,8,11,12,17 and one that does not (the SPC/E model)37,38,39. We select these models to provide clearly contrasting behavior, rather than for their accuracy (or lack thereof) in reproducing quantitative experimental behavior of water. Our simulations show that, in the absence of a LLPT (the SPC/E model), the glass transition loci Tg(P) of HDA and LDA are nearly indistinguishable and that Tg(P) varies smoothly with pressure. In marked contrast, when there is a LLPT (the ST2 model), the Tg(P) loci of LDA and HDA have opposite pressure dependences. By also evaluating Tg(P) from glasses obtained by hyperquenching the equilibrium liquid under pressure, we show that this Tg(P) (which is directly related to liquid states) roughly coincides with that of LDA at low pressure and with that of HDA at high pressure; this Tg(P) exhibits a sharp change from LDA- to HDA-like behavior at the intersection with the known LLPT line. The qualitative differences between the results obtained with the SPC/E and ST2 models provide a template to interpret experiments measuring Tg(P) from amorphous ices and hence to distinguish whether or not the behavior of water is indicative of an LLPT. We also discuss the available experimental data, which are qualitatively consistent with the LLPT scenario shown by the ST2 model and inconsistent with the SPC/E model; further experiments are needed for confirmation.
Results
Glassy States under Consideration
The many glassy states of water can be broadly categorized into low- and high-density forms. The properties of the glassy states of the SPC/E and ST2 models have been previously examined and it has been shown that both models qualitatively reproduce the LDA and HDA states40,41,42,43,44,45. In the following sections, we will examine hyperquenched glassy water (HGW) under compression and HDA under both compression and decompression; we also examine LDA, obtained by heating HDA at low pressure. We follow procedures similar to those used in experiments24,31,46 to prepare these states, although the cooling/heating and compression/decompression rates accessible in simulations are significantly faster than those used in experiments. See the Methods section for a complete description of the preparation of these glasses.
For the ST2 model case, we also examine glasses formed by direct hyperquenches of the liquid at different pressures. Since these hyperquenched glasses (HQG) are obtained by quenching the liquid under pressure, they are directly related to the liquid state at the same pressure. Note that for the particular case of P = 0.1 MPa (and only for this pressure) HQG is the same as HGW. Rapid quenching of bulk samples under pressure is experimentally very challenging; this approach has been pursued experimentally only for μm size droplets of emulsified water where crystallization is suppressed34.
For each glass we form, we evaluate the glass transition temperature locus, Tg(P). For a given glass at pressure P, we determine Tg(P) following a procedure analogous to that employed in differential scanning calorimetry (DSC) experiments (see Methods section). The data presented are an average over twenty independent simulations for each glass.
Glass Transition in the Absence of a Liquid-Liquid Phase Transition
We first consider the behavior of the various glasses in the SPC/E model, where there is no interference of an LLPT to complicate the behavior of Tg(P). Figure 1 shows the Tg(P) loci for (i) compressed HGW,  , (ii) HDA obtained during compression of ice Ih and (iii) decompressed HDA,
, (ii) HDA obtained during compression of ice Ih and (iii) decompressed HDA,  (again, averaged over twenty independent simulations). All glasses are heated from an initial temperature of T = 80 K.
(again, averaged over twenty independent simulations). All glasses are heated from an initial temperature of T = 80 K.
Pressure-dependence of the glass transition temperature Tg(P) for the SPC/E model.
The behavior of Tg(P) is nearly independent of the glass preparation procedure. At pressures  , compression of HGW yields HDA (see methods section on preparation of glasses). The maximum diffusivity line (Dmax-line) is taken from Ref.38.
, compression of HGW yields HDA (see methods section on preparation of glasses). The maximum diffusivity line (Dmax-line) is taken from Ref.38.
Remarkably, Fig. 1 shows that in the absence of an LLPT, the Tg loci are almost completely independent of the starting glassy state, i.e.,  and
and  (under compression or decompression) all roughly collapse onto a single Tg(P) locus. This is particularly surprising given that the initial density of these glasses can differ by more than 20%, such as in the case of compressed HGW and decompressed HDA at
(under compression or decompression) all roughly collapse onto a single Tg(P) locus. This is particularly surprising given that the initial density of these glasses can differ by more than 20%, such as in the case of compressed HGW and decompressed HDA at  (see Methods section to compare the initial densities). However, it should be noted that, while Tg(P) of the various glasses are nearly the same, the behavior of cP(T) (from which Tg(P) is determined) and ρ(T) upon heating differs significantly for T < Tg (Fig. 2).
(see Methods section to compare the initial densities). However, it should be noted that, while Tg(P) of the various glasses are nearly the same, the behavior of cP(T) (from which Tg(P) is determined) and ρ(T) upon heating differs significantly for T < Tg (Fig. 2).
Isobaric specific heat and density for glasses of the SPC/E model on heating.
(a) Isobaric specific heat, cP(T) and (b) density, ρ(T), upon heating compressed HGW (solid lines) and decompressed HDA (dashed lines) at various pressures. Tg(P) (defined by the inflection of cP(T) below its maximum temperature) occurs at nearly the same temperature for compressed HGW and decompressed HDA. However, the behavior of the cP(T) and ρ(T) differ significantly in the glassy state (e.g., at T < 200 K for all pressures). For clarity, curves in (a) have been successively shifted by 30 kJ/(K·mol) for increasing pressure.
Interestingly, Fig. 1 shows that Tg(P) has a minimum at P ≈ 300 MPa. Such a minimum occurs at the intersection of the Tg(P) locus and the maximum diffusivity line (Dmax-line). The Dmax-line is defined as the set of pressures at which the diffusion coefficient D exhibits a maximum upon isothermal compression; its presence is a consequence of the well-known anomalous pressure-dependence of water diffusivity. The coincidence of the Tg(P) minimum and the Dmax-line is a reassuring consistency of the definition of Tg employed in this work, since we define Tg by a thermodynamic (rather than dynamic) criterion.
We can quantitatively explain the observed relation between the minimum of Tg(P) and the Dmax locus by considering the diffusive relaxation time τ ∝ (D/T)−1 47 as an explicit function of T and P. Consider the total differential

Experimentally, Tg(P) is often defined as the temperature at which τ(T, P) reaches a fixed value (typically 100 s) for a given cooling/heating rate48. Since all glasses in our simulations are formed using the same cooling/heating rate at all pressures, it follows that τ should be constant along the Tg(P) locus and thus dτ = 0 along Tg(P). Therefore, rearranging eq. (1),

along the Tg(P) locus. The denominator (∂τ/∂T)P < 0 and finite, since τ is a monotonically decreasing function of T. Hence, dTg(P)/dP = 0 (where Tg(P) is minimum) only if the numerator (∂τ/∂P)T = 0 at Tg and hence τ (and correspondingly D) must be an extremum as a function of P. Moreover, it can be shown from eq. (2) that a maximum in D corresponds to a minimum in Tg(P) and vice versa. Accordingly, the Dmax-line must intersect the Tg(P) locus at the pressure at which Tg(P) has a minimum. Additionally, at very low pressure approaching the liquid spinodal, it is known that the SPC/E model exhibits a minimum of D, due to “tearing apart” hydrogen bonds at very low density38,49. Equation 2 requires that the associated Dmin-line must intersect with a maximum on Tg(P). We do not observe this feature, since Dmin occurs at negative pressures that are not explored in this work.
Glass Transition in the Presence of a Liquid-Liquid Phase Transition
We now turn our attention to the ST2 model where the LLPT is accessible in equilibrium simulations and can significantly impact the behavior of glassy states. Due to vast differences in the behavior of Tg(P) for various glasses, we examine the Tg(P) locus for each glass separately.
Tg from Hyperquenching under Pressure
To unambiguously associate a glass with a liquid for a given pressure, the glass should be formed by isobaric quench from the liquid state at that pressure. So far, cooling under pressure is only experimentally feasible for emulsion samples34. Fortunately, the experimental complications pose no problem in computational studies. Therefore, we first examine Tg(P) obtained by hyperquenching the equilibrium liquid under pressure and reheating at the same P using our standard cooling/heating rate; we refer to the glass transition temperature of the resulting hyperquenched glasses (i.e. HQG) as  . The behavior of the Tg(P) formed in this straight-forward approach provides a useful baseline to interpret the Tg(P) behavior we will see for compressed HGW and decompressed HDA.
. The behavior of the Tg(P) formed in this straight-forward approach provides a useful baseline to interpret the Tg(P) behavior we will see for compressed HGW and decompressed HDA.
The  locus is shown in Fig. 3a. Qualitatively similar to the Tg(P) locus of SPC/E water, we see that
locus is shown in Fig. 3a. Qualitatively similar to the Tg(P) locus of SPC/E water, we see that  for the ST2 model is also negatively sloped at low pressure (due to the diffusion anomaly) and positively sloped at high pressures. The data for the ST2 model does not extend to very high pressures due to crystallization on heating that complicates determination of Tg. Interestingly, unlike the SPC/E model case, there is a sharp change in Tg(P) at P = 250 MPa.
for the ST2 model is also negatively sloped at low pressure (due to the diffusion anomaly) and positively sloped at high pressures. The data for the ST2 model does not extend to very high pressures due to crystallization on heating that complicates determination of Tg. Interestingly, unlike the SPC/E model case, there is a sharp change in Tg(P) at P = 250 MPa.
Pressure-dependence of the glass transition temperature Tg(P) for glasses of the ST2 model.
(a)  determined by hyper-quenching the equilibrium liquid at the desired pressure P and reheating the resulting glass at that same P. As discussed in the text, the dramatic change of TgHQG(P) around 250 MPa can be understood from the location of the equilibrium LLPT line (taken from Ref.13). The Dmax locus is taken from Ref.50. The color gradient represents the density ρ of HQG on heating. (b) Comparison of the glass transition temperature of compressed HGW,
determined by hyper-quenching the equilibrium liquid at the desired pressure P and reheating the resulting glass at that same P. As discussed in the text, the dramatic change of TgHQG(P) around 250 MPa can be understood from the location of the equilibrium LLPT line (taken from Ref.13). The Dmax locus is taken from Ref.50. The color gradient represents the density ρ of HQG on heating. (b) Comparison of the glass transition temperature of compressed HGW,  , with
, with  . At low P,
. At low P,  while at high P, compressed HGW converts to a high density glass (HDA′) before a glass transition to HDL occurs (violet solid line). The dotted portion of the
while at high P, compressed HGW converts to a high density glass (HDA′) before a glass transition to HDL occurs (violet solid line). The dotted portion of the  locus indicates the temperature at which compressed HGW transforms to HDA′. Included is the HDL-to-LDL spinodal line from Ref.12. Here the color gradient represents ρ of compressed HGW on heating. (c) Comparison of the glass transition temperature of decompressed HDA,
locus indicates the temperature at which compressed HGW transforms to HDA′. Included is the HDL-to-LDL spinodal line from Ref.12. Here the color gradient represents ρ of compressed HGW on heating. (c) Comparison of the glass transition temperature of decompressed HDA,  , with
, with  . At high P,
. At high P,  while at low P, decompressed HDA converts to LDA (dashed line) before a glass transition to the liquid occurs at TgLDA(P) (green solid line). Here the color gradient is ρ of decompressed HDA on heating, which helps to identify the transformation to LDA.
while at low P, decompressed HDA converts to LDA (dashed line) before a glass transition to the liquid occurs at TgLDA(P) (green solid line). Here the color gradient is ρ of decompressed HDA on heating, which helps to identify the transformation to LDA.
The sharp change of  at P = 250 MPa can be understood by superimposing the known location of the LL coexistence line for ST2 (taken from ref.13). Figure 3a shows that, for P < 250 MPa, the
at P = 250 MPa can be understood by superimposing the known location of the LL coexistence line for ST2 (taken from ref.13). Figure 3a shows that, for P < 250 MPa, the  locus is at higher T than the liquid-liquid coexistence line. Accordingly, on hyperquenching, the liquid falls out of equilibrium (into a low-density glass) before the LLPT occurs and so
locus is at higher T than the liquid-liquid coexistence line. Accordingly, on hyperquenching, the liquid falls out of equilibrium (into a low-density glass) before the LLPT occurs and so  is smooth at all P < 250 MPa. At P = 250 MPa, the
is smooth at all P < 250 MPa. At P = 250 MPa, the  locus intersects the LL coexistence line and consequently the system is now sensitive to the equilibrium LLPT and so there should be a LLPT before vitrification (into a high-density glass). Accordingly, the large difference in the dynamical properties of LDL (for
locus intersects the LL coexistence line and consequently the system is now sensitive to the equilibrium LLPT and so there should be a LLPT before vitrification (into a high-density glass). Accordingly, the large difference in the dynamical properties of LDL (for  ) and HDL (for
) and HDL (for  ) is reflected by a large change in
) is reflected by a large change in  at P = 250 MPa. A similar discontinuity in Tg(P) has been observed in computer simulations of a polyamorphic monatomic liquid33.
at P = 250 MPa. A similar discontinuity in Tg(P) has been observed in computer simulations of a polyamorphic monatomic liquid33.
