Abstract
Unconventional T cells are a diverse and underappreciated group of relatively rare lymphocytes that are distinct from conventional CD4+ and CD8+ T cells, and that mainly recognize antigens in the absence of classical restriction through the major histocompatibility complex (MHC). These non-MHC-restricted T cells include mucosal-associated invariant T (MAIT) cells, natural killer T (NKT) cells, γδ T cells and other, often poorly defined, subsets. Depending on the physiological context, unconventional T cells may assume either protective or pathogenic roles in a range of inflammatory and autoimmune responses in the kidney. Accordingly, experimental models and clinical studies have revealed that certain unconventional T cells are potential therapeutic targets, as well as prognostic and diagnostic biomarkers. The responsiveness of human Vγ9Vδ2 T cells and MAIT cells to many microbial pathogens, for example, has implications for early diagnosis, risk stratification and targeted treatment of peritoneal dialysis-related peritonitis. The expansion of non-Vγ9Vδ2 γδ T cells during cytomegalovirus infection and their contribution to viral clearance suggest that these cells can be harnessed for immune monitoring and adoptive immunotherapy in kidney transplant recipients. In addition, populations of NKT, MAIT or γδ T cells are involved in the immunopathology of IgA nephropathy and in models of glomerulonephritis, ischaemia–reperfusion injury and kidney transplantation.
Key points
-
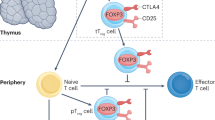
Unconventional T cells, such as γδ T cells, mucosal-associated invariant T (MAIT) cells and invariant natural killer T (iNKT) cells, are distinct from classical CD4+ and CD8+ T cells and can have either protective or pathogenic roles in a range of inflammatory and autoimmune conditions related to acute and chronic kidney disease.
-
Vγ9Vδ2 T cells and MAIT cells respond to metabolites shared by a wide range of microbial pathogens, which may have implications for early diagnosis, risk stratification and targeted treatment of peritoneal dialysis-related peritonitis.
-
Non-Vγ9Vδ2 γδ T cells expand during cytomegalovirus (CMV) infection in kidney transplant recipients and contribute to viral clearance, which suggests that they can be harnessed for immune monitoring and for adoptive immunotherapy in refractory CMV infections.
-
IgA nephropathy is accompanied by oligoclonal expansion of γδ T cells in blood and the kidneys; this expansion correlates with disease progression and may contribute to immunopathology.
-
In murine models of glomerulonephritis, kidney γδ T cells are an important source of IL-17A, which is necessary for the recruitment of macrophages, neutrophils and T cells, and contributes to the development of kidney fibrosis.
-
Murine type I and type II NKT cells have opposite roles in ischaemia–reperfusion injury and may be relevant for allograft tolerance, as well as kidney protection in lupus nephritis or crescentic glomerulonephritis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
DuPage, M. & Bluestone, J. A. Harnessing the plasticity of CD4+ T cells to treat immune-mediated disease. Nat. Rev. Immunol. 16, 149–163 (2016).
Mittrücker, H. W., Visekruna, A. & Huber, M. Heterogeneity in the differentiation and function of CD8+ T cells. Arch. Immunol. Ther. Exp. 62, 449–458 (2014).
Godfrey, D. I., Uldrich, A. P., McCluskey, J., Rossjohn, J. & Moody, D. B. The burgeoning family of unconventional T cells. Nat. Immunol. 16, 1114–1123 (2015).
Pellicci, D. G., Koay, H. F. & Berzins, S. P. Thymic development of unconventional T cells: how NKT cells, MAIT cells and γδ T cells emerge. Nat. Rev. Immunol. 20, 756–770 (2020).
Rhodes, D. A., Reith, W. & Trowsdale, J. Regulation of immunity by butyrophilins. Annu. Rev. Immunol. 34, 151–172 (2016).
Hayday, A. C. & Vantourout, P. The innate biologies of adaptive antigen receptors. Annu. Rev. Immunol. 38, 487–510 (2020).
Godfrey, D. I., Koay, H. F., McCluskey, J. & Gherardin, N. A. The biology and functional importance of MAIT cells. Nat. Immunol. 20, 1110–1128 (2019).
Corbett, A. J., Awad, W., Wang, H. & Chen, Z. Antigen recognition by MR1-reactive T cells; MAIT cells, metabolites, and remaining mysteries. Front. Immunol. 11, 1961 (2020).
Adams, E. J. Lipid presentation by human CD1 molecules and the diverse T cell populations that respond to them. Curr. Opin. Immunol. 26, 1–6 (2014).
Lepore, M., Mori, L. & De Libero, G. The conventional nature of non-MHC-restricted T cells. Front. Immunol. 9, 1365 (2018).
Willcox, C. R., Mohammed, F. & Willcox, B. E. The distinct MHC-unrestricted immunobiology of innate-like and adaptive-like human γδ T cell subsets-Nature’s CAR-T cells. Immunol. Rev. 298, 25–46 (2020).
Papadopoulou, M., Sanchez Sanchez, G. & Vermijlen, D. Innate and adaptive γδ T cells: How, when, and why. Immunol. Rev. 298, 99–116 (2020).
Godfrey, D. I., Le Nours, J., Andrews, D. M., Uldrich, A. P. & Rossjohn, J. Unconventional T cell targets for cancer immunotherapy. Immunity 48, 453–473 (2018).
Silva-Santos, B., Mensurado, S. & Coffelt, S. B. γδ T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat. Rev. Cancer 19, 392–404 (2019).
Turner, J. E. et al. IL-17A production by renal γδ T cells promotes kidney injury in crescentic GN. J. Am. Soc. Nephrol. 23, 1486–1495 (2012). This study shows that in the mouse, kidney-resident γδ T cells produce IL-17A, promote neutrophil recruitment and contribute to the immunopathogenesis of crescentic glomerulonephritis.
Uchida, T. et al. Repeated administration of alpha-galactosylceramide ameliorates experimental lupus nephritis in mice. Sci. Rep. 8, 8225 (2018).
Eberl, M., Friberg, I. M., Liuzzi, A. R., Morgan, M. P. & Topley, N. Pathogen-specific immune fingerprints during acute infection: The diagnostic potential of human γδ T-cells. Front. Immunol. 5, 572 (2014).
Khairallah, C., Déchanet-Merville, J. & Capone, M. γδ T cell-mediated immunity to cytomegalovirus infection. Front. Immunol. 8, 105 (2017).
Lefranc, M. P. & Rabbitts, T. H. A nomenclature to fit the organization of the human T-cell receptor γ and δ genes. Res. Immunol. 141, 615–618 (1990).
Heilig, J. S. & Tonegawa, S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature 322, 836–840 (1986).
Morita, C. T., Jin, C., Sarikonda, G. & Wang, H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol. Rev. 215, 59–76 (2007).
Falk, M. C. et al. Infiltration of the kidney by αβ and γδ T cells: effect on progression in IgA nephropathy. Kidney Int. 47, 177–185 (1995). This pioneering study shows that αβ T cells are found in both stable and progressive IgA nephropathy, whereas γδ T cells are associated with progressive IgA nephropathy.
Turner, J. E., Becker, M., Mittrücker, H. W. & Panzer, U. Tissue-resident lymphocytes in the kidney. J. Am. Soc. Nephrol. 29, 389–399 (2018).
Dusseaux, M. et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 117, 1250–1259 (2011).
Nel, I., Bertrand, L., Toubal, A. & Lehuen, A. MAIT cells, guardians of skin and mucosa? Mucosal Immunol. 22, 1–12 (2021).
Terpstra, M. L. et al. Tissue-resident mucosal-associated invariant T (MAIT) cells in the human kidney represent a functionally distinct subset. Eur. J. Immunol. 50, 1783–1797 (2020).
Gambón-Deza, F. & Olivieri, D. N. Immunoglobulin and T cell receptor genes in Chinese crocodile lizard Shinisaurus crocodilurus. Mol. Immunol. 101, 160–166 (2018).
La Gruta, N. L., Gras, S., