Key Points
-
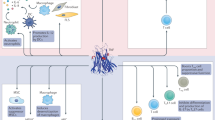
B cells, T cells and cytokines are key in the pathogenesis of systemic lupus erythematosus (SLE), and numerous biologic agents that target these cells and factors are in various stages of development
-
B-cell-directed biologic therapies include agents targeting CD20, CD19, CD22, CD40, and FcγRIIb, and some of these agents have already shown promising results
-
T-cell-directed biologic agents are aimed at promoting T cell tolerance, blocking T cell activation and differentiation, altering T cell trafficking, or generating regulatory T cells
-
Cytokine-directed biologic therapies include agents targeting BAFF, type I interferons, IL-6, and TNF, with an anti-BAFF monoclonal antibody (belimumab) already approved by the FDA for treatment of SLE
Abstract
With the approval by the FDA in 2011 of a biologic agent (namely belimumab) for the treatment of systemic lupus erythematosus (SLE), optimism abounds that additional biologic (and nonbiologic) agents will be similarly endorsed. Given the numerous immune-based abnormalities associated with SLE, the potential therapeutic targets for biologic agents and the candidate biologic approaches are also numerous. These approaches include: biologic agents that promote B-cell depletion, B-cell inactivation, or the generation of regulatory B cells; biologic agents that induce T-cell tolerance, block T-cell activation and differentiation, or alter T-cell trafficking; biologic agents that target the B-cell activating factor (BAFF) axis, type I interferons, IL-6 and its receptor, or TNF; and the adoptive transfer of ex vivo-generated regulatory T cells. Owing to the great heterogeneity inherent to SLE, no single approach should be expected to be effective in all patients. As our understanding of the pathogenic mechanisms of SLE continues to expand, additional therapeutic targets and approaches will undoubtedly be identified and should be fully exploited.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Shlomchik, M. J., Madaio, M. P., Ni, D., Trounstein, M. & Huszar, D. The role of B cells in lpr/lpr-induced autoimmunity. J. Exp. Med. 180, 1295–1306 (1994).
Jacob, N. et al. B cell and BAFF dependence of IFN-α-exaggerated disease in systemic lupus erythematosus-prone NZM 2328 mice. J. Immunol. 186, 4984–4993 (2011).
Chan, O. T. M., Hannum, L. G., Haberman, A. M., Madaio, M. P. & Shlomchik, M. J. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J. Exp. Med. 189, 1639–1647 (1999).
Sanz, I. & Lee, F. E.-H. B cells as therapeutic targets in SLE. Nat. Rev. Rheumatol. 6, 326–337 (2010).
Cragg, M. S., Walshe, C. A., Ivanov, A. O. & Glennie, M. J. The biology of CD20 and its potential as a target for mAb therapy. Curr. Dir. Autoimmun. 8, 140–174 (2005).
Leandro, M. J., Edwards, J. C., Cambridge, G., Ehrenstein, M. R. & Isenberg, D. A. An open study of B lymphocyte depletion in systemic lupus erythematosus. Arthritis Rheum. 46, 2673–2677 (2002).
Looney, R. J. et al. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum. 50, 2580–2589 (2004).
Merrill, J. T. et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 62, 222–233 (2010).
Rovin, B. H. et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the lupus nephritis assessment with rituximab study. Arthritis Rheum. 64, 1215–1226 (2012).
Ramos-Casals, M., Díaz-Lagares, C. & Khamashta, M. A. Rituximab and lupus: good in real life, bad in controlled trials. Comment on the article by Lu et al. Arthritis Rheum. 61, 1281–1282 (2009).
Carson, K. R. et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 113, 4834–4840 (2009).
US National Library of Medicine. ClinicalTrials.gov [online].
US National Library of Medicine. ClinicalTrials.gov [online].
US National Library of Medicine. ClinicalTrials.gov [online].
Mysler, E. F. et al. Efficacy and safety of ocrelizumab, a humanized antiCD20 antibody, in patients with active proliferative lupus nephritis (LN): results from the randomized, double-blind phase III BELONG study. Arthritis Rheum. 62, S606–S607 (2010).
Tedder, T. F. et al. The CD19–CD21 complex regulates signal transduction thresholds governing humoral immunity and autoimmunity. Immunity 6, 107–118 (1997).
Herbst, R. et al. B-cell depletion in vitro and in vivo with an afucosylated anti-CD19 antibody. J. Pharmacol. Exp. Ther. 335, 213–222 (2010).
Cardarelli, P. M. et al. A nonfucosylated human antibody to CD19 with potent B-cell depletive activity for therapy of B-cell malignancies. Cancer Immunol. Immunother. 59, 257–265 (2010).
US National Library of Medicine. ClinicalTrials.gov [online].
US National Library of Medicine. ClinicalTrials.gov [online].
Nitschke, L. CD22 and Siglec-G: B-cell inhibitory receptors with distinct functions. Immunol. Rev. 230, 128–143 (2009).
Carnahan, J. et al. Epratuzumab, a CD22-targeting recombinant humanized antibody with a different mode of action from rituximab. Mol. Immunol. 44, 1331–1341 (2007).
Dörner, T. et al. Initial clinical trial of epratuzumab (humanized anti-CD22 antibody) for immunotherapy of systemic lupus erythematosus. Arthritis Res. Ther. 8, R74 (2006).
Wallace, D. J. et al. Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placeo-controlled multicentre study. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2012-202760.
US National Library of Medicine. ClinicalTrials.gov [online].
US National Library of Medicine. ClinicalTrials.gov [online].
Heyman, B. Feedback regulation by IgG antibodies. Immunol. Lett. 88, 157–161 (2003).
Bolland, S. & Ravetch, J. V. Spontaneous autoimmune disease in FcγRIIB-deficient mice results from strain-specific epistasis. Immunity 13, 277–285 (2000).
Brownlie, R. J. et al. Distinct cell-specific control of autoimmunity and infection by FcγRIIb. J. Exp. Med. 205, 883–895 (2008).
Mackay, M. et al. Selective dysregulation of the FcγIIB receptor of memory B cells in SLE. J. Exp. Med. 203, 2157–2164 (2006).
Su, K. et al. Expression profile of FcγRIIb on leukocytes and its dysregulation in systemic lupus erythematosus. J. Immunol. 178, 3272–3280 (2007).
Floto, R. A. et al. Loss of function of a lupus-associated FcγRIIb polymorphism through exclusion from lipid rafts. Nat. Med. 11, 1056–1058 (2005).
Veri, M.-C. et al. Therapeutic control of B cell activation via recruitment of Fcγ receptor IIb (CD32B) inhibitory function with a novel bispecific antibody scaffold. Arthritis Rheum. 62, 1933–1943 (2010).
Depoil, D. et al. CD19 is essential for B cell activation by promoting B cell receptor–antigen microcluster formation in response to membrane-bound ligand. Nat. Immunol. 9, 63–72 (2008).
Chu, S. Y. et al. Inhibition of B cell receptor-mediated activation of primary human B cells by coengagement of CD19 and FcγRIIb with Fc-engineered antibodies. Mol. Immunol. 45, 3926–3933 (2008).
Horton, H. M. et al. Antibody-mediated coengagement of FcγRIIb and B cell receptor complex suppresses humoral immunity in systemic lupus erythematosus. J. Immunol. 186, 4223–4233 (2011).
EU Clinical Trials Register. Clinicaltrialsregister.eu [online].
Current Controlled Trials. Controlled-trials.com [online].
Fillatreau, S., Sweenie, C. H., McGeachy, M. J., Gray, D. & Anderton, S. M. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 3, 944–950 (2002).
Mizoguchi, A., Mizoguchi, E., Takedatsu, H., Blumberg, R. S. & Bhan, A. K. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 16, 219–230 (2002).
Haas, K. M. et al. Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice. J. Immunol. 184, 4789–4800 (2010).
Watanabe, R. et al. Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J. Immunol. 184, 4801–4809 (2010).
Blair, P. A. et al. CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals by are functionally impaired in systemic lupus erythematosus patients. Immunity 32, 129–140 (2010).
Iwata, Y. et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 117, 530–541 (2011).
Blair, P. A. et al. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J. Immunol. 182, 3492–3502 (2009).
US National Library of Medicine. ClinicalTrials.gov [online].
US National Library of Medicine. ClinicalTrials.gov [online].
Mihara, M. et al. Immunologic abnormality in NZB/NZW F1 mice: thymus-independent occurrence of B cell abnormality and requirement for T cells in the development of autoimmune disease, as evidenced by an analysis of the athymic nude individuals. J. Immunol. 141, 85–90 (1988).
Wofsy, D. & Seaman, W. E. Successful treatment of autoimmunity in NZB/NZW F1 mice with monoclonal antibody to L3T4. J. Exp. Med. 161, 378–391 (1985).
Chesnutt, M. S. et al. Enhanced lymphoproliferation and diminished autoimmunity in CD4-deficient MRL/lpr mice. Clin. Immunol. Immunopathol. 87, 23–32 (1998).
