Key Points
-
Autoantibodies directed against or crossreacting with kidney antigens form immune complexes in the periphery or in situ and activate complement; immune complexes deposited in the kidney can alter the structure and function of resident kidney cells and facilitate immune-cell infiltration
-
Various T-cell subtypes exist: T regulatory cells prevent autoimmunity, whereas T follicular helper (TFH) cells, T-helper (TH) type 1 and type 2 cells, CD8+ T cells and resident memory T cells are implicated in the pathogenesis of autoimmune nephropathies
-
IL-17-producing T cells (γδT cells, TH17 cells and double-negative T cells) promote renal impairment by supporting self-reactive B-cell survival, differentiation, and subsequent antibody production, and by promoting inflammation in infiltrated tissues
-
Autoimmune kidney disease is also influenced by molecular aberrations in T cells; for example, increased expression of the transcription factors CREM and STAT3 drives differentiation of TH17 and TFH cells
-
Alteration in molecules such as CaMKIV, ROCK and mTOR also influence the inflammatory response and contribute to kidney cell damage in autoimmune disease
-
Some of these molecules control the function of glomerular epithelial cells and their inhibition has been shown to have therapeutic value in preclinical studies; they might therefore represent suitable targets for clinical trials
Abstract
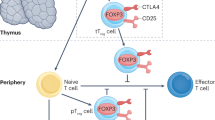
Glomerulonephritis is traditionally considered to result from the invasion of the kidney by autoantibodies and immune complexes from the circulation or following their formation in situ, and by cells of the innate and the adaptive immune system. The inflammatory response leads to the proliferation and dysfunction of cells of the glomerulus, and invasion of the interstitial space with immune cells, resulting in tubular cell malfunction and fibrosis. T cells are critical drivers of autoimmunity and related organ damage, by supporting B-cell differentiation and antibody production or by directly promoting inflammation and cytotoxicity against kidney resident cells. T cells might become activated by autoantigens in the periphery and become polarized to secrete inflammatory cytokines before entering the kidney where they have the opportunity to expand owing to the presence of costimulatory molecules and activating cytokines. Alternatively, naive T cells could enter the kidney where they become activated after encountering autoantigen and expand locally. As not all individuals with a peripheral autoimmune response to kidney antigens develop glomerulonephritis, the contribution of local kidney factors expressed or produced by kidney cells is probably of crucial importance. Improved understanding of the biochemistry and molecular biology of T cells in patients with glomerulonephritis offers unique opportunities for the recognition of treatment targets for autoimmune kidney disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Zhang, Q. & Vignali, D. A. A. Co-stimulatory and co-inhibitory pathways in autoimmunity. Immunity 44, 1034–1051 (2016).
Holdsworth, S. R., Gan, P.-Y. & Kitching, A. R. Biologics for the treatment of autoimmune renal diseases. Nat. Rev. Nephrol. 12, 217–231 (2016).
Tsokos, G. C. Systemic lupus erythematosus. N. Engl. J. Med. 365, 2110–2121 (2011).
Ronco, P. & Debiec, H. Membranous nephropathy: a fairy tale for immunopathologists, nephrologists and patients. Mol. Immunol. 68, 57–62 (2015).
Cui, Z. & Zhao, M.-H. Advances in human antiglomerular basement membrane disease. Nat. Rev. Nephrol. 7, 697–705 (2011).
Jennette, J. C. & Falk, R. J. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat. Rev. Rheumatol. 10, 463–473 (2014).
Zhao, Z. et al. Cross-reactivity of human lupus anti-DNA antibodies with alpha-actinin and nephritogenic potential. Arthritis Rheum. 52, 522–530 (2005).
Suzuki, N., Otuka, I., Harada, T., Mizushima, Y. & Sakane, T. Preferential adsorption of cationic anti-DNA antibodies with immobilized polyanionic compounds, dextran sulfate. Autoimmunity 19, 105–112 (1994).
Mastroianni-Kirsztajn, G., Hornig, N. & Schlumberger, W. Autoantibodies in renal diseases — clinical significance and recent developments in serological detection. Front. Immunol. http://dx.doi.org/10.3389/fimmu.2015.00221 (2015).
Flierman, R. & Daha, M. R. Pathogenic role of anti-C1q autoantibodies in the development of lupus nephritis — a hypothesis. Mol. Immunol. 44, 133–138 (2007).
Couser, W. G. Basic and translational concepts of immune-mediated glomerular diseases. J. Am. Soc. Nephrol. 23, 381–399 (2012).
Kurts, C., Panzer, U., Anders, H.-J. & Rees, A. J. The immune system and kidney disease: basic concepts and clinical implications. Nat. Rev. Immunol. 13, 738–753 (2013).
Alexopoulos, E., Seron, D., Hartley, R. B., Nolasco, F. & Cameron, J. S. Immune mechanisms in idiopathic membranous nephropathy: the role of the interstitial infiltrates. Am. J. Kidney Dis. 13, 404–412 (1989).
Turner, J.-E. et al. IL-17A production by renal γδ T cells promotes kidney injury in crescentic GN. J. Am. Soc. Nephrol. 23, 1486–1495 (2012).
Mayadas, T. N., Rosetti, F., Ernandez, T. & Sethi, S. Neutrophils: game changers in glomerulonephritis? Trends Mol. Med. 16, 368–378 (2010).
Lood, C. et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 22, 146–153 (2016).
Gupta, S. & Kaplan, M. J. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat. Rev. Nephrol. 12, 402–413 (2016).
Mistry, P. & Kaplan, M. J. Cell death in the pathogenesis of systemic lupus erythematosus and lupus nephritis. Clin. Immunol. http://dx.doi.org/10.1016/j.clim.2016.08.010 (2016).