This dramatic change in the liquid properties is indicated by the presence of two distinctive features in cP(T) upon heating HQG at P = 250 MPa (Fig. 4) (similar characteristics are observed in ρ(T), see Fig. 5). Specifically, in addition to the typical cP peak, characteristic of the glass-to-liquid transition observed at P < 250 MPa, a shoulder in cP(T) develops at slightly lower temperature, T ≈ 220 K (Fig. 4a). As we discuss below, this lower-T feature of cP(T) can be associated to the transformation of the high-density glass (HDA) to the low-density glass (LDA); the upper-T feature of cP(T) can be related to a glass-to-liquid transition, from the low-density glass to the liquid. At P = 250 MPa, this last transformation occurs at the intersection of the  and liquid-liquid coexistence TLL(P) loci. At higher pressure, a simpler behavior reemerges. Specifically, for P > 250 MPa, hyperquenching the liquid yields a high-density glassy state that connects smoothly with the behavior of the liquid at high pressure (Fig. 3a)43.
and liquid-liquid coexistence TLL(P) loci. At higher pressure, a simpler behavior reemerges. Specifically, for P > 250 MPa, hyperquenching the liquid yields a high-density glassy state that connects smoothly with the behavior of the liquid at high pressure (Fig. 3a)43.
Isobaric specific heat for glasses of the ST2 model on heating.
Comparison of the isobaric specific heat cP(T) for (a) HQG (solid lines) and compressed HGW (dashed lines) and (b) HQG (solid lines) and decompressed HDA (dashed lines) at various pressures. For clarity of the figure, curves for increasing pressure have been successively shifted vertically by 100 kJ/(K·mol) and horizontally by-10 K. For the case of compressed HGW [panel (a)], there are two features at high pressures. For example, at P = 500 MPa, compressed HGW converts to a high density glass similar to HDA at T ≈ 190 K, which on further heating converts to an equilibrium liquid at T ≈ 240 K. Similarly, for decompressed HDA at low pressure [panel (b)], there are two features at low pressures. For example, at P = 0.1 MPa, HDA converts to LDA (similar to HGW) at T ≈ 150 K, which on further heating converts to an equilibrium liquid at T = 275 K.
A minimum in  apparently develops at approximately 250 ≤ P ≤ 300 MPa, close to the P where
apparently develops at approximately 250 ≤ P ≤ 300 MPa, close to the P where  it exhibits a discontinuity – at the intersection with the TLL line. This discontinuity is consistent with the fact that, across the TLL locus, τ will show a discontinuity with the change in phase. Like the SPC/E model, Fig. 3a also shows that the Dmax-line approaches the minimum in
it exhibits a discontinuity – at the intersection with the TLL line. This discontinuity is consistent with the fact that, across the TLL locus, τ will show a discontinuity with the change in phase. Like the SPC/E model, Fig. 3a also shows that the Dmax-line approaches the minimum in  .
.
Tg of Compressed HGW
We next compare the behavior of  with that of
with that of  . Since HGW is formed by hyperquenching the liquid at atmospheric pressure, it is natural to expect that, at low pressure, the Tg resulting from heating compressed HGW should be very similar to that of the glass formed by hyperquenching the pressurized equilibrium liquid (i.e., HQG). For P ≤ 200 MPa, Fig. 3b confirms that indeed
. Since HGW is formed by hyperquenching the liquid at atmospheric pressure, it is natural to expect that, at low pressure, the Tg resulting from heating compressed HGW should be very similar to that of the glass formed by hyperquenching the pressurized equilibrium liquid (i.e., HQG). For P ≤ 200 MPa, Fig. 3b confirms that indeed  . Moreover, the behavior of cP(T) of the two glasses, even at T < Tg, is nearly identical in this pressure range (Fig. 4a). For P > 250 MPa, the
. Moreover, the behavior of cP(T) of the two glasses, even at T < Tg, is nearly identical in this pressure range (Fig. 4a). For P > 250 MPa, the  and
and  loci differ significantly. Specifically, while
loci differ significantly. Specifically, while  is affected by the proximity to the LLPT,
is affected by the proximity to the LLPT,  is nearly insensitive to the presence of the liquid-liquid transition.
is nearly insensitive to the presence of the liquid-liquid transition.
We can understand the difference between the  and
and  loci at high pressure based on the preparation of compressed HGW. Since we compress HGW at very low T, compressed HGW cannot relax during compression; thus, compressed HGW retains the characteristics of the low-density glass state, even at pressures where the high-density state may have lower free energy. This is analogous to the fact that HDA can be decompressed to atmospheric pressure and retain its high density, provided the temperature is kept sufficiently low. Since the equilibrium LLPT occurs at temperatures below
loci at high pressure based on the preparation of compressed HGW. Since we compress HGW at very low T, compressed HGW cannot relax during compression; thus, compressed HGW retains the characteristics of the low-density glass state, even at pressures where the high-density state may have lower free energy. This is analogous to the fact that HDA can be decompressed to atmospheric pressure and retain its high density, provided the temperature is kept sufficiently low. Since the equilibrium LLPT occurs at temperatures below  (for the present heating/cooling rate), the
(for the present heating/cooling rate), the  locus continues as a smooth extension of its low pressure behavior to P much larger than 250 MPa (where the Tg(P) of HQG has an apparent discontinuity).
locus continues as a smooth extension of its low pressure behavior to P much larger than 250 MPa (where the Tg(P) of HQG has an apparent discontinuity).
Since compressed HGW remains mechanically stable for P > Pc (Pc = 190 MPa), it is natural to ask to what liquid compressed HGW transforms on heating at high pressures. We separately consider the cases Pc < P < 400 MPa and P > 400 MPa, since at P ≈ 400 MPa the  and
and  loci intersect one another.
loci intersect one another.
In the range Pc < P < 400 MPa, compressed HGW transforms to HDL without intermediate phases. That the final liquid is HDL can be understood by noting the location of the LDL-to-HDL spinodal (see Fig. 3b). It can be seen that  is greater than the spinodal temperature. Therefore, on heating, the glass transition must be from HGW to HDL, since HDL is the only (meta)stable liquid at these (P,T). This is confirmed by the dramatic increase in density at
is greater than the spinodal temperature. Therefore, on heating, the glass transition must be from HGW to HDL, since HDL is the only (meta)stable liquid at these (P,T). This is confirmed by the dramatic increase in density at  shown in Fig. 5a for Pc < P ≤ 400 MPa.
shown in Fig. 5a for Pc < P ≤ 400 MPa.
At P > 400 MPa, the final liquid resulting from heating compressed HGW is also HDL. However, the progression to the HDL state is more complex at these pressures. At P > 400 MPa,  and, as a result, when compressed HGW reaches Tg, no metastable liquid state is accessible. Thus, HGW makes a glass-glass transition to an HDA-like glass – the glassy extension of HDL. We refer to this form as HDA′ since it has similar (but not identical) properties to HDA on decompression. The HGW→HDA′ transformation is dominated by an orientational reordering of the molecules and does not require significant translational displacements. For
and, as a result, when compressed HGW reaches Tg, no metastable liquid state is accessible. Thus, HGW makes a glass-glass transition to an HDA-like glass – the glassy extension of HDL. We refer to this form as HDA′ since it has similar (but not identical) properties to HDA on decompression. The HGW→HDA′ transformation is dominated by an orientational reordering of the molecules and does not require significant translational displacements. For  – the glass-glass and glass-liquid transitions are well separated and we can detect two distinct transformations in cP(T) (Fig. 4a) – the first being HGW→HDA′, followed on heating by HDA→liquid, defining
– the glass-glass and glass-liquid transitions are well separated and we can detect two distinct transformations in cP(T) (Fig. 4a) – the first being HGW→HDA′, followed on heating by HDA→liquid, defining  which roughly coincides with
which roughly coincides with  . It is important to notice that the glass-glass HGW→HDA′ transition is a natural continuation of the
. It is important to notice that the glass-glass HGW→HDA′ transition is a natural continuation of the  from lower P, showing that the shape of the
from lower P, showing that the shape of the  depends only on the form of the glass and not the liquid (or glass) to which HGW transforms. Hence, the HGW-to-HDA′ transition is a result of HGW reaching its kinetic (not thermodynamic) limit of stability and becoming caught in another out-of-equilibrium state, HDA′, before the liquid becomes available upon further heating. Considering that LDA and HGW are largely the same, it follows that the LDA-to-HDA′ transition upon heating at high pressures is not related to the LDL-to-HDL spinodal, but instead to the LDA Tg. A similar scenario holds for the case of decompressed HDA, as discussed in the next section.
depends only on the form of the glass and not the liquid (or glass) to which HGW transforms. Hence, the HGW-to-HDA′ transition is a result of HGW reaching its kinetic (not thermodynamic) limit of stability and becoming caught in another out-of-equilibrium state, HDA′, before the liquid becomes available upon further heating. Considering that LDA and HGW are largely the same, it follows that the LDA-to-HDA′ transition upon heating at high pressures is not related to the LDL-to-HDL spinodal, but instead to the LDA Tg. A similar scenario holds for the case of decompressed HDA, as discussed in the next section.
Tg of Decompressed HDA
We finally examine the Tg(P) locus of decompressed HDA. This form of HDA is most closely related to that of experimentally prepared uHDA51. The behavior of  is easiest to understand at high pressure since HDA is formed under high pressure conditions. Specifically, we expect that the high pressure behavior of
is easiest to understand at high pressure since HDA is formed under high pressure conditions. Specifically, we expect that the high pressure behavior of  should approximately follow that of the glass from the hyper-quenched liquid at the same pressure. Indeed, Fig. 3c shows that, for
should approximately follow that of the glass from the hyper-quenched liquid at the same pressure. Indeed, Fig. 3c shows that, for  ,
,  . We note that at P ≈ 260 MPa, the
. We note that at P ≈ 260 MPa, the  locus intersects the HDL-to-LDL spinodal on decompression. Therefore, the liquid to which HDA transforms at
locus intersects the HDL-to-LDL spinodal on decompression. Therefore, the liquid to which HDA transforms at  is HDL.
is HDL.
We next consider the behavior of  at P < 250 MPa, where the equilibrium liquid forms a low-density glass on isobaric quenching. Just as in experiments, HDA in our simulations is mechanically stable at liquid N2 temperatures down to atmospheric pressure. Furthermore, as known experimentally, but not observed for the SPC/E model, decompressed HDA shows a clear conversion to a low density amorphous glass upon heating, normally referred to as LDA. The conversion to LDA, as well as the conversion of LDA to a liquid upon further heating, appear as two distinct features in the specific heat (Fig. 4b). This is particularly clear at P = 0.1 and 200 MPa where cP(T) exhibits minima at T ≈ 190 and 230 K (respectively) below the temperature at which the cP maximum, that is characteristic of the glass transition, occurs.
at P < 250 MPa, where the equilibrium liquid forms a low-density glass on isobaric quenching. Just as in experiments, HDA in our simulations is mechanically stable at liquid N2 temperatures down to atmospheric pressure. Furthermore, as known experimentally, but not observed for the SPC/E model, decompressed HDA shows a clear conversion to a low density amorphous glass upon heating, normally referred to as LDA. The conversion to LDA, as well as the conversion of LDA to a liquid upon further heating, appear as two distinct features in the specific heat (Fig. 4b). This is particularly clear at P = 0.1 and 200 MPa where cP(T) exhibits minima at T ≈ 190 and 230 K (respectively) below the temperature at which the cP maximum, that is characteristic of the glass transition, occurs.
As observed in experiments, the HDA-to-LDA (glass-glass) transformation, readily seen by a similar exothermic feature (Fig. 4b), is accompanied by a dramatic decrease of density (Fig. 5b). Like the conversion of compressed HGW to HDA′ at high pressure discussed in the previous subsection, the HDA-to-LDA transformation is primarily associated with a reorientation of the molecules without significant translational motion. Figure 3c shows that the locus of the HDA-to-LDA (dashed violet line) connects smoothly with the  locus (solid violet line) at high pressure. This demonstrates that the HDA-to-LDA transition is a result of HDA reaching its Tg; rather than transforming to the liquid at Tg, the system becomes caught in another out-of-equilibrium state, LDA, before the liquid becomes available upon further heating. It follows that the HDA-to-LDA transition is not related to the thermodynamic HDL-to-LDL spinodal. However, the HDL-to-LDL spinodal on decompression does signal the terminal pressure of the HDA-to-LDA conversion locus. At large pressures,
locus (solid violet line) at high pressure. This demonstrates that the HDA-to-LDA transition is a result of HDA reaching its Tg; rather than transforming to the liquid at Tg, the system becomes caught in another out-of-equilibrium state, LDA, before the liquid becomes available upon further heating. It follows that the HDA-to-LDA transition is not related to the thermodynamic HDL-to-LDL spinodal. However, the HDL-to-LDL spinodal on decompression does signal the terminal pressure of the HDA-to-LDA conversion locus. At large pressures,  , HDA converts directly to HDL.
, HDA converts directly to HDL.
Experimentally, LDA is known to have similar Tg to HGW at atmospheric pressure52. Therefore, we expect that the Tg(P) locus from heating LDA should be similar to both Tg(P) for compressed HGW and HQG. Indeed, Figs. 3b and 3c show that for P < 100 MPa,  . Nearing the region of the LLPT, the behavior of the Tg loci for the different low-density glasses have similar pressure dependence but no longer coincide – not surprising since the density of LDA is noticeably different from compressed HGW at P ≈ 200 MPa and T < 260 K (Fig. 5b).
. Nearing the region of the LLPT, the behavior of the Tg loci for the different low-density glasses have similar pressure dependence but no longer coincide – not surprising since the density of LDA is noticeably different from compressed HGW at P ≈ 200 MPa and T < 260 K (Fig. 5b).
Relationship to Experiments
The results obtained for the SPC/E (without a LLPT) and the ST2 (with a LLPT) models present clearly distinguishable scenarios for the behavior of the Tg(P) loci of the different glasses. In this section, we compare available experimental data for real water with these scenarios. In short, we find that the available experimental data for (i) the Tg(P) loci of LDA and HDA, (ii) the pressure-dependence of the LDA-to-HDA and HDA-to-LDA transition temperatures and (iii) the location of the Dmax-line for liquid water are consistent with the LLPT scenario of the ST2 model and inconsistent with the simulations using the SPC/E model that lack an accessible LLPT. We explain the comparisons in the following. To simplify the discussion, we use Tg(P) to refer to both the locus of glass-glass and glass-liquid transitions.
We first consider the ST2 model scenario, where HDA and LDA (or HGW) exhibit distinct Tg(P) loci. Experimental data for the transitions of the amorphous solids are shown in Fig. 6, which can be compared with Fig. 3. We observe several key qualitative similarities. Specifically, we see that (i) the experimental Tg(P) locus of HDA on heating35 and the decompression induced HDA-to-LDA transition line26 are both positively sloped and can be identified with the qualitative behavior of the  locus of the ST2 model; (ii) conversely, the compression induced LDA-to-HDA transition line26 is negatively sloped, and, if we associate this line with a pressure induced glass-glass transition, the behavior is consistent with the ST2 model; (iii) the location of the Dmax line is consistent with a change of the Tg(P) locus from one like LDA at low pressure (negative slope), to one like HDA (positive slope) at high pressure; (iv) the calorimetric Tg of LDA is higher than the calorimetric Tg of HDA at ambient pressure35. Based on the similarity between the ST2 results and experiments, we can make a crude prediction for the experimental Tg(P) locus of LDA (or HGW) on heating (Fig. 6). The negative slope of the predicted Tg(P) locus is supported by the fact that D has anomalous pressure dependence in this region. Confirmation of such qualitative behavior would strongly support the possibility of a LLPT.
locus of the ST2 model; (ii) conversely, the compression induced LDA-to-HDA transition line26 is negatively sloped, and, if we associate this line with a pressure induced glass-glass transition, the behavior is consistent with the ST2 model; (iii) the location of the Dmax line is consistent with a change of the Tg(P) locus from one like LDA at low pressure (negative slope), to one like HDA (positive slope) at high pressure; (iv) the calorimetric Tg of LDA is higher than the calorimetric Tg of HDA at ambient pressure35. Based on the similarity between the ST2 results and experiments, we can make a crude prediction for the experimental Tg(P) locus of LDA (or HGW) on heating (Fig. 6). The negative slope of the predicted Tg(P) locus is supported by the fact that D has anomalous pressure dependence in this region. Confirmation of such qualitative behavior would strongly support the possibility of a LLPT.
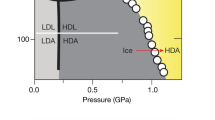
The experimental behavior of Tg(P) for LDA and HDA.
Data for Tg are primarily from ref.35. We also compare with data for the isothermal, pressure driven LDA/HDA transition26. The difference in path and rate between these experiments precludes a quantitative comparison. However, the data show the same qualitative features as the ST2 model that exhibits a LLPT (Fig. 3). Given these facts, the dashed green line offers a prediction for the approximate behavior of Tg(P) for LDA. The prediction is determined by scaling and shifting  from the ST2 model to match the experimental
from the ST2 model to match the experimental  (the resulting locus is indicated by the dashed violet line); this scaling factor is then applied to
(the resulting locus is indicated by the dashed violet line); this scaling factor is then applied to  from the ST2 model and shifted so that it matches experiments at atmospheric pressure.
from the ST2 model and shifted so that it matches experiments at atmospheric pressure.
While the similarities are favorable for the scenario of the ST2 model, there are complicating factors based on differences with the experimental details that we should consider. The first complication regards possible differences between the behavior of eHDA and uHDA. The experimental Tg(P) locus35 for HDA (Fig. 6) is derived from eHDA (annealed at low pressure), while our simulations examine uHDA. Tg for these two forms is very similar. However, they apparently differ in the nature of the transformation: careful annealing just above Tg for eHDA results in an apparently high-density liquid state for P ≤ 300 MPa35, while uHDA transforms immediately to LDA on heating at low pressure53 (as found here for the ST2 model). Fig. 6 includes the compression-induced LDA-to-HDA and decompression-induced HDA-to-LDA transition line from ref.26. These experiments were performed using uHDA. The slope of the experimental  locus35 is very similar to the slope of the experimental HDA-to-LDA transition line26 (see Fig. 6). This is consistent with the conclusion that the HDA-to-LDA transition is a result of HDA reaching its Tg, rather than being associated with the HDL-to-LDL spinodal. The experimental difference between eHDA and uHDA seems to be that the HDA-to-LDA transition is shifted to higher temperature in the former29. This difference opens a window where HDL is accessible in isobaric heating experiments done with eHDA35, whereas uHDA transforms to LDA without access to HDL. Another complication is that (above atmospheric pressure) the experimental
locus35 is very similar to the slope of the experimental HDA-to-LDA transition line26 (see Fig. 6). This is consistent with the conclusion that the HDA-to-LDA transition is a result of HDA reaching its Tg, rather than being associated with the HDL-to-LDL spinodal. The experimental difference between eHDA and uHDA seems to be that the HDA-to-LDA transition is shifted to higher temperature in the former29. This difference opens a window where HDL is accessible in isobaric heating experiments done with eHDA35, whereas uHDA transforms to LDA without access to HDL. Another complication is that (above atmospheric pressure) the experimental  35 has been obtained volumetrically. However, simulations indicate that Tg measured from calorimetry and volume experiments are very close to each other54; we made a similar analysis using our volume data and find consistent results with the calorimetric method (see supplementary information).
35 has been obtained volumetrically. However, simulations indicate that Tg measured from calorimetry and volume experiments are very close to each other54; we made a similar analysis using our volume data and find consistent results with the calorimetric method (see supplementary information).
We next consider a comparison of experimental data with the SPC/E scenario. In addition to the glass transition temperatures of LDA and HDA, Fig. 6 also shows the location of the experimental Dmax line1,55. To show that the experimental data is inconsistent with the SPC/E scenario, we note that in the SPC/E scenario: (i) there is a single Tg(P) locus for all amorphous ices and (ii) the Tg(P) locus exhibits a single minimum that intersects the Dmax-line. Figure 6 indicates that points (i) and (ii) cannot be simultaneously satisfied. Specifically, if there were a single Tg(P) locus, then its minimum must occur at approximately P ≤ 115 MPa in order to intersect the LDA and HDA Tg loci in Fig. 6. However, the pressure of the Dmax-line, which should coincide with a minimum of Tg(P) locus, clearly extrapolates to much larger pressure, P ≥ 190 MPa. Hence, conditions (i) and (ii) cannot both be satisfied and so the SPC/E picture is not supported by the experiments. In other words, observation of two distinct values of Tg(LDA) and Tg(HDA) at approximately 200 MPa, where the Dmax line intersects, can be used as a criterion to positvely identify a liquid-liquid phase transition scenario and to rule out a scenario without such a transition. The predictions made here combined with the experimental scenario strongly suggest this to be the case, but definite experimental confirmation remains an open issue.