Molina, J. F. et al. Coexistence of human immunodeficiency virus infection and systemic lupus erythematosus. J. Rheumatol. 22, 347–350 (1995).
Diri, E., Lipsky, P. E. & Berggren, R. E. Emergence of lupus erythematosus after initiation of highly active antiretroviral therapy for human immunodeficiency virus infection. J. Rheumatol. 27, 2711–2714 (2000).
Groom, J. R. et al. BAFF and MyD88 signals promote a lupus-like disease independent of T cells. J. Exp. Med. 204, 1959–1971 (2007).
Crispin, J. C. et al. T cells as therapeutic targets in SLE. Nat. Rev. Rheumatol. 6, 317–325 (2010).
Hiepe, F., Volk, H.-D., Apostoloff, E., von Baehr, R. & Emmrich, F. Treatment of severe systemic lupus erythematosus with anti-CD4 monoclonal antibody. Lancet 338, 1529–1530 (1991).
Herold, K. C. et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N. Engl. J. Med. 346, 1692–1698 (2002).
Keymeulen, B. et al. Insulin needs after anti-CD3 antibody therapy in new-onset type 1 diabetes. N. Engl. J. Med. 352, 2598–2608 (2005).
Arbuckle, M. R. et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 349, 1526–1533 (2003).
Freeman, G. J. et al. B7, a new member of the Ig superfamily with unique expression on activated and neoplastic B cells. J. Immunol. 143, 2714–2722 (1989).
Linsley, P. S. et al. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J. Exp. Med. 173, 721–730 (1991).
Linsley, P. S. et al. CTLA-4 is a second receptor for the B cell activation antigen B7. J. Exp. Med. 174, 561–569 (1991).
Razi-Wolf, Z., Galvin, F., Gray, G. & Reiser, H. Evidence for an additional ligand, distinct from B7, for the CTLA-4 receptor. Proc. Natl Acad. Sci. USA 90, 11182–11186 (1993).
Azuma, M. et al. B70 antigen is a second ligand for CTLA-4 and CD28. Nature 366, 76–79 (1993).
Hathcock, K. S. et al. Identification of an alternative CTLA-4 ligand costimulatory for T cell activation. Science 262, 905–907 (1993).
Freeman, G. J. et al. Cloning of B7–2: a CTLA-4 counter-receptor that costimulated human T cell proliferation. Science 262, 909–911 (1993).
Linsley, P. S. et al. Human B7–1 (CD80) and B7–2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity 1, 793–801 (1994).
Finck, B. K., Linsley, P. S. & Wofsy, D. Treatment of murine lupus with CTLA4Ig. Science 265, 1225–1227 (1994).
Merrill, J. T. et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 62, 3077–3087 (2010).
Furie, R. et al. Efficacy and safety of abatacept over 12 months in patients with lupus nephritis: results from a multicenter, randomized, double-blind, placebo-controlled phase II/III study. Arthritis Rheum. 63, S962–S963 (2011).
Wofsy, D., Hillson, J. L. & Diamond, B. Abatacept for lupus nephritis: alternative definitions of complete response support conflicting conclusions. Arthritis Rheum. 64, 3660–3665 (2012).
US National Library of Medicine. ClinicalTrials.gov [online].
Buhlmann, J. E. et al. In the absence of a CD40 signal, B cells are tolerogenic. Immunity 2, 645–653 (1995).
Desai-Mehta, A., Lu, L., Ramsey-Goldman, R. & Datta, S. K. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J. Clin. Invest. 97, 2063–2073 (1996).
Koshy, M., Berger, D. & Crow, M. K. Increased expression of CD40 ligand on systemic lupus erythematosus lymphocytes. J. Clin. Invest. 98, 826–837 (1996).
Mohan, C., Shi, Y., Laman, J. D. & Datta, S. K. Interaction between CD40 and its ligand gp39 in the development of murine lupus nephritis. J. Immunol. 154, 1470–1480 (1995).
Early, G. S., Zhao, W. & Burns, C. M. Anti-CD40 ligand antibody treatment prevents the development of lupus-like nephritis in a subset of New Zealand Black X New Zealand White mice. J. Immunol. 157, 3159–3164 (1996).
Russell, J. Q. et al. Anti-CD40L accelerates renal disease and adenopathy in MRL-lpr mice in parallel with decreased thymocyte apoptosis. J. Immunol. 161, 729–739 (1998).