Boilard, E. & Fortin, P. R. Connective tissue diseases: mitochondria drive NETosis and inflammation in SLE. Nat. Rev. Rheumatol. 12, 195–196 (2016).
Campbell, A. M., Kashgarian, M. & Shlomchik, M. J. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci. Transl Med. 4, 157ra141 (2012).
Rosenzweig, S. D. Inflammatory manifestations in chronic granulomatous disease (CGD). J. Clin. Immunol. 28 (Suppl. 1), S67–S72 (2008).
Spada, R., Rojas, J. M. & Barber, D. F. Recent findings on the role of natural killer cells in the pathogenesis of systemic lupus erythematosus. J. Leukoc. Biol. 98, 479–487 (2015).
Davidson, A. What is damaging the kidney in lupus nephritis? Nat. Rev. Rheumatol. 12, 143–153 (2016).
Chen, J. et al. Immunoregulation of NKT cells in systemic lupus erythematosus, immunoregulation of NKT cells in systemic lupus erythematosus. J. Immunol. Res. 2015, e206731 (2015).
Liarski, V. M. et al. Cell distance mapping identifies functional T follicular helper cells in inflamed human renal tissue. Sci. Transl Med. 6, 230ra46 (2014).
Hiepe, F. & Radbruch, A. Plasma cells as an innovative target in autoimmune disease with renal manifestations. Nat. Rev. Nephrol. 12, 232–240 (2016).
Lalor, S. J. & McLoughlin, R. M. Memory γδ T cells-newly appreciated protagonists in infection and immunity. Trends Immunol. 37, 690–702 (2016).
Paul, S., Shilpi & Lal, G. Role of gamma-delta (γδ) T cells in autoimmunity. J. Leukoc. Biol. 97, 259–271 (2015).
Rajagopalan, S., Zordan, T., Tsokos, G. C. & Datta, S. K. Pathogenic anti-DNA autoantibody-inducing T helper cell lines from patients with active lupus nephritis: isolation of CD4-8- T helper cell lines that express the gamma delta T-cell antigen receptor. Proc. Natl Acad. Sci. USA 87, 7020–7024 (1990).
Peng, X. et al. IL-17A produced by both γδ T and Th17 cells promotes renal fibrosis via RANTES-mediated leukocyte infiltration after renal obstruction. J. Pathol. 235, 79–89 (2015).
Yin, S. et al. Hyperactivation and in situ recruitment of inflammatory Vδ2 T cells contributes to disease pathogenesis in systemic lupus erythematosus. Sci. Rep. 5, 14432 (2015).
Chen, H. et al. Chaperonin-containing T-complex protein 1 subunit ζ serves as an autoantigen recognized by human Vδ2 γδ T cells in autoimmune diseases. J. Biol. Chem. 291, 19985–19993 (2016).
Thomson, C. W., Lee, B. P.-L. & Zhang, L. Double-negative regulatory T cells: non-conventional regulators. Immunol. Res. 35, 163–178 (2006).
Rodríguez-Rodríguez, N. et al. Programmed cell death 1 and Helios distinguish TCR-αβ+ double-negative (CD4-CD8-) T cells that derive from self-reactive CD8 T cells. J. Immunol. 194, 4207–4214 (2015).
Crispín, J. C. et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J. Immunol. 181, 8761–8766 (2008).
Oliveira, J. B. et al. Revised diagnostic criteria and classification for the autoimmune lymphoproliferative syndrome (ALPS): report from the 2009 NIH International Workshop. Blood 116, e35–e40 (2010).
Alunno, A. et al. CD4−CD8− T-cells in primary Sjögren's syndrome: association with the extent of glandular involvement. J. Autoimmun. 51, 38–43 (2014).
Tarbox, J. A. et al. Elevated double negative T cells in pediatric autoimmunity. J. Clin. Immunol. 34, 594–599 (2014).
Rodríguez-Rodríguez, N. et al. Pro-inflammatory self-reactive T cells are found within murine TCR-αβ+ CD4− CD8− PD-1+ cells. Eur. J. Immunol. 46, 1383–1391 (2016).
Yamagata, T., Skepner, J. & Yang, J. Targeting Th17 effector cytokines for the treatment of autoimmune diseases. Arch. Immunol. Ther. Exp. (Warsz.) 63, 405–414 (2015).
Gaffen, S. L., Jain, R., Garg, A. V. & Cua, D. J. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 14, 585–600 (2014).
Turner, J.-E., Paust, H.-J., Steinmetz, O. M. & Panzer, U. The Th17 immune response in renal inflammation. Kidney Int. 77, 1070–1075 (2010).
Deteix, C. et al. Intragraft Th17 infiltrate promotes lymphoid neogenesis and hastens clinical chronic rejection. J. Immunol. 184, 5344–5351 (2010).
Mitsdoerffer, M. et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc. Natl Acad. Sci. USA 107, 14292–14297 (2010).
Schaffert, H. et al. IL-17-producing CD4+ T cells contribute to the loss of B-cell tolerance in experimental autoimmune myasthenia gravis. Eur. J. Immunol. 45, 1339–1347 (2015).
Krebs, C. F. et al. Autoimmune renal disease is exacerbated by S1P-receptor-1-dependent intestinal Th17 cell migration to the kidney. Immunity 45, 1078–1092 (2016).
Velden, J. et al. Renal IL-17 expression in human ANCA-associated glomerulonephritis. Am. J. Physiol. Renal Physiol. 302, F1663–F1673 (2012).