Discussion
We have evaluated the pressure dependence of Tg for various forms of amorphous water in two simulation models, one with an accessible liquid-liquid transition and one without. Our findings indicate a much richer behavior for Tg(P) in the presence of a LLPT and that this behavior appears to be consistent with the available experimental data. The behavior of Tg from simulations in the absence of an LLPT is inconsistent with the available experimental data. Hence our findings support the possibility of multiple liquid states and a critical point in supercooled water. Based on these results, we have made a rough prediction for Tg(P) of LDA. Further experimental work to evaluate the Tg(P) loci of HGW or LDA, will be valuable to confirm or refute these hypotheses.
Methods
Simulation models and methods
Our findings are based on extensive molecular dynamics (MD) simulations of water using two different models: (i) the extended simple point charge (SPC/E) model56 and (ii) the Stillinger “ST2” potential57. These models have been extensively applied to the study of thermodynamic and dynamic properties of supercooled water and, to a lesser degree, to the study of glassy water28. Both models reproduce qualitatively the most common anomalies characteristic of liquid water. The primary difference between the models and the reason we select them, is that the ST2 model exhibits a LLPT that is readily accessible by computer simulation4,11,12,17, while the SPC/E model does not. If it exhibits a LLPT, it occurs at a temperature well below the lowest temperatures accessible in equilibrium simulations37,38,39.
The basic potential parameters and simulation protocols, such as simulation time step and cutoff distance used for the truncation of the interactions, etc. are described in previous studies: we follow precisely the parameterization described in refs.38 and12 for the SPC/E and ST2 models, respectively. The temperature and pressure are controlled using the Berendsen technique58. All data are averaged over 20 independent samples to improve statistics. For glasses obtained from an initially liquid state, we use a 1000-molecule cubic system with periodic boundary conditions. For glasses obtained from the amorphization of ice Ih, we use a system of 1024 molecules in a rectangular unit cell (with periodic boundary conditions) that matches the unit cell geometry of ice Ih. Proton disorder in the initial ice configuration is generated by identifying closed hydrogen bond loops and exchanging hydrogens between molecules, as described in Ref.59. For the ST2 model, some samples crystallize on heating the glass for  . We have excluded these samples from our analysis.
. We have excluded these samples from our analysis.
Preparation of Glassy States
The glass obtained by rapidly quenching bulk water at ambient pressure is referred to as hyperquenched glassy water (HGW)31. To obtain HGW in simulation, we first equilibrate liquid water at relatively high temperature (T = 300 K for SPC/E and T = 350 K for ST2) and P = 0.1 MPa. We isobarically cool the liquid to T = 80 K at a rate qT = 30 K/ns. To study the pressure dependence of Tg for the case of HGW, we first isothermally compress HGW at a rate qP = 300 MPa/ns from P = 0.1 MPa to the desired P at T = 80 K; we then isobarically heat the sample at the same P and recover a pressurized liquid state. We note that HGW can be compressed at T = 80 K to P ≈ 700 MPa, for the SPC/E model case and to P ≈ 1000 MPa, for the ST2 model case; further compression causes HGW to collapse to an HDA-type glass for both models (Fig. 7). A similar collapse from an LDA-type glass to an HDA-type glass is observed experimentally26,60.
Compression/Decompression behavior of LDA, HDA and ice Ih.
Evolution of density at T = 80 K upon compressing ice Ih (green line) and HGW (black line) to form HDA, followed by decompression of HDA (red and blue lines correspond to decompression of HDA obtained from ice Ih and HGW, respectively). Results are for (a) the SPC/E and (b) the ST2 model. In both models, the sudden changes in density correspond to the ice Ih-to-HDA (green line) or HGW-to-HDA (black line) transition, respectively. Symbols indicate the starting densities for glass configurations that are heated at fixed P to determine Tg, shown in subsequent figures.
We also obtain HDA by following the corresponding experimental procedure46, i.e., we isothermally compress proton-disordered ice Ih (at the rate qP) at T = 80 K to high pressure. Since we do not anneal HDA, the closest experimental counterpart to our procedure is “unrelaxed” HDA (uHDA), as opposed to relaxed (rHDA) or expanded (eHDA) treatments51. Figure 7 shows the density as function of pressure during compression of ice Ih; the results differ significantly between the two models. For the SPC/E model, the collapse to HDA happens over a narrow, but gradual and continuous range of pressure,  , while for the ST2 model, the collapse happens over a very narrow window around P = 3200 MPa. Given that these results are an average over independent compressions, examining individual runs shows that the collapse of ice Ih is indeed discontinuous in the case of the ST2 model; the width in the average density change in Fig. 7a is a result of the fact that different realizations collapse at slightly different P. An examination of individual runs for SPC/E water shows that the behavior of each realization has the same gradual behavior as the average. The difference in the sharpness in the two models is presumably a consequence of the fact that ST2 water has a clear LLPT and LLCP, accessible to equilibrium simulations, while SPC/E water does not (at least for our rapid cooling and compression rates). We note that the experimental amorphization of ice Ih46 more closely resembles the behavior of ST2 water, albeit at significantly smaller P. The ST2 model shows a larger transition pressure, both due to the rapid pressurization in the simulation and because ST2 so strongly emphasizes tetrahedral (ice-like) structure. We note that for both water models and also in experiments28, the conversion of ice Ih to HDA occurs at higher pressures than the pressures at which HGW converts to HDA. However, the structure of HDA obtained by compression of HGW or ice Ih is indistinguishable. As a result, decompression (at rate qP) of HDA formed from ice or HGW shows the same P(ρ) dependence (Fig. 7).
, while for the ST2 model, the collapse happens over a very narrow window around P = 3200 MPa. Given that these results are an average over independent compressions, examining individual runs shows that the collapse of ice Ih is indeed discontinuous in the case of the ST2 model; the width in the average density change in Fig. 7a is a result of the fact that different realizations collapse at slightly different P. An examination of individual runs for SPC/E water shows that the behavior of each realization has the same gradual behavior as the average. The difference in the sharpness in the two models is presumably a consequence of the fact that ST2 water has a clear LLPT and LLCP, accessible to equilibrium simulations, while SPC/E water does not (at least for our rapid cooling and compression rates). We note that the experimental amorphization of ice Ih46 more closely resembles the behavior of ST2 water, albeit at significantly smaller P. The ST2 model shows a larger transition pressure, both due to the rapid pressurization in the simulation and because ST2 so strongly emphasizes tetrahedral (ice-like) structure. We note that for both water models and also in experiments28, the conversion of ice Ih to HDA occurs at higher pressures than the pressures at which HGW converts to HDA. However, the structure of HDA obtained by compression of HGW or ice Ih is indistinguishable. As a result, decompression (at rate qP) of HDA formed from ice or HGW shows the same P(ρ) dependence (Fig. 7).