Kalunian, K. C. et al. Treatment of systemic lupus erythematosus by inhibition of T cell costimulation with anti-CD154. Arthritis Rheum. 46, 3251–3258 (2002).
Boumpas, D. T. et al. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 48, 719–727 (2003).
Hutloff, A. et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397, 263–266 (1999).
Yoshinaga, S. K. et al. T-cell co-stimulation through B7RP-1 and ICOS. Nature 402, 827–832 (1999).
Akiba, H. et al. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J. Immunol. 175, 2340–2348 (2005).
Dong, C. et al. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature 409, 97–101 (2001).
McAdam, A. J. et al. ICOS is critical for CD40-mediated antibody class switching. Nature 409, 102–105 (2001).
Tafuri, A. et al. ICOS is essential for effective T-helper-cell responses. Nature 409, 105–109 (2001).
Grimbacher, B. et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat. Immunol. 4, 261–268 (2003).
Vinuesa, C. G. et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 435, 452–458 (2005).
US National Library of Medicine. ClinicalTrials.gov [online].
US National Library of Medicine. ClinicalTrials.gov [online].
Usmani, N. & Goodfield, M. Efalizumab in the treatment of discoid lupus erythematosus. Arch. Dermatol. 143, 873–877 (2007).
Genentech. Press release: Genentech announces voluntary withdrawal of Raptiva from the U.S. market. Genentech [online].
Kappos, L. et al. Natalizumab treatment for multiple sclerosis: updated recommendations for patient selection and monitoring. Lancet Neurol. 10, 745–758 (2011).
Zhang, Y., Chen, Y.-C. M., Krummel, M. F. & Rosen, S. D. Autotaxin through lysophosphatidic acid stimulates polarization, motility, and transendothelial migration of naive T cells. J. Immunol. 189, 3914–3924 (2012).
Pappu, R. et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 316, 295–298 (2007).
Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336 (2003).
Khattri, R., Cox, T., Yasayko, S.-A. & Ramsdell, F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4, 337–342 (2003).
Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003).
Lee, J.-H. et al. Inverse correlation between CD4+ regulatory T-cell population and autoantibody levels in paediatric patients with systemic lupus erythematosus. Immunology 117, 280–286 (2006).
Alvarado-Sánchez, B. et al. Regulatory T cells in patients with systemic lupus erythematosus. J. Autoimmun. 27, 110–118 (2006).
Valencia, X., Yarboro, C., Illei, G. & Lipsky, P. E. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J. Immunol. 178, 2579–2588 (2007).
Zheng, S. G. et al. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-β suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J. Immunol. 172, 1531–1539 (2004).
Scalapino, K. J., Tang, Q., Bluestone, J. A., Bonyhadi, M. L. & Daikh, D. I. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J. Immunol. 177, 1451–1459 (2006).
Lan, Q. et al. Polyclonal CD4+Foxp3+ Treg cells induce TGFβ-dependent tolerogenic dendritic cells that suppress the murine lupus-like syndrome. J. Mol. Cell Biol. 4, 409–419 (2012).
US National Library of Medicine. ClinicalTrials.gov [online].
Abdulahad, W. H. et al. FoxP3+ CD4+ T cells in systemic autoimmune diseases: the delicate balance between true regulatory T cells and effector Th-17 cells. Rheumatology 50, 646–656 (2011).
Jacob, N. & Stohl, W. Cytokine disturbances in systemic lupus erythematosus. Arthritis Res. Ther. 13, 228 (2011).
Moore, P. A. et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science 285, 260–263 (1999).
Schneider, P. et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J. Exp. Med. 189, 1747–1756 (1999).
Nardelli, B. et al. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood 97, 198–204 (2001).
Laabi, Y. et al. The BCMA gene, preferentially expressed during B lymphoid maturation, is bidirectionally transcribed. Nucleic Acids Res. 22, 1147–1154 (1994).
von Bülow, G.-U. & Bram, R. J. NF-AT activation induced by a CAML-interacting member of the tumor necrosis factor receptor superfamily. Science 278, 138–141 (1997).
Thompson, J. S. et al. BAFF-R, a novel TNF receptor that specifically interacts with BAFF. Science 293, 2108–2111 (2001).
Yan, M. et al. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr. Biol. 11, 1547–1552 (2001).
Hahne, M. et al. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J. Exp. Med. 188, 1185–1190 (1998).
Marsters, S. A. et al. Interaction of the TNF homologues BLyS and APRIL with the receptor homologues BCMA and TACI. Curr. Biol. 10, 785–788 (2000).