Koga, T., Ichinose, K. & Tsokos, G. C. T cells and IL-17 in lupus nephritis. Clin. Immunol. http://dx.doi.org/10.1016/j.clim.2016.04.010 (2016).
Krebs, C. F. et al. Plasticity of Th17 cells in autoimmune kidney diseases. J. Immunol. 197, 449–457 (2016).
Hünemörder, S. et al. TH1 and TH17 cells promote crescent formation in experimental autoimmune glomerulonephritis. J. Pathol. 237, 62–71 (2015).
Craft, J. E. Follicular helper T cells in immunity and systemic autoimmunity. Nat. Rev. Rheumatol. 8, 337–347 (2012).
Ueno, H. T follicular helper cells in human autoimmunity. Curr. Opin. Immunol. 43, 24–31 (2016).
Morita, R. et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 34, 108–121 (2011).
Locci, M. et al. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 39, 758–769 (2013).
Steinmetz, O. M. et al. CXCR3 mediates renal Th1 and Th17 immune response in murine lupus nephritis. J. Immunol. 183, 4693–4704 (2009).
Paust, H.-J. et al. CXCR3+ regulatory T cells control TH1 responses in crescentic GN. J. Am. Soc. Nephrol. 27, 1933–1942 (2016).
Shimizu, S. et al. Membranous glomerulonephritis development with Th2-type immune deviations in MRL/lpr mice deficient for IL-27 receptor (WSX-1). J. Immunol. 175, 7185–7192 (2005).
Masutani, K. et al. Predominance of Th1 immune response in diffuse proliferative lupus nephritis. Arthritis Rheum. 44, 2097–2106 (2001).
Yap, D. Y. H. & Lai, K. N. Pathogenesis of renal disease in systemic lupus erythematosus — the role of autoantibodies and lymphocytes subset abnormalities. Int. J. Mol. Sci. 16, 7917–7931 (2015).
Peterson, R. A. Regulatory T-cells: diverse phenotypes integral to immune homeostasis and suppression. Toxicol. Pathol. 40, 186–204 (2012).
Ferretti, C. & La Cava, A. Adaptive immune regulation in autoimmune diabetes. Autoimmun. Rev. 15, 236–241 (2016).
Ghali, J. R., Wang, Y. M., Holdsworth, S. R. & Kitching, A. R. Regulatory T cells in immune-mediated renal disease. Nephrology (Carlton) 21, 86–96 (2016).
Klatzmann, D. & Abbas, A. K. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat. Rev. Immunol. 15, 283–294 (2015).
Chandran, S. & Feng, S. Current status of tolerance in kidney transplantation. Curr. Opin. Nephrol. Hypertens. 25, 591–601 (2016).
Kasper, I. R., Apostolidis, S. A., Sharabi, A. & Tsokos, G. C. Empowering regulatory T cells in autoimmunity. Trends Mol. Med. 22, 784–797 (2016).
Roccatello, D. et al. New insights into immune mechanisms underlying response to Rituximab in patients with membranous nephropathy: a prospective study and a review of the literature. Autoimmun. Rev. 15, 529–538 (2016).
Saadoun, D. et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N. Engl. J. Med. 365, 2067–2077 (2011).
Arpaia, N. et al. A distinct function of regulatory T cells in tissue protection. Cell 162, 1078–1089 (2015).
Comte, D. et al. Engagement of SLAMF3 enhances CD4+ T-cell sensitivity to IL-2 and favors regulatory T-cell polarization in systemic lupus erythematosus. Proc. Natl Acad. Sci. USA 113, 9321–9326 (2016).
Chang, J. et al. CD8+ T cells effect glomerular injury in experimental anti-myeloperoxidase GN. J. Am. Soc. Nephrol. 28, 47–55 (2017).
McKinney, E. F. et al. A CD8+ T cell transcription signature predicts prognosis in autoimmune disease. Nat. Med. 16, 586–591 (2010).
Kopetschke, K. et al. The cellular signature of urinary immune cells in Lupus nephritis: new insights into potential biomarkers. Arthritis Res. Ther. 17, 94 (2015).
Park, C. O. & Kupper, T. S. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat. Med. 21, 688–697 (2015).
Zhou, G. et al. Identification of systemically expanded activated T cell clones in MRL/lpr and NZB/W F1 lupus model mice. Clin. Exp. Immunol. 136, 448–455 (2004).
Kato, T. et al. Analysis of accumulated T cell clonotypes in patients with systemic lupus erythematosus. Arthritis Rheum. 43, 2712–2721 (2000).
Hu, S.-Y. et al. T cell infiltration is associated with kidney injury in patients with anti-glomerular basement membrane disease. Sci. China Life Sci. 59, 1282–1289 (2016).
Riedel, J.-H. et al. IL-17F promotes tissue injury in autoimmune kidney diseases. J. Am. Soc. Nephrol. 27, 3666–3677 (2016).
Ifuku, M. et al. Various roles of Th cytokine mRNA expression in different forms of glomerulonephritis. Am. J. Nephrol. 38, 115–123 (2013).
Schmidt, T. et al. Function of the Th17/interleukin-17A immune response in murine lupus nephritis. Arthritis Rheumatol. 67, 475–487 (2015).
Song, X., He, X., Li, X. & Qian, Y. The roles and functional mechanisms of interleukin-17 family cytokines in mucosal immunity. Cell. Mol. Immunol. 13, 418–431 (2016).
Luger, D. et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J. Exp. Med. 205, 799–810 (2008).
Amarilyo, G., Lourenço, E. V., Shi, F.-D. & La Cava, A. IL-17 promotes murine lupus. J. Immunol. 193, 540–543 (2014).
