Abstract
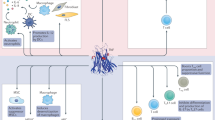
The failure of many new, mostly biologic, drugs to meet their primary end points in double-blind clinical trials in patients with systemic lupus erythematosus (SLE) has caused a profound sense of disappointment among both physicians and patients. Arguably, the success of B cell depletion with rituximab in open-label studies and in patients with lupus nephritis in the USA and in difficult-to-treat patients with SLE in the UK, together with the approval of belimumab (which blocks B cell-activating factor (BAFF)) for use in patients with SLE and the recognition that clinical trial design can be improved, have given some cause for hope. However, changes to therapies in current use and the development of new approaches are urgently needed. The results of the latest studies investigating the use of several new approaches to treating SLE are discussed in this Review, including: fully humanized anti-CD20 and anti-CD19 monoclonal antibodies; inhibition of tyrosine-protein kinase BTK; CD40 ligand blockade; interfering with the presentation of antigen to autoreactive T cells using a peptide approach; a receptor decoy approach using an analogue of Fcγ receptor IIB; dual blockade of IL-12 and IL-23; and inhibition of Janus kinases.
Key points
-
The approval of new therapies, especially biologic drugs, for systemic lupus erythematosus (SLE) has been scarce in comparison to rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis.
-
Belimumab (FDA approved) and rituximab (National Health Service England approved) are available for use in some countries, although the cost (particularly of belimumab) mitigates their universal uptake.
-
Clinical trial design for SLE is problematic, and success in phase II trials is not often followed by success in phase III trials.
-
Several new approaches are under investigation that target B cells, cytokines or intracellular signalling pathways, providing hope that new therapies will be approved for SLE.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Change history
03 July 2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Bernatsky, S. et al. in Dubois’ Lupus Erythematosus and Related Syndromes 8th edn Ch. 57 (eds Wallace, D. J. & Hahn, B. H.) 666–675 (Elsevier, 2013).
Carli, L. et al. Risk factors for osteoporosis and fragility fractures in patients with systemic lupus erythematosus. Lupus Sci. Med. 3, e000098 (2016).
Goldblatt, F., Chambers, S., Rahman, A. & Isenberg, D. A. Serious infections in British patients with systemic lupus erythematosus: hospitalisations and mortality. Lupus 18, 682–689 (2009).
Stojan, G. & Petri, M. Atherosclerosis in systemic lupus erythematosus. J. Cardiovasc. Pharmacol. 62, 255–262 (2013).
Croca, S. C., Rodrigues, T. & Isenberg, D. A. Assessment of a lupus nephritis cohort over a 30-year period. Rheumatology 50, 1424–1430 (2011).
Isenberg, D. A. & Rahman, A. Systemic lupus erythematosus in 2013. Taking a closer look at biologic therapy for SLE. Nat. Rev. Rheumatol. 10, 71–72 (2014).
Touma, Z. & Gladman, D. D. Current and future therapies for SLE: obstacles and recommendations for the development of novel treatments. Lupus Sci. Med. 4, e000239 (2017).
Ciurtin, C. & Isenberg, D. (eds) Biologics in Rheumatology: New Developments, Clinical Uses and Health Implication (Nova Science Publishers, 2016).
Nashi, E., Wang, Y. & Diamond, B. The role of B cells in lupus pathogenesis. Int. J. Biochem. Cell Biol. 42, 543–550 (2010).
Chan, V. S., Tsang, H. H., Tam, R. C., Lu, L. & Lau, C. S. B cell-targeted therapies in systemic lupus erythematosus. Cell. Mol. Immunol. 10, 133–142 (2013).
Ramos, L. & Isenberg, D. Rituximab: the lupus journey. Curr. Treat. Opt. Rheum. 1, 30–41 (2015).
Carreira, P. L. & Isenberg, D. A. Recent developments in biologic therapies for the treatment of patients with systemic lupus erythematosus. Rheumatology 58, 382–387 (2019).
McCarthy, E. M. et al. Short-term efficacy and safety of rituximab therapy in refractory systemic lupus erythematosus: results from the British Isles Lupus Assessment Group Biologics Register. Rheumatology 57, 410–479 (2018).
Hahn, B. H. et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res. 64, 797–808 (2012).
Bertsias, G. K. et al. Joint European League Against Rheumatism and European Renal Association–European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann. Rheum. Dis. 71, 1771–1782 (2012).
NHS England. Clinical commissioning policy statement: rituximab for the treatment of systemic lupus erythematosus in adults. NHS England www.england.nhs.uk/wp-content/uploads/2018/07/Rituximab-for-the-treatment-of-systemic-lupus-erythematosus-in-adults.pdf (2013).
Reddy, V., Martinez, L., Isenberg, D. A., Leandro, M. J. & Cambridge, G. Pragmatic treatment of patients with systemic lupus erythematosus with rituximab: long-term effects on serum immunoglobulins. Arthritis Care Res. 69, 857–866 (2017).
Aguiar, R., Araújo, C., Martins-Coelho, G. & Isenberg, D. Use of rituximab in systemic lupus erythematosus: a single centre experience over 14 years. Arthritis Care Res. 69, 257–262 (2017).
Hennessey, A., Lukawaska, J., Cambridge, G., Isenberg, D. & Leandro, M. AB0443 Infusion reactions to rituximab in systemic lupus erythematosus [abstract]. Ann. Rheum. Dis. 76 (Suppl. 2), 1205 (2017).
Furie, R. et al. A phase III randomized placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 63, 3918–3930 (2011).
Navarra, S. V. et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 377, 721–731 (2011).
Zhang, F. et al. A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Ann. Rheum. Dis. 77, 355–363 (2018).
Stohl, W. et al. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: a fifty-two-week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. 69, 1016–1027 (2017).
Iaccarino, L. et al. Clinical predictors of response and discontinuation of belimumab in patients with systemic lupus erythematosus in real life setting. Results of a large, multicentric, nationwide study. J. Autoimmun. 86, 1–8 (2018).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01639339 (2019).
Feld, J. & Isenberg, D. Why and how should we measure disease activity and damage in lupus? Presse Med. 43, e151–e156 (2014).
Isenberg, D. et al. Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial). Ann. Rheum. Dis. 74, 2006–2015 (2015).
Merrill, J. T. et al. Safety profile in SLE patients treated with atacicept in a phase IIb study (ADDRESS II) and its extension study [abstract]. Arthritis Rheumatol. 69 (Suppl. 10), 2585 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT00626197 (2017).
Isenberg, D. A. et al. Efficacy and safety of subcutaneous tabalumab in patients with systemic lupus erythematosus: results from ILLUMINATE-1, a 52-week, phase III, multicentre randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 75, 323–331 (2016).
Merrill, J. et al. Efficacy and safety of subcutaneous tabalumab, a monoclonal antibody to B cell activating factor, in patients with systemic lupus erythematosus: results from ILLUMINATE-2, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 75, 332–340 (2016).
Du, F. H., Mills, E. A. & Mao-Draayer, Y. Next-generation anti-CD20 monoclonal antibodies in autoimmune disease treatment. Auto Immun. Highlights 8, 12 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT00539838 (2017).
Mysler, E. F. et al. Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: results from a randomized, double-blind, phase III study. Arthritis Rheum. 65, 2368–2379 (2013).
Reddy, V. et al. Obinutuzumab induces superior B cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples. Rheumatology 56, 1227–1237 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02550652 (2019).
Montillo, M. et al. Autoimmune hemolytic anemia and immune mediated thrombocytopenia in the phase III RESONATETM study of ibrutinib versus ofatumumab in relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma, including a case report. Blood 124, 5654 (2014).