To obtain LDA we follow the typical experimental procedure24, i.e., we first decompress HDA to low pressures (at rate qP) at T = 80 K and then, heat decompressed HDA at constant pressure (at rate qT). As in the experimental case, HDA does not convert to LDA at P = 0.1 MPa at low T. Conversion to LDA only occurs on heating decompressed-HDA. The starting HDA configurations are obtained by isothermal compression of ice Ih at T = 80 K, as previously explained. The main text shows that the HDA-to-LDA transformation can be clearly identified for the ST2 case; for SPC/E, the HDA-to-LDA transformation is more gradual and not easily delineated44. We note that for both the ST2 and SPC/E potentials, the decompressed HDA prepared from an initial compression of ice or HGW for each model is nearly indistinguishable in terms of density (see Fig. 7) and structure (as measured by pair correlations).
Determination of Tg
Experimentally, Tg is typically determined from differential scanning calorimetry experiments where the sample is heated at a fixed rate at ambient pressure30,32. The glass transition is apparent from a peak in the specific heat cP(T), the height and width of which depend on fragility48. The specific value of Tg can be assigned by a variety of methods; we assign Tg as the temperature corresponding to the inflection point of cP(T) on heating. More specifically, we evaluate the (non-equilibrium) isobaric specific heat, cP(T) = (∂H/∂T)P, by heating glasses at the same rate qT = 30 K/ns used to cool initial samples. To calculate cP(T), we calculate the energy U(T) and volume V(T) of the system as function of T, the enthalpy being H(T) = U(T) + P V(T). We associate Tg(P) with the inflection point of cP(T) prior to the glass transition maximum. Thus, Tg(P) can be obtained from the location of the maximum in dcP(T)/dT that occurs at temperature below the cP(T) glass transition maximum.
We have tested several common definitions for Tg, for example by the intersection of a linear extrapolation of the low T glass behavior of cP(T) with its linear rising part at higher T, before the cP(T) maximum occurs. This is usually referred as the “onset” glass transition temperature, Tg,onset. While our Tg values are slightly higher than the corresponding Tg,onset values, the qualitative pressure dependence of Tg we report is robust for any reasonable definition of Tg. Note that DSC methods typically do not operate at low T and high P at the same time; consequently, there is no experimental standard method to measure Tg at large pressure. In these cases, Tg can be measured from dilatometry experiments (see SI).
References
Angell, C. A. In Franks, F. (ed.) Water: A Comprehensive Treatise vol. 7, 1–81 (Plenum, New York, 1982).
Mishima, O. & Stanley, H. E. The relationship between liquid, supercooled and glassy water. Nature 396, 329 (1998).
Debenedetti, P. G. Supercooled and glassy water. J. Phys.: Condens. Matter 15, R1669–R1726 (2003).
Poole, P. H., Sciortino, F., Essmann, U. & Stanley, H. Phase behaviour of metastable water. Nature 360, 324–328 (1992).
Mishima, O. & Stanley, H. E. Decompression-induced melting of ice iv and the liquid-liquid transition in water. Nature 392, 192 (1998).
Speedy, R. J. Stability-limit conjecture: An interpretation of the properties of water. J. Phys. Chem. 86, 982–991 (1982).
Sastry, S., Debenedetti, P., Sciortino, F. & Stanley, H. E. Singularity-free interpretation of the thermodynamics of supercooled water. Phys. Rev. E 53, 6144–6154 (1996).
Poole, P. H., Sciortino, F., Essmann, U. & Stanley, H. E. Spinodal of liquid water. Phys. Rev. E 48, 3799 (1993).
Tanaka, H. A self-consistent phase diagram for supercooled water. Nature 380, 328–330 (1996).
Sciortino, F., Poole, P. H., Essmann, U. & Stanley, H. E. Line of compressibility maxima in the phase diagram of supercooled water. Phys. Rev. E 55, 727–737 (1997).
Harrington, S., Zhang, R., Poole, P. H., Sciortino, F. & Stanley, H. Liquid-liquid phase transition: Evidence from simulations. Phys. Rev. Lett. 78, 2409–2412 (1997).
Poole, P. H., Saika-Voivod, I. & Sciortino, F. Density minimum and liquid-liquid phase transition. J. Phys.: Condens. Matter 17, L431–L437 (2005).
Cuthbertson, M. J. & Poole, P. H. Mixturelike behavior near a liquid-liquid phase transition in simulations of supercooled water. Phys. Rev. Lett. 106, 115706 (2011).
Yamada, M., Mossa, S., Stanley, H. E. & Sciortino, F. Interplay between time-temperature-transformation and the liquid-liquid phase transition in water. Phys. Rev. Lett. 88, 195701 (2002).
Brovchenko, I., Geiger, A. & Oleinikova, A. Multiple liquid-liquid transitions in supercooled water. J. Chem. Phys. 118, 9473 (2003).
Paschek, D. How the liquid-liquid transition affects hydrophobic hydration in deeply supercooled water. Phys. Rev. Lett. 94, 217802 (2005).
Liu, Y., Panagiotopoulos, A. Z. & Debenedetti, P. G. Low-temperature fluid-phase behavior of ST2 water. J. Chem. Phys. 131, 104508 (2009).
Sastry, S. & Angell, C. A. Liquid-liquid phase transition in supercooled silicon. Nat. Materials 2, 739–43 (2003).
Vasisht, V. V., Saw, S. & Sastry, S. Liquid-liquid critical point in supercooled silicon. Nat. Phys. 7, 549 (2011).
Hsu, C. W., Largo, J., Sciortino, F. & Starr, F. W. Hierarchies of networked phases induced by multiple liquid-liquid critical points. Proc. Natl. Acad. Sci. USA 105, 13711–13715 (2008).
Dai, W., Hsu, C. W., Sciortino, F. & Starr, F. W. Valency dependence of polymorphism and polyamorphism in dna-functionalized nanoparticles. Langmuir 26, 3601–3608 (2010).
Zhang, Y. et al. Density hysteresis of heavy water confined in a nanoporous silica matrix. Proc. Natl. Acad. Sci. USA 108, 12206–12211 (2011).
Mancinelli, R., Bruni, F. & Ricci, M. A. Controversial evidence on the point of minimum density in deeply supercooled confined water. J. Phys. Chem. Lett. 1, 1277–1282 (2010).
Mishima, O., Calvert, L. D. & Whalley, E. An apparently first-order transition between two amorphous phases of ice induced by pressure. Nature 314, 76 (1985).
Mishima, O., Takemura, K. & Aoki, K. Visual observations of the amorphous-amorphous transition in H2O under pressure. Science 254, 406–408 (1991).
Mishima, O. Reversible first-order transition between two H2O amorphs at ∼0.2 GPa and ∼125 K. J. Chem. Phys. 100, 5910–5912 (1994).
Winkel, K., Elsaesser, M. S., Mayer, E. & Loerting, T. Water polyamorphism: reversibility and (dis)continuity. J. Chem. Phys. 128, 044510 (2008).
Loerting, T. & Giovambattista, N. Amorphous ices: experiments and numerical simulations. J. Phys.: Condens. Matter 18, R919–R977 (2006).