Wang, Y. et al. Laser microdissection-based analysis of cytokine balance in the kidneys of patients with lupus nephritis. Clin. Exp. Immunol. 159, 1–10 (2010).
Wen, Z. et al. Interleukin-17 expression positively correlates with disease severity of lupus nephritis by increasing anti-double-stranded DNA antibody production in a lupus model induced by activated lymphocyte derived DNA. PLoS ONE 8, e58161 (2013).
Zickert, A. et al. IL-17 and IL-23 in lupus nephritis — association to histopathology and response to treatment. BMC Immunol. 16, 7 (2015).
Ghali, J. R., Holdsworth, S. R. & Kitching, A. R. Targeting IL-17 and IL-23 in immune mediated renal disease. Curr. Med. Chem. 22, 4341–4365 (2015).
Ouyang, W., Kolls, J. K. & Zheng, Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28, 454–467 (2008).
Disteldorf, E. M. et al. CXCL5 drives neutrophil recruitment in TH17-mediated GN. J. Am. Soc. Nephrol. 26, 55–66 (2015).
Li, D. et al. Interleukin-17 in systemic lupus erythematosus: a comprehensive review. Autoimmunity 48, 353–361 (2015).
Subbarayal, B., Chauhan, S. K., Di Zazzo, A. & Dana, R. IL-17 augments B cell activation in ocular surface autoimmunity. J. Immunol. 197, 3464–3470 (2016).
Liu, Y. et al. Induction of C-Mip by IL-17 plays an important role in adriamycin-induced podocyte damage. Cell. Physiol. Biochem. 36, 1274–1290 (2015).
Suárez-Fueyo, A., Bradley, S. J. & Tsokos, G. C. T cells in systemic lupus erythematosus. Curr. Opin. Immunol. 43, 32–38 (2016).
Goto, K. et al. Leptin deficiency down-regulates IL-23 production in glomerular podocytes resulting in an attenuated immune response in nephrotoxic serum nephritis. Int. Immunol. 28, 197–208 (2016).
Riol-Blanco, L. et al. IL-23 receptor regulates unconventional IL-17-producing T cells that control bacterial infections. J. Immunol. 184, 1710–1720 (2010).
Reinhardt, A. et al. Interleukin-23-dependent γ/δ T cells produce interleukin-17 and accumulate in the enthesis, aortic valve, and ciliary body in mice. Arthritis Rheumatol. 68, 2476–2486 (2016).
Kyttaris, V. C., Zhang, Z., Kuchroo, V. K., Oukka, M. & Tsokos, G. C. Cutting edge: IL-23 receptor deficiency prevents the development of lupus nephritis in C57BL/6-lpr/lpr mice. J. Immunol. 184, 4605–4609 (2010).
Kyttaris, V. C., Kampagianni, O. & Tsokos, G. C. Treatment with anti-interleukin 23 antibody ameliorates disease in lupus-prone mice. BioMed Res. Int. 2013, 861028 (2013).
Teng, M. W. L. et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat. Med. 21, 719–729 (2015).
Ichinose, K. et al. Lupus nephritis IgG induction of calcium/calmodulin-dependent protein kinase IV expression in podocytes and alteration of their function. Arthritis Rheumatol. 68, 944–952 (2016).
Lee, Y. et al. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 13, 991–999 (2012).
Huang, M. Y., Chaturvedi, L. S., Koul, S. & Koul, H. K. Oxalate stimulates IL-6 production in HK-2 cells, a line of human renal proximal tubular epithelial cells. Kidney Int. 68, 497–503 (2005).
Acosta-Rodriguez, E. V., Napolitani, G., Lanzavecchia, A. & Sallusto, F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 8, 942–949 (2007).
Qi, H. T follicular helper cells in space-time. Nat. Rev. Immunol. 16, 612–625 (2016).
Jourdan, M. et al. IL-6 supports the generation of human long-lived plasma cells in combination with either APRIL or stromal cell-soluble factors. Leukemia 28, 1647–1656 (2014).
Zhang, X. L., Topley, N., Ito, T. & Phillips, A. Interleukin-6 regulation of transforming growth factor (TGF)-beta receptor compartmentalization and turnover enhances TGF-beta1 signaling. J. Biol. Chem. 280, 12239–12245 (2005).
Lin, G. et al. Interleukin-6 inhibits regulatory T cells and improves the proliferation and cytotoxic activity of cytokine-induced killer cells. J. Immunother. 35, 337–343 (2012).
Jones, S. A., Fraser, D. J., Fielding, C. A. & Jones, G. W. Interleukin-6 in renal disease and therapy. Nephrol. Dial. Transplant. 30, 564–574 (2015).
Chen, S.-Y. et al. Effect of IL-6 C-572G polymorphism on idiopathic membranous nephropathy risk in a Han Chinese population. Ren. Fail. 32, 1172–1176 (2010).
Wallace, D. J. et al. Efficacy and safety of an interleukin 6 monoclonal antibody for the treatment of systemic lupus erythematosus: a phase II dose-ranging randomised controlled trial. Ann. Rheum. Dis. 76, 534–542 (2017).
Yoshida, N. et al. ICER is requisite for Th17 differentiation. Nat. Commun. 7, 12993 (2016).
Shevach, E. M. & Thornton, A. M. tTregs, pTregs, and iTregs: similarities and differences. Immunol. Rev. 259, 88–102 (2014).
Gray, M. & Gray, D. Regulatory B cells mediate tolerance to apoptotic self in health: implications for disease. Int. Immunol. 27, 505–511 (2015).