Haarhaus, M. L., Svenungsson, E. & Gunnarsson, I. Ofatumumab treatment in lupus nephritis patients. Clin. Kidney J. 9, 552–555 (2016).
Lavie, F. et al. Increase of B cell-activating factor of the TNF family (BAFF) after rituximab treatment: insights into a new regulating system of BAFF production. Ann. Rheum. Dis. 66, 700–703 (2007).
Dall’Era, M. et al. Phase 2 trial of induction therapy with anti-CD20 (rituximab) followed by maintenance therapy with anti-BAFF (belimumab) in patients with active lupus nephritis [abstract]. Arthritis Rheumatol. 70 (Suppl. 10), 1870 (2018).
Kraaij, T. et al. PS7:129 synergetic B cell immunomodulation with rituximab and belimumab combination treatment in severe, refractory SLE [abstract]. Lupus Sci. Med. 5 (Suppl. 1), A99 (2018).
ISRCTN Registry. Belimumab after B cell depletion therapy as a new treatment for patients with systemic lupus erythematosus (SLE). ISRCTN.com http://www.isrctn.com/ISRCTN47873003 (2019).
Merrill, J. T. et al. Top-line results of a phase 2, double-blind, randomized, placebo-controlled study of a reversible B cell inhibitor, XmAb®5871, in systemic lupus erythematous (SLE) [abstract]. Arthritis Rheumatol. 70 (Suppl. 10), L19 (2018).
López-Herrera, G. et al. Bruton’s tyrosine kinase — an integral protein of B cell development that also has an essential role in the innate immune system. J. Leukoc. Biol. 95, 243–250 (2014).
Rankin, A. L. et al. Selective inhibition of BTK prevents murine lupus and antibody-mediated glomerulonephritis. J. Immunol. 191, 4540–4550 (2013).
Kil, L. P. et al. Btk levels set the threshold for B cell activation and negative selection of autoreactive B cells in mice. Blood 119, 3744–3756 (2012).
Hutcheson, J. et al. Modulating proximal cell signaling by targeting Btk ameliorates humoral autoimmunity and end-organ disease in murine lupus. Arthritis Res. Ther. 14, R243 (2012).
Katsumato, T. et al. Safety, pharmacokinetics, and biomarker profile from phase 1 clinical trials of healthy volunteers treated with GDC-0853, a highly selective reversible oral Bruton’s tyrosine kinase (BTK) inhibitor [abstract]. Arthritis Rheumatol. 68 (Suppl. 10), 2622 (2016).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02908100 (2019).
Elgueta, R. et al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 229, 152–172 (2009).
Koshy, M., Berger, D. & Crow, M. K. Increased expression of CD40 ligand on systemic lupus erythematosus lymphocytes. J. Clin. Invest. 98, 826–837 (1996).
Higuchi, T. et al. Cutting edge. Ectopic expression of CD40 ligand on B cells induces lupus-like autoimmune disease. J. Immunol. 168, 9–12 (2002).
Early, G. S., Zhao, W. & Burns, C. M. Anti-CD40 ligand antibody treatment prevents the development of lupus-like nephritis in a subset of New Zealand black × New Zealand white mice. Response correlates with the absence of an anti-antibody response. J. Immunol. 157, 3159–3164 (1996).
Boumpas, D. T. et al. A short course of BG9588 (anti-CD40 ligand antibody) improves serological activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 48, 719–727 (2003).
Kalunian, K. C. et al. Treatment of systemic lupus erythematosus by inhibition of T cell costimulation with anti-CD154: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 46, 3251–3258 (2002).
Law, C.-L. et al. in Therapeutic Targets of the TNF Superfamily Ch. 2 (ed. Grewal, I. S.) 8–36 (Springer, 2009).
Langer, F. et al. The role of CD40 in CD40L- and antibody-mediated platelet activation. Thromb. Haemost. 93, 1137–1146 (2005).