Yu, G. et al. APRIL and TALL-1 and receptors BCMA and TACI: system for regulating humoral immunity. Nat. Immunol. 1, 252–256 (2000).
Rennert, P. et al. A soluble form of B cell maturation antigen, a receptor for the tumor necrosis factor family member APRIL, inhibits tumor cell growth. J. Exp. Med. 192, 1677–1683 (2000).
Roschke, V. et al. BLyS and APRIL form biologically active heterotrimers that are expressed in patients with systemic immune-based rheumatic diseases. J. Immunol. 169, 4314–4321 (2002).
Dillon, S. R. et al. B-lymphocyte stimulator/a proliferation-inducing ligand heterotrimers are elevated in the sera of patients with autoimmune disease and are neutralized by atacicept and B cell maturation antigen-immunoglobulin. Arthritis Res. Ther. 12, R48 (2010).
Mackay, F. et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 190, 1697–1710 (1999).
Khare, S. D. et al. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc. Natl Acad. Sci. USA 97, 3370–3375 (2000).
Gross, J. A. et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature 404, 995–999 (2000).
Cheema, G. S., Roschke, V., Hilbert, D. M. & Stohl, W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 44, 1313–1319 (2001).
Zhang, J. et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J. Immunol. 166, 6–10 (2001).
Petri, M. et al. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum. 58, 2453–2459 (2008).
Lesley, R. et al. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity 20, 441–453 (2004).
Thien, M. et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity 20, 785–798 (2004).
Ota, M. et al. Regulation of the B cell receptor repertoire and self-reactivity by BAFF. J. Immunol. 185, 4128–4136 (2010).
Nikbakht, N., Migone, T.-S., Ward, C. P. & Manser, T. Cellular competition independent of BAFF/B lymphocyte stimulator results in low frequency of an autoreactive clonotype in mature polyclonal B cell compartments. J. Immunol. 187, 37–46 (2011).
Kayagaki, N. et al. BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF-κB2. Immunity 17, 515–524 (2002).
Ramanujam, M. et al. Similarities and differences between selective and nonselective BAFF blockade in murine SLE. J. Clin. Invest. 116, 724–734 (2006).
Jacob, C. O. et al. Paucity of clinical disease despite serological autoimmunity and kidney pathology in lupus-prone New Zealand Mixed 2328 mice deficient in BAFF. J. Immunol. 177, 2671–2680 (2006).
Navarra, S. V. et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 377, 721–731 (2011).
Furie, R. et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 63, 3918–3930 (2011).
US National Library of Medicine. ClinicalTrials.gov [online].
US National Library of Medicine. ClinicalTrials.gov [online].
Bossen, C. et al. Mutation of the BAFF furin cleavage site impairs B-cell homeostasis and antibody response. Eur. J. Immunol. 41, 787–797 (2011).
Furie, R. A. et al. Blisibimod, an inhibitor of B cell activating factor, in patients with moderate-to-severe systemic lupus erythematosus. Arthritis Rheum. 64, 4169 (2012).
US National Library of Medicine. ClinicalTrials.gov [online].
Genovese, M. C. et al. Tabalumab in rheumatoid arthritis patients with an inadequate response to methotrexate and naive to biologic therapy: a phase II, randomized, placebo-controlled trial. Arthritis Rheum. 65, 880–889 (2013).
Genovese, M. C. et al. Tabalumab, an anti-BAFF monoclonal antibody, in patients with active rheumatoid arthritis with an inadequate response to TNF inhibitors. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2012-202775.
Lilly. Press release: Lilly discontinues phase 3 rheumatoid arthritis program for tabalumab based on efficacy results. Lilly [online].
US National Library of Medicine. ClinicalTrials.gov [online].
US National Library of Medicine. ClinicalTrials.gov [online].
Stein, J. V. et al. APRIL modulates B and T cell immunity. J. Clin. Invest. 109, 1587–1598 (2002).
Jacob, C. O. et al. Dispensability of APRIL to the development of systemic lupus erythematosus in NZM 2328 mice. Arthritis Rheum. 64, 1610–1619 (2012).
Lee, Y. H., Ota, F., Kim-Howard, X., Kaufman, K. M. & Nath, S. K. APRIL polymorphism and systemic lupus erythematosus (SLE) susceptibility. Rheumatology 46, 1274–1276 (2007).
Stohl, W. et al. Inverse association between circulating APRIL levels and serologic and clinical disease activity in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 63, 1096–1103 (2004).
Morel, J. et al. Serum levels of tumour necrosis factor family members a proliferation-inducing ligand (APRIL) and B lymphocyte stimulator (BLyS) are inversely correlated in systemic lupus erythematosus. Ann. Rheum. Dis. 68, 997–1002 (2009).