Hänninen, A. & Harrison, L. C. Gamma delta T cells as mediators of mucosal tolerance: the autoimmune diabetes model. Immunol. Rev. 173, 109–119 (2000).
Raker, V. K., Domogalla, M. P. & Steinbrink, K. Tolerogenic dendritic cells for regulatory T cell induction in man. Front. Immunol. 6, 569 (2015).
Tard, C., Rouxel, O. & Lehuen, A. Regulatory role of natural killer T cells in diabetes. Biomed. J. 38, 484–495 (2015).
Hofmann, S. R., Rösen-Wolff, A., Tsokos, G. C. & Hedrich, C. M. Biological properties and regulation of IL-10 related cytokines and their contribution to autoimmune disease and tissue injury. Clin. Immunol. 143, 116–127 (2012).
Zeid, S. A., Khalifa, G. & Nabil, M. IL10 in lupus nephritis: detection and relationship with disease activity. Electron. Physician 7, 1680–1685 (2015).
Sinuani, I., Beberashvili, I., Averbukh, Z. & Sandbank, J. Role of IL-10 in the progression of kidney disease. World J. Transplant. 3, 91–98 (2013).
Liao, W., Lin, J.-X. & Leonard, W. J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 38, 13–25 (2013).
Parackova, Z. et al. T regulatory lymphocytes in type 1 diabetes: impaired CD25 expression and IL-2 induced STAT5 phosphorylation in pediatric patients. Autoimmunity 49, 523–531 (2016).
Mizui, M. & Tsokos, G. C. Low-dose IL-2 in the treatment of lupus. Curr. Rheumatol. Rep. 18, 68 (2016).
Pérol, L. et al. Loss of immune tolerance to IL-2 in type 1 diabetes. Nat. Commun. 7, 13027 (2016).
Humrich, J. Y. et al. Homeostatic imbalance of regulatory and effector T cells due to IL-2 deprivation amplifies murine lupus. Proc. Natl Acad. Sci. USA 107, 204–209 (2010).
Sharma, R., Sung, S.-S. J., Gaskin, F., Fu, S. M. & Ju, S.-T. A novel function of IL-2: chemokine/chemoattractant/retention receptor genes induction in Th subsets for skin and lung inflammation. J. Autoimmun. 38, 322–331 (2012).
Ray, J. P. et al. The interleukin-2-mTORc1 kinase axis defines the signaling, differentiation, and metabolism of T helper 1 and follicular B helper T cells. Immunity 43, 690–702 (2015).
Mizui, M. et al. IL-2 protects lupus-prone mice from multiple end-organ damage by limiting CD4-CD8-IL-17-producing T cells. J. Immunol. 193, 2168–2177 (2014).
Spence, A. & Tang, Q. Restoring regulatory T Cells in type 1 diabetes. Curr. Diab. Rep. 16, 110 (2016).
Koreth, J. et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N. Engl. J. Med. 365, 2055–2066 (2011).
He, J. et al. Low-dose interleukin-2 treatment selectively modulates CD4+ T cell subsets in patients with systemic lupus erythematosus. Nat. Med. 22, 991–993 (2016).
von Spee-Mayer, C. et al. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 75, 1407–1415 (2016).
Humrich, J. Y. et al. Rapid induction of clinical remission by low-dose interleukin-2 in a patient with refractory SLE. Ann. Rheum. Dis. 74, 791–792 (2015).
Hartemann, A. et al. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 1, 295–305 (2013).
Comte, D. et al. CD4+ T cells from SLE patients respond poorly to exogenous IL-2. Arthritis Rheum. http://dx.doi.org/10.1002/art.40014 (2016).
Moulton, V. R. & Tsokos, G. C. T cell signaling abnormalities contribute to aberrant immune cell function and autoimmunity. J. Clin. Invest. 125, 2220–2227 (2015).
Li, P. et al. TCR-CD3ζ gene polymorphisms and expression profile in rheumatoid arthritis. Autoimmunity 49, 466–471 (2016).
Zayed, H. Genetic epidemiology of type 1 diabetes in the 22 Arab countries. Curr. Diab. Rep. 16, 37 (2016).
Zhang, Z. et al. TCRzetadim lymphocytes define populations of circulating effector cells that migrate to inflamed tissues. Blood 109, 4328–4335 (2007).
Yoshimoto, K., Setoyama, Y., Tsuzaka, K., Abe, T. & Takeuchi, T. Reduced expression of TCR zeta is involved in the abnormal production of cytokines by peripheral T cells of patients with systemic lupus erythematosus. J. Biomed. Biotechnol. 2010, 509021 (2010).
Ferraccioli, G. & Zizzo, G. The potential role of Th17 in mediating the transition from acute to chronic autoimmune inflammation: rheumatoid arthritis as a model. Discov. Med. 11, 413–424 (2011).
Deng, G.-M., Beltran, J., Chen, C., Terhorst, C. & Tsokos, G. C. T. Cell CD3ζ deficiency enables multiorgan tissue inflammation. J. Immunol. 191, 3563–3567 (2013).
Sionov, R. V. & Naor, D. Calcium- and calmodulin-dependent PMA-activation of the CD44 adhesion molecule. Cell Adhes. Commun. 6, 503–523 (1998).
Crispín, J. C. et al. Expression of CD44 variant isoforms CD44v3 and CD44v6 is increased on T cells from patients with systemic lupus erythematosus and is correlated with disease activity. Arthritis Rheum. 62, 1431–1437 (2010).
Biswas, P. S. et al. Phosphorylation of IRF4 by ROCK2 regulates IL-17 and IL-21 production and the development of autoimmunity in mice. J. Clin. Invest. 120, 3280–3295 (2010).