Chamberlain, C. et al. Repeated administration of dapirolizumab pegol in a randomised phase I study is well tolerated and accompanied by improvements in several composite measures of systemic lupus erythematosus disease activity and changes in whole blood transcriptomic profiles. Ann. Rheum. Dis. 76, 1837–1844 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02804763 (2019).
UCB. UCB and Biogen announce topline results from a phase 2b study of dapirolizumab pegol in systemic lupus erythematosus. UCB https://www.ucb.com/stories-media/Press-Releases/article/UCB-and-Biogen-Announce-Topline-Results-from-a-Phase-2b-Study-of-Dapirolizumab-Pegol-in-Systemic-Lupus-Erythematosus (2018).
Hutloff, A. et al. ICOS is an inducible T cell co-stimulator structurally and functionally related to CD28. Nature 397, 263–266 (1999).
Yang, J. H. et al. Expression and function of inducible costimulator on peripheral blood T cells in patients with systemic lupus erythematosus. Rheumatology 44, 1245–1254 (2005).
Sullivan, B. A. et al. Inducible T cell co-stimulator ligand (ICOSL) blockade leads to selective inhibition of anti-KLH IgG responses in subjects with systemic lupus erythematosus. Lupus Sci. Med. 3, e000146 (2016).
Daëron, M. Fc receptor biology. Annu. Rev. Immunol. 15, 203–234 (1997).
Niederer, H. A., Clatworthy, M. R., Willcocks, L. C. & Smith, K. G. FcγRIIB, FcγRIIIB, and systemic lupus erythematosus. Ann. NY Acad. Sci. 1183, 69–88 (2010).
Tillmans, S. et al. SM101, a novel recombinant, soluble, human FcγIIB receptor, in the treatment of systemic lupus erythematosus: results of a double-blind, placebo-controlled multicenter study [abstract]. Arthritis Rheum. 66 (Suppl. 10), 2833 (2014).
Monneaux, F., Lozano, J. M., Patarroyo, M. E., Briand, J. P. & Muller, S. T cell recognition and therapeutic effect of a phosphorylated synthetic peptide of the 70K snRNP protein administered in MR/lpr mice. Eur. J. Immunol. 33, 287–296 (2003).
Page, N. et al. The spliceosomal phosphopeptide P140 controls the lupus disease by interacting with the HSC70 protein and via a mechanism mediated by γδ T cells. PLOS ONE 4, e5273 (2009).
Zimmer, R., Scherbarth, H. R., Rillo, O. L., Gomez-Reino, J. J. & Muller, S. Lupuzor/P140 peptide in patients with systemic lupus erythematosus: a randomised, double-blind, placebo-controlled phase IIb clinical trial. Ann. Rheum. Dis. 72, 1830–1835 (2013).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02504645 (2019).
ImmuPharma. Top line results of Lupuzor™ pivotal phase III trial. ImmuPharma https://www.immupharma.co.uk/top-line-results-lupuzor-pivotal-phase-iii-trial/ (2018).
Figueiredo, M. Add-on Lupuzor fails primary goal but shows some positive results in phase 3 trial for SLE. Lupus News Today https://lupusnewstoday.com/2018/04/23/lupuzor-shows-promising-results-in-phase-3-study-in-lupus-patients (2018).
Bennett, L. et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 197, 711–723 (2003).
Bronson, P. G., Chaivorapol, C., Ortmann, W., Behrens, T. W. & Graham, R. R. The genetics of type I interferon in systemic lupus erythematosus. Curr. Opin. Immunol. 24, 530–537 (2012).
Niewold, T. B. Interferon alpha-induced lupus: proof of principle. J. Clin. Rheumatol. 14, 131–132 (2008).
de Weerd, N. A., Samarajiwa, S. A. & Hertzog, P. J. Type I interferon receptors: biochemistry and biological functions. J. Biol. Chem. 282, 20053–20057 (2007).