Dall'Era, M. et al. Reduced B lymphocyte and immunoglobulin levels after atacicept treatment in patients with systemic lupus erythematosus: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating trial. Arthritis Rheum. 56, 4142–4150 (2007).
Ginzler, E. M. et al. Atacicept in combination with MMF and corticosteroids in lupus nephritis: results of a prematurely terminated trial. Arthritis Res. Ther. 14, R33 (2012).
US National Library of Medicine. ClinicalTrials.gov [online].
Sasaki, Y., Casola, S., Kutok, J. L., Rajewski, K. & Schmidt-Supprian, M. TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J. Immunol. 173, 2245–2252 (2004).
Shulga-Morskaya, S. et al. B cell-activating factor belonging to the TNF family acts through separate receptors to support B cell survival and T cell-independent antibody formation. J. Immunol. 173, 2331–2341 (2004).
Xu, S. & Lam, D.-P. B-cell maturation protein, which binds the tumor necrosis factor family members BAFF and APRIL, is dispensable for humoral immune responses. Mol. Cell. Biol. 21, 4067–4074 (2001).
Schiemann, B. et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science 293, 2111–2114 (2001).
von Bülow, G.-U., van Deursen, J. M. & Bram, R. J. Regulation of the T-independent humoral response by TACI. Immunity 14, 573–582 (2001).
Yan, M. et al. Activation and accumulation of B cells in TACI-deficient mice. Nat. Immunol. 2, 638–643 (2001).
Lee, C. V. et al. Synthetic anti-BR3 antibodies that mimic BAFF binding and target both human and murine B cells. Blood 108, 3103–3111 (2006).
Lin, W. Y. et al. Anti-BR3 antibodies: a new class of B-cell immunotherapy combining cellular depletion and survival blockade. Blood 110, 3959–3967 (2007).
Jacob, C. O. et al. Development of systemic lupus erythematosus in NZM 2328 mice in the absence of any single BAFF receptor. Arthritis Rheum. 65, 1043–1054 (2013).
Santiago-Raber, M.-L. et al. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J. Exp. Med. 197, 777–788 (2003).
Agrawal, H. et al. Deficiency of type I IFN receptor in lupus-prone New Zealand Mixed 2328 mice decreases dendritic cell numbers and activation and protects from disease. J. Immunol. 183, 6021–6029 (2009).
Mathian, A., Weinberg, A., Gallegos, M., Banchereau, J. & Koutouzov, S. IFN-α induces early lethal lupus in preautoimmune (New Zealand Black X New Zealand White)F1 but not in BALB/c mice. J. Immunol. 174, 2499–2506 (2005).
Fairhurst, A.-M. et al. Systemic IFN-α drives kidney nephritis in B6.SLE123 mice. Eur. J. Immunol. 38, 1948–1960 (2008).
Ramanujam, M. et al. Interferon-α treatment of female (NZW X BXSB)F1 mice mimics some but not all features associated with the Yaa mutation. Arthritis Rheum. 60, 1096–1101 (2009).
Hron, J. D. & Peng, S. L. Type I IFN protects against murine lupus. J. Immunol. 173, 2134–2142 (2004).
Baechler, E. C. et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl Acad. Sci. USA 100, 2610–2615 (2003).
Bennett, L. et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 197, 711–723 (2003).
Kirou, K. A. et al. Activation of the interferon-α pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 52, 1491–1503 (2005).
Peterson, K. S. et al. Characterization of heterogeneity in the molecular pathogenesis of lupus nephritis from transcriptional profiles of laser-captured glomeruli. J. Clin. Invest. 113, 1722–1733 (2004).
Nzeusseu Toukap, A. et al. Identification of distinct gene expression profiles in the synovium of patients with systemic lupus erythematosus. Arthritis Rheum. 56, 1579–1588 (2007).
Rönnblom, L. E., Alm, G. V. & Öberg, K. E. Possible induction of systemic lupus erythematosus by interferon-α treatment in a patient with a malignant carcinoid tumor. J. Intern. Med. 227, 207–210 (1990).
Niewold, T. B. & Swedler, W. I. Systemic lupus erythematosus arising during interferon-α therapy for cryoglobulinemic vasculitis associated with hepatitis C. Clin. Rheumatol. 24, 178–181 (2005).
Thacker, S. G. et al. Type I interferons modulate vascular function, repair, thrombosis, and plaque progression in murine models of lupus and atherosclerosis. Arthritis Rheum. 64, 2975–2985 (2012).