Li, Y. et al. Phosphorylated ERM is responsible for increased T cell polarization, adhesion, and migration in patients with systemic lupus erythematosus. J. Immunol. 178, 1938–1947 (2007).
Loirand, G. Rho kinases in health and disease: from basic science to translational research. Pharmacol. Rev. 67, 1074–1095 (2015).
Nishikimi, T. & Matsuoka, H. Molecular mechanisms and therapeutic strategies of chronic renal injury: renoprotective effect of rho-kinase inhibitor in hypertensive glomerulosclerosis. J. Pharmacol. Sci. 100, 22–28 (2006).
Komers, R. Rho kinase inhibition in diabetic kidney disease. Br. J. Clin. Pharmacol. 76, 551–559 (2013).
Isgro, J. et al. Enhanced rho-associated protein kinase activation in patients with systemic lupus erythematosus. Arthritis Rheum. 65, 1592–1602 (2013).
Hayashi, K. et al. Molecular mechanisms and therapeutic strategies of chronic renal injury: role of rho-kinase in the development of renal injury. J. Pharmacol. Sci. 100, 29–33 (2006).
Stirzaker, R. A. et al. Administration of fasudil, a ROCK inhibitor, attenuates disease in lupus-prone NZB/W F1 female mice. Lupus 21, 656–661 (2012).
Meyer-Schwesinger, C. et al. Rho-kinase inhibition prevents proteinuria in immune-complex-mediated antipodocyte nephritis. Am. J. Physiol. Renal Physiol. 303, F1015–F1025 (2012).
Xie, X. et al. Berberine ameliorates experimental diabetes-induced renal inflammation and fibronectin by inhibiting the activation of RhoA/ROCK signaling. Mol. Cell. Endocrinol. 381, 56–65 (2013).
Kiely, M. & Kiely, P. A. PP2A: the wolf in sheep's clothing? Cancers (Basel) 7, 648–669 (2015).
Apostolidis, S. A. et al. Phosphatase PP2A is requisite for the function of regulatory T cells. Nat. Immunol. 17, 556–564 (2016).
Crispín, J. C., Apostolidis, S. A., Finnell, M. I. & Tsokos, G. C. Induction of PP2A Bβ, a regulator of IL-2 deprivation-induced T-cell apoptosis, is deficient in systemic lupus erythematosus. Proc. Natl Acad. Sci. USA 108, 12443–12448 (2011).
Juang, Y.-T. et al. PP2A dephosphorylates Elf-1 and determines the expression of CD3zeta and FcRgamma in human systemic lupus erythematosus T cells. J. Immunol. 181, 3658–3664 (2008).
Katsiari, C. G., Kyttaris, V. C., Juang, Y.-T. & Tsokos, G. C. Protein phosphatase 2A is a negative regulator of IL-2 production in patients with systemic lupus erythematosus. J. Clin. Invest. 115, 3193–3204 (2005).
Breuer, R. et al. The protein phosphatase 2A regulatory subunit B56γ mediates suppression of T cell receptor (TCR)-induced nuclear factor-κB (NF-κB) activity. J. Biol. Chem. 289, 14996–15004 (2014).
Apostolidis, S. A., Rauen, T., Hedrich, C. M., Tsokos, G. C. & Crispin, J. C. Protein phosphatase 2A enables expression of interleukin 17 (IL-17) through chromatin remodeling. J. Biol. Chem. 288, 26775–26784 (2013).
Ottenlinger, F. et al. Fingolimod targeting protein phosphatase 2A differently affects IL-33 induced IL-2 and IFN-γ production in CD8+ lymphocytes. Eur. J. Immunol. 46, 941–951 (2016).
Sunahori, K. et al. The catalytic subunit of protein phosphatase 2A (PP2Ac) promotes DNA hypomethylation by suppressing the phosphorylated mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) kinase (MEK)/phosphorylated ERK/DNMT1 protein pathway in t-cells from controls and systemic lupus erythematosus patients. J. Biol. Chem. 288, 21936–21944 (2013).
Juang, Y.-T. et al. Transcriptional activation of the cAMP-responsive modulator promoter in human T cells is regulated by protein phosphatase 2A-mediated dephosphorylation of SP-1 and reflects disease activity in patients with systemic lupus erythematosus. J. Biol. Chem. 286, 1795–1801 (2011).
Crispín, J. C. et al. Cutting edge: protein phosphatase 2A confers susceptibility to autoimmune disease through an IL-17-dependent mechanism. J. Immunol. 188, 3567–3571 (2012).
Rauen, T., Hedrich, C. M., Tenbrock, K. & Tsokos, G. C. cAMP responsive element modulator: a critical regulator of cytokine production. Trends Mol. Med. 19, 262–269 (2013).
Hedrich, C. M. et al. cAMP response element modulator α controls IL2 and IL17A expression during CD4 lineage commitment and subset distribution in lupus. Proc. Natl Acad. Sci. USA 109, 16606–16611 (2012).
Hedrich, C. M. et al. cAMP-responsive element modulator α (CREMα) trans-represses the transmembrane glycoprotein CD8 and contributes to the generation of CD3+CD4-CD8- T cells in health and disease. J. Biol. Chem. 288, 31880–31887 (2013).
Hedrich, C. M. et al. cAMP responsive element modulator (CREM) mediates chromatin remodeling of CD8 during the generation of CD3+CD4-CD8- T cells. J. Biol. Chem. 289, 2361–2370 (2014).
Naz, H., Islam, A., Ahmad, F. & Hassan, M. I. Calcium/calmodulin-dependent protein kinase IV: a multifunctional enzyme and potential therapeutic target. Prog. Biophys. Mol. Biol. 121, 54–65 (2016).
Gringhuis, S. I., de Leij, L. F., Wayman, G. A., Tokumitsu, H. & Vellenga, E. The Ca2+/calmodulin-dependent kinase type IV is involved in the CD5-mediated signaling pathway in human T lymphocytes. J. Biol. Chem. 272, 31809–31820 (1997).
Juang, Y.-T. et al. Systemic lupus erythematosus serum IgG increases CREM binding to the IL-2 promoter and suppresses IL-2 production through CaMKIV. J. Clin. Invest. 115, 996–1005 (2005).
Koga, T., Ichinose, K., Mizui, M., Crispín, J. C. & Tsokos, G. C. Calcium/calmodulin-dependent protein kinase IV suppresses IL-2 production and regulatory T cell activity in lupus. J. Immunol. 189, 3490–3496 (2012).
Koga, T. et al. CaMK4-dependent activation of AKT/mTOR and CREM-α underlies autoimmunity-associated Th17 imbalance. J. Clin. Invest. 124, 2234–2245 (2014).
Koga, T. et al. CaMK4 facilitates the recruitment of IL-17-producing cells to target organs through the CCR6/CCL20 axis in Th17-driven inflammatory diseases. Arthritis Rheumatol. 68, 1981–1988 (2016).
Koga, T. et al. KN-93, an inhibitor of calcium/calmodulin-dependent protein kinase IV, promotes generation and function of Foxp3+ regulatory T cells in MRL/lpr mice. Autoimmunity 47, 445–450 (2014).
Ichinose, K. et al. Cutting edge: calcium/calmodulin-dependent protein kinase type IV is essential for mesangial cell proliferation and lupus nephritis. J. Immunol. 187, 5500–5504 (2011).
Perl, A. Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases. Nat. Rev. Rheumatol. 12, 169–182 (2016).
Choi, S.-C., Titov, A. A., Sivakumar, R., Li, W. & Morel, L. Immune cell metabolism in systemic lupus erythematosus. Curr. Rheumatol. Rep. 18, 66 (2016).
Delgoffe, G. M. et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 12, 295–303 (2011).
Kato, H. & Perl, A. Mechanistic target of rapamycin complex 1 expands Th17 and IL-4+ CD4-CD8-double-negative T cells and contracts regulatory T cells in systemic lupus erythematosus. J. Immunol. 192, 4134–4144 (2014).
Suárez-Fueyo, A., Barber, D. F., Martínez-Ara, J., Zea-Mendoza, A. C. & Carrera, A. C. Enhanced phosphoinositide 3-kinase δ activity is a frequent event in systemic lupus erythematosus that confers resistance to activation-induced T cell death. J. Immunol. 187, 2376–2385 (2011).
Kshirsagar, S. et al. Akt-dependent enhanced migratory capacity of Th17 cells from children with lupus nephritis. J. Immunol. 193, 4895–4903 (2014).
Lai, Z.-W. et al. Mechanistic target of rapamycin activation triggers IL-4 production and necrotic death of double-negative T cells in patients with systemic lupus erythematosus. J. Immunol. 191, 2236–2246 (2013).
Oaks, Z., Winans, T., Huang, N., Banki, K. & Perl, A. Activation of the mechanistic target of rapamycin in SLE: explosion of evidence in the last five years. Curr. Rheumatol. Rep. 18, 73 (2016).
Saleiro, D. & Platanias, L. C. Intersection of mTOR and STAT signaling in immunity. Trends Immunol. 36, 21–29 (2015).
Harada, T. et al. Increased expression of STAT3 in SLE T cells contributes to enhanced chemokine-mediated cell migration. Autoimmunity 40, 1–8 (2007).
Yiu, G. et al. Development of Th17-associated interstitial kidney inflammation in lupus-prone mice lacking the gene encoding STAT-1. Arthritis Rheumatol. 68, 1233–1244 (2016).
Ge, Y. et al. Cgnz1 allele confers kidney resistance to damage preventing progression of immune complex-mediated acute lupus glomerulonephritis. J. Exp. Med. 210, 2387–2401 (2013).
Xie, C. et al. Lupus-prone strains vary in susceptibility to antibody-mediated end organ disease. Genes Immun. 14, 170–178 (2013).
Liu, K. et al. Kallikrein genes are associated with lupus and glomerular basement membrane-specific antibody-induced nephritis in mice and humans. J. Clin. Invest. 119, 911–923 (2009).
Arakawa, T. et al. Activation of signal transducer and activator of transcription 3 correlates with cell proliferation and renal injury in human glomerulonephritis. Nephrol. Dial. Transplant. 23, 3418–3426 (2008).
Velagapudi, C. et al. The tuberin/mTOR pathway promotes apoptosis of tubular epithelial cells in diabetes. J. Am. Soc. Nephrol. 22, 262–273 (2011).
Yung, S., Cheung, K. F., Zhang, Q. & Chan, T. M. Mediators of inflammation and their effect on resident renal cells: implications in lupus nephritis. Clin. Dev. Immunol. 2013, 317682 (2013).
Zhao, J. et al. P2X7 blockade attenuates murine lupus nephritis by inhibiting activation of the NLRP3/ASC/caspase 1 pathway. Arthritis Rheum. 65, 3176–3185 (2013).
Zhao, J. et al. Lupus nephritis: glycogen synthase kinase 3β promotion of renal damage through activation of the NLRP3 inflammasome in lupus-prone mice. Arthritis Rheumatol. 67, 1036–1044 (2015).