Winkel, K., Mayer, E. & Loerting, T. Equilibrated high-density amorphous ice and its first-order transition to the low-density form. J. Phys. Chem. B 115, 14141–14148 (2011).
Johari, G. P., Hallbrucker, A. & Mayer, E. The glass-liquid transition of hyperquenched water. Nature 330, 552–553 (1987).
Brüggeller, P. & Mayer, E. Complete vitrification in pure liquid water and dilute aqueous solutions. Nature 288, 569–571 (1980).
Kohl, I., Bachmann, L., Hallbrucker, A., Mayer, E. & Loerting, T. Liquid-like relaxation in hyperquenched water at ≤ 140 K. Phys. Chem. Chem. Phys. 7, 3210–3220 (2005).
Xu, L., Buldyrev, S. V., Giovambattista, N., Angell, C. A. & Stanley, H. E. A monatomic system with a liquid-liquid critical point and two distinct glassy states. J. Chem. Phys. 130, 054505 (2009).
Mishima, O. & Suzuki, Y. Vitrification of emulsified liquid water under pressure. J. Chem. Phys. 115, 4199–4202 (2001).
Seidl, M. et al. Volumetric study consistent with a glass-to-liquid transition in amorphous ices under pressure. Phys. Rev. B 83, 100201 (2011).
Andersson, O. Glass-liquid transition of water at high pressure. Proc. Natl. Acad. Sci. USA 108, 11013–11016 (2011).
Harrington, S., Poole, P. H., Sciortino, F. & Stanley, H. E. Equation of state of supercooled water simulated using the extended simple point charge intermolecular potential. J. Chem. Phys. 107, 7443–7450 (1997).
Starr, F. W., Sciortino, F. & Stanley, H. E. Dynamics of simulated water under pressure. Phys. Rev. E 60, 6757–6768 (1999).
Scala, A., Starr, F. W., La Nave, E., Stanley, H. E. & Sciortino, F. The free energy surface of supercooled water. Phys. Rev. E 62, 8016–8020 (2000).
Poole, P. H., Essmann, U., Sciortino, F. & Stanley, H. E. Phase diagram for amorphous solid water. Phys. Rev. E 48, 4605–4610 (1993).
Starr, F. W., Bellissent-Funel, M.-C. & Stanley, H. E. Structure of supercooled and glassy water under pressure. Phys. Rev. E 60, 1084–1087 (1999).
Giovambattista, N., Stanley, H. E. & Sciortino, F. A Potential Energy Landscape Study of the Amorphous-Amorphous Transformation in H2O. Phys. Rev. Lett. 91, 115504 (2003).
Giovambattista, N., Stanley, H. E. & Sciortino, F. Relation between the high-density phase and the very high-density phase of amorphous solid water. Phys. Rev. Lett. 94, 107803 (2005).
Giovambattista, N., Stanley, H. & Sciortino, F. Phase diagram of amorphous solid water: Low-density, high-density and very-high-density amorphous ices. Phys. Rev. E 72 (2005).
Giovambattista, N., Debenedetti, P., Sciortino, F. & Stanley, H. Structural order in glassy water. Phys. Rev. E 71 (2005).
Mishima, O., Calvert, L. D. & Whalley, E. ‘Melting ice’ I at 77 K and 10 kbar: a new method of making amorphous solids. Nature 310, 393–395 (1984).
Hansen, J. P. & McDonald, I. R. Theory of Simple Liquids (Academic Press, London, 1986).
Angell, C. A. Formation of glasses from liquids and biopolymers. Science 267, 1924–1935 (1995).
Errington, J. & Debenedetti, P. G. Relationship between structural order and the anomalies of liquid water. Nature 409, 318–321 (2001).
Poole, P. H., Becker, S. R., Sciortino, F. & Starr, F. W. Dynamical behavior near a liquid-liquid phase transition in simulations of supercooled water. J. Phys. Chem. B 115, 14176–14183 (2011).
Nelmes, R. J. et al. Annealed high-density amorphous ice under pressure. Nat. Phys. 2, 414–418 (2006).
Elsaesser, M. S., Winkel, K., Mayer, E. & Loerting, T. Reversibility and isotope effect of the calorimetric glass → liquid transition of low-density amorphous ice. Phys. Chem. Chem. Phys. 12, 708–712 (2010).
Handa, Y., Mishima, O. & Whalley, E. High Density Amorphous ice III. Thermal Properties. J. Chem. Phys. 84, 2766–2770 (1986).
Seidl, M., Loerting, T. & Zifferer, G. High-density amorphous ice: Molecular dynamics simulations of the glass transition at 0.3 GPa. J. Chem. Phys. 131, 114502 (2009).
Prielmeier, F. X., Lang, E. W., Speedy, R. J. & Lüdemann, H.-D. The pressure dependence of self-diffusion in supercooled light and heavy water. Ber. Bunsenges. Phys. Chem. 92, 1111 (1988).
Berendsen, H. J. C., Grigera, J. R. & Stroatsma, T. P. The missing term in effective pair potentials. J. Phys. Chem. 91, 6269–6271 (1987).
Stillinger, F. H. & Rahman, A. Improved simulation of liquid water by molecular dynamics. J. Chem. Phys. 60, 1545–1557 (1974).
Berendsen, H. J. C., Postma, J. P. M., Gunsteren, W. F. V., Dinola, A. & Haak, J. R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684 (1984).
Rahman, A. & Stillinger, F. H. Proton distribution in ice and kirkwood correlation factor. J. Chem. Phys. 57, 4009–4017 (1972).
Winkel, K. et al. In Kuhs, W. F. (ed.) Physics and Chemistry of Ice chap. Isothermal amorphous-amorphous-amorphous transitions in water, 641–648 (The Royal Society of Chemistry, Cambridge, 2007).
Acknowledgements
Support of this project was provided by a PSC-CUNY Award to NG, jointly funded by The Professional Staff Congress and The City University of New York. TL is grateful for financial support by the Austrian Science Fund FWF (START award Y391) and the European Research Council (ERC) from grant SULIWA. FWS thanks the NSF for support from grant number CNS-0959856.
Author information
Authors and Affiliations
Contributions
NG, TL, FS designed research and wrote manuscript. All authors performed research and reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Suplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Giovambattista, N., Loerting, T., Lukanov, B. et al. Interplay of the Glass Transition and the Liquid-Liquid Phase Transition in Water. Sci Rep 2, 390 (2012). https://doi.org/10.1038/srep00390
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00390
This article is cited by
-
Evidence of a liquid–liquid phase transition in H\(_2\)O and D\(_2\)O from path-integral molecular dynamics simulations
Scientific Reports (2022)
-
Manifestations of metastable criticality in the long-range structure of model water glasses
Nature Communications (2021)
-
Polymorphism in glassy silicon: Inherited from liquid-liquid phase transition in supercooled liquid
Scientific Reports (2015)
-
Crystal-clear transition
Nature Physics (2014)
-
Liquid-liquid phase transition in water
Science China Physics, Mechanics & Astronomy (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.