Denny, M. F. et al. Interferon-α promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood 110, 2907–2915 (2007).
Somers, E. C. et al. Type I interferons are associated with subclinical markers of cardiovascular disease in a cohort of systemic lupus erythematosus patients. PLoS ONE 7, e37000 (2012).
Morimoto, A. M. et al. Association of endogenous anti-interferon-α autoantibodies with decreased interferon-pathway and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 63, 2407–2415 (2011).
Yao, Y. et al. Neutralization of interferon-α/β-inducible genes and downstream effect in a phase I trial of an anti-interferon-α monoclonal antibody in systemic lupus erythematosus. Arthritis Rheum. 60, 1785–1796 (2009).
Merrill, J. T. et al. Safety profile and clinical activity of sifalimumab, a fully human anti-interferon α monoclonal antibody, in systemic lupus erythematosus: a phase I, muticentre, double-blind randomised study. Ann. Rheum. Dis. 70, 1905–1913 (2011).
Petri, M. et al. Sifalimumab, a human anti-interferon-α monoclonal antibody, in systemic lupus erythematosus: a phase I randomized, controlled, dose-escalation study. Arthritis Rheum. 65, 1011–1021 (2013).
Tcherepanova, I., Curtis, M., Sale, M., Miesowicz, F. & Nicolette, C. Results of a randomized placebo controlled phase IA study of AGS-009, a humanized anti-interferon-α monoclonal antibody in subjects with systemic lupus erythematosus. Ann. Rheum. Dis. 71 (Suppl. 3), 536 (2012).
McBride, J. M. et al. Safety and pharmacodynamics of rontalizumab in patients with systemic lupus erythematosus: results of a phase I, placebo-controlled, double-blind, dose-escalation study. Arthritis Rheum. 64, 3666–3676 (2012).
Kalunian, K. et al. Efficacy and safety of rontalizumab (anti-interferon α) in SLE subjects with restricted immunosuppressant use: results of a randomized, double-blind placebo-controlled phase 2 study. Arthritis Rheum. 64, S1111 (2012).
US National Library of Medicine. ClinicalTrials.gov [online].
Mathian, A. et al. Active immunisation of human interferon α transgenic mice with a human interferon α kinoid induces antibodies that neutralise interferon α in sera from patients with systemic lupus erythematosus. Ann. Rheum. Dis. 70, 1138–1143 (2011).
US National Library of Medicine. ClinicalTrials.gov [online].
US National Library of Medicine. ClinicalTrials.gov [online].
US National Library of Medicine. ClinicalTrials.gov [online].
Cash, H. et al. Interleukin 6 (IL-6) deficiency delays lupus nephritis in MRL-Faslpr mice: The IL-6 pathway as a new therapeutic target in treatment of autoimmune kidney disease in systemic lupus erythematosus. J. Rheumatol. 37, 60–70 (2010).
Ryffel, B. et al. Interleukin-6 exacerbates glomerulonephritis in (NZB x NZM)F1 mice. Am. J. Pathol. 144, 927–937 (1994).
Finck, B. K., Chan, B. & Wofsy, D. Interleukin 6 promotes murine lupus in NZB/NZW F1 mice. J. Clin. Invest. 94, 585–591 (1994).
Mihara, M., Takagi, N., Takeda, Y. & Ohsugi, Y. IL-6 receptor blockage inhibits the onset of autoimmune kidney disease in NZB/W F1 mice. Clin. Exp. Immunol. 112, 397–402 (1998).
Linker-Israeli, M. et al. Elevated levels of endogenous IL-6 in systemic lupus erythematosus: a putative role in pathogenesis. J. Immunol. 147, 117–123 (1991).
Gröndal, G. et al. Cytokine production, serum levels and disease activity in systemic lupus erythematosus. Clin. Exp. Rheumatol. 18, 565–570 (2000).
Hirohata, S. et al. Accuracy of cerebrospinal fluid IL-6 testing for diagnosis of lupus psychosis. A multicenter retrospective study. Clin. Rheumatol. 28, 1319–1323 (2009).
Herrera-Esparza, R., Barbosa-Cisneros, O., Villalobos-Hurtado, R. & Avalos-Díaz, E. Renal expression of IL-6 and TNFα genes in lupus nephritis. Lupus 7, 154–158 (1998).
García-Hernández, F. J., González-León, R., Castillo-Palma, M. J., Ocaña-Medina, C. & Sánchez-Román, J. Tocilizumab for treating refractory haemolytic anaemia in a patient with systemic lupus erythematosus. Rheumatology 51, 1918–1919 (2012).
Kamata, Y. & Minota, S. Successful treatment of massive intractable pericardial effusion in a patient with systemic lupus erythematosus with tocilizumab. BMJ Case Rep. http://dx.doi.org/10.1136/bcr-2012-007834.
Illei, G. G. et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 62, 542–552 (2010).
US National Library of Medicine. ClinicalTrials.gov [online].
US National Library of Medicine. ClinicalTrials.gov [online].
Jacob, C. O. & McDevitt, H. O. Tumour necrosis factor-α in murine autoimmune 'lupus' nephritis. Nature 331, 356–358 (1988).
Gordon, C., Ranges, G. E., Greenspan, J. S. & Wofsy, D. Chronic therapy with recombinant tumor necrosis factor-α in autoimmune NZB/NZW F1 mice. Clin. Immunol. Immunopathol. 52, 421–434 (1989).
Kontoyiannis, D. & Kollias, G. Accelerated autoimmunity and lupus nephritis in NZB mice with an engineered heterozygous deficiency in tumor necrosis factor. Eur. J. Immunol. 30, 2038–2047 (2000).
Jacob, N. et al. Accelerated pathological and clinical nephritis in systemic lupus erythematosus-prone New Zealand Mixed 2328 mice doubly deficient in TNF receptor 1 and TNF receptor 2 via a Th17-associated pathway. J. Immunol. 182, 2532–2541 (2009).
Studnicka-Benke, A., Steiner, G., Petera, P. & Smolen, J. S. Tumour necrosis factor α and its soluble receptors parallel clinical disease and autoimmune activity in systemic lupus erythematosus. Br. J. Rheumatol. 35, 1067–1074 (1996).
Zhu, L.-J. et al. Altered expression of TNF-α signaling pathway proteins in systemic lupus erythematosus. J. Rheumatol. 37, 1658–1666 (2010).
Soforno, E. et al. Induction of systemic lupus erythematosus with tumor necrosis factor blockers. J. Rheumatol. 37, 204–205 (2010).
Aringer, M., Graninger, W. B., Steiner, G. & Smolen, J. S. Safety and efficacy of tumor necrosis factor α blockade in systemic lupus erythematosus: an open-label study. Arthritis Rheum. 50, 3161–3169 (2004).
Uppal, S. S., Hayat, S. J. & Raghupathy, R. Efficacy and safety of infliximab in active SLE: a pilot study. Lupus 18, 690–697 (2009).
Matsumura, R. et al. Anti-tumor necrosis factor therapy in patients with difficult-to-treat lupus nephritis: a prospective series of nine patients. Clin. Exp. Rheumatol. 27, 416–421 (2009).
Aringer, M. et al. Adverse events and efficacy of TNF-α blockade with infliximab in patients with systemic lupus erythematosus: long-term follow-up of 13 patients. Rheumatology 48, 1451–1454 (2009).
US National Library of Medicine. ClinicalTrials.gov [online].
US National Library of Medicine. ClinicalTrials.gov [online].
Stohl, W. & Hilbert, D. M. The discovery and development of belimumab: the anti-BLyS–lupus connection. Nat. Biotechnol. 30, 69–77 (2012).
Acknowledgements
The author's research is funded in part by a grant from the Alliance for Lupus Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares that he is a member of a scientific advisory board for Eli Lilly, has consulted for Novartis, and has received preclinical grant support from Xencor.
Rights and permissions
About this article
Cite this article
Stohl, W. Future prospects in biologic therapy for systemic lupus erythematosus. Nat Rev Rheumatol 9, 705–720 (2013). https://doi.org/10.1038/nrrheum.2013.136
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2013.136
This article is cited by
-
Effect and safety profile of belimumab and tacrolimus combination therapy in thirty-three patients with systemic lupus erythematosus
Clinical Rheumatology (2022)
-
BAFF-neutralizing interaction of belimumab related to its therapeutic efficacy for treating systemic lupus erythematosus
Nature Communications (2018)
-
Beyond TNF: TNF superfamily cytokines as targets for the treatment of rheumatic diseases
Nature Reviews Rheumatology (2017)
-
A meta-analysis of public microarray data identifies gene regulatory pathways deregulated in peripheral blood mononuclear cells from individuals with Systemic Lupus Erythematosus compared to those without
BMC Medical Genomics (2016)
-
Diagnostic value of progranulin in patients with lupus nephritis and its correlation with disease activity
Rheumatology International (2016)