Benz, P. S., Fan, X. & Wüthrich, R. P. Enhanced tubular epithelial CD44 expression in MRL-lpr lupus nephritis. Kidney Int. 50, 156–163 (1996).
Kuipers, H. F. et al. Hyaluronan synthesis is necessary for autoreactive T-cell trafficking, activation, and Th1 polarization. Proc. Natl Acad. Sci. USA 113, 1339–1344 (2016).
Nagy, N. et al. Inhibition of hyaluronan synthesis restores immune tolerance during autoimmune insulitis. J. Clin. Invest. 125, 3928–3940 (2015).
Acknowledgements
S.J.B. is supported by a T32 training grant from NIAID, NIH to G.C.T. The work of D.K. is funded by French state funds within the Investissements d'Avenir programme (ANR-11-IDEX-0004-02; LabEx Transimmunom); the European Research Council Advanced Grant (ERC-2012-AdG, TRiPoD, Agreement number 322856); the Assistance Publique – Hopitaux de Paris, France; the Sorbonne University, Pierre and Marie Curie Medical School, Paris, France; and the Institut National de la Santé et de la Recherche Médicale (INSERM). The work of G.C.T. is supported by grants from the NIH.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to researching data for the article, discussion of the content, and revising or editing the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- γδT cells
-
T-cell subtype characterized by the expression of a unique T-cell receptor composed of one γ and one δ chain. Although their prevalence is small compared to that of αβT cells, they are frequently present in tissues at high risk of infection and are involved in the initiation and propagation of immune responses in physiological and pathological conditions.
- NETosis
-
A specific process of cell death mediated by neutrophils, which generate neutrophil extracellular traps (NETs).
- T-helper (TH) type 1 cells
-
Subtype of CD4+ αβT cell characterized by the secretion of IFNγ, IL-2 and TNFβ. Their principal transcription factor, T-bet, is expressed following activation of STAT-1 through the IL-12 receptor.
- NKT cells
-
(Natural killer T cells). An innate-like lymphocyte population expressing markers associated with T cells and NK cells, with an important role in immune regulation through the production of cytokines.
- Plasma cells
-
Long-lived effector B cells responsible for humoral memory.
- T follicular helper (TFH) cells
-
Subtype of CD4+ T cells that express BCL6 and support B-cell activation and differentiation in germinal centres.
- Plasmablasts
-
Short-lived effector B cells that rapidly produce antibodies upon activation. They can differentiate into plasma cells.
- Peripheral T-cell tolerance
-
Combination of mechanisms that control autoreactive T-cell responses in the periphery. These mechanisms can be either cell-intrinsic (anergy and activation-induced cell death) or extrinsically mediated by T regulatory cells.
- Vδ2 T cells
-
Proinflammatory subtype of γδT cells, which are activated through phospho-antigens.
- Double-negative (DN) T cells
-
A subtype of TCRαβ T cells, which differentiate from autoreactive CD8+ T cells. They are found at increased levels in several autoimmune and chronic inflammatory diseases. In lupus nephritis kidneys they have been found to produce IL-17.
- αβT cells
-
Subtype of T cells that express a T-cell receptor composed of an α and a β chain. They express the co-receptors CD4 or CD8.
- Ectopic lymphoid follicles
-
Discrete structures in which B cells and T cells interact in non-lymphoid tissues. The follicles are formed during autoimmunity or chronic inflammation as a consequence of immune cell infiltration.
- Immunoglobulin class-switching
-
Biological mechanism whereby activated B cells change the isotype of antibodies that they produce. This process is also known as class-switch recombination.
- TH2 cells
-
Subtype of CD4+ αβT cell characterized by the secretion of IL-4, IL-5, IL-6, IL-9, IL-13, and IL-17E (IL-25), which support the humoral response. GATA-3 is their principal transcripton factor, which is expressed following activation of STAT-6 by IL-4.
- Tissue-resident memory T cells
-
T cells, which after tissue infiltration, activate an mTOR-dependent mechanism that leads to a change in phenotype to resident memory T cells. They can have either a protective or harmful role in physiology and pathology.
- Central memory T cells
-
Long-term surviving memory T cells characterized by the expression of L-selectin and CCR7 and the secretion of several cytokines. They present self-renewal capacity and localize in lymph nodes.
- T cell clonotypes
-
T cells that share the same T-cell receptor.
- Activation-induced cell death
-
Mechanism of progammed cell death mediated by the interaction of Fas and FasL in restimulated T cells. It represents one of the peripheral tolerance mechanisms for T cells.
- CD3 complex
-
CD3 is a complex of molecules associated with and used by the T-cell receptor to instigate T-cell signalling. It comprizes six molecules: an δ-chain, a γ-chain, two ε chains and two ζ chains.
Rights and permissions
About this article
Cite this article
Suárez-Fueyo, A., Bradley, S., Klatzmann, D. et al. T cells and autoimmune kidney disease. Nat Rev Nephrol 13, 329–343 (2017). https://doi.org/10.1038/nrneph.2017.34
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2017.34
This article is cited by
-
Neddylation is a novel therapeutic target for lupus by regulating double negative T cell homeostasis
Signal Transduction and Targeted Therapy (2024)
-
Renal manifestations in inflammatory bowel disease: a systematic review
Journal of Gastroenterology (2022)
-
Alteration of N6-methyladenosine epitranscriptome profile in lipopolysaccharide-induced mouse mesangial cells
Naunyn-Schmiedeberg's Archives of Pharmacology (2022)
-
Different renal manifestations associated with very early onset pediatric inflammatory bowel disease: case report and review of literature
BMC Nephrology (2021)
-
T helper cell trafficking in autoimmune kidney diseases
Cell and Tissue Research (2021)