Kalunian, K. C. et al. A phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-α) in patients with systemic lupus erythematosus (ROSE). Ann. Rheum. Dis. 75, 196–202 (2016).
Khamastha, M. et al. Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo controlled study. Ann. Rheum. Dis. 75, 1909–1916 (2016).
Furie, R. et al. Anifrolumab, an anti-interferon-α receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol. 69, 376–386 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02446899 (2018).
AstraZeneca. Update on TULIP 1 phase III trial for anifrolumab in systemic lupus erythematosus. AstraZeneca https://www.astrazeneca.com/investor-relations/stock-exchange-announcements/2018/update-on-tulip-1-phase-iii-trial-for-anifrolumab-in-systemic-lupus-erythematosus-31082018.html (2018).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02547922 (2018).
Ducreux, J. et al. Interferon α kinoid induces neutralizing anti-interferon α antibodies that decrease the expression of interferon-induced and B cell activation associated transcripts: analysis of extended follow-up data from the interferon α kinoid phase I/II study. Rheumatology 55, 1901–1905 (2016).
Lauwerys, B. R. et al. Down-regulation of interferon signature in systemic lupus erythematosus patients by active immunization with interferon α-kinoid. Arthritis Rheum. 65, 447–456 (2013).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02665364 (2019).
Yin, Q. et al. Comprehensive assessment of the association between genes on JAK–STAT pathway (IFIH1, TYK2, IL-10) and systemic lupus erythematosus: a meta-analysis. Arch. Dermatol. Res. 310, 711–728 (2018).
Fragoulis, G. E., McInnes, I. B. & Siebert, S. JAK-inhibitors. New players in the field of immune-mediated diseases, beyond rheumatoid arthritis. Rheumatology 58, i43–i54 (2019).
Furumoto, Y. et al. Tofacitinib ameliorates murine lupus and its associated vascular dysfunction. Arthritis Rheumatol. 69, 148–160 (2017).
Wallace, D. J. et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 392, 222–231 (2018).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT03616964 (2019).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT03616912 (2019).
Kavanaugh, A. et al. Ustekinumab, an anti-Il-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann. Rheum. Dis. 73, 1000–1006 (2014).
Leng, R. X. et al. IL-23: a promising therapeutic target for systemic lupus erythematosus. Arch. Med. Res. 41, 221–225 (2010).
van Vollenhoven, R. F. et al. Efficacy and safety of ustekinumab an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet 392, 1330–1339 (2018).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT03517722 (2019).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02535689 (2018).
Acknowledgements
D.A.I. acknowledges the support of the Biomedical Research Centre award to University College Hospital and University College London.
Reviewer information
Nature Reviews Rheumatology thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
D.A.I. declares that he has received honoraria from AstraZeneca, Celgene, Eli Lilly, Merck Serono, Roche, UCB and XTL Biopharmaceuticals. G.M. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Murphy, G., Isenberg, D.A. New therapies for systemic lupus erythematosus — past imperfect, future tense. Nat Rev Rheumatol 15, 403–412 (2019). https://doi.org/10.1038/s41584-019-0235-5
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-019-0235-5
This article is cited by
-
Natural language processing to identify lupus nephritis phenotype in electronic health records
BMC Medical Informatics and Decision Making (2024)
-
Regulatory T cells expressing CD19-targeted chimeric antigen receptor restore homeostasis in Systemic Lupus Erythematosus
Nature Communications (2024)
-
Cytokines inhibitory mechanism of Prunus domestica L. (Plum) peptides as potential immunomodulators against systemic lupus erythematosus: an in-silico screening
In Silico Pharmacology (2024)
-
Safety and efficacy of biologics in childhood systemic lupus erythematosus: a critical systematic review
Clinical Rheumatology (2024)
-
hUC-MSC transplantation therapy effects on lupus-prone MRL/lpr mice at early disease stages
Stem Cell Research & Therapy (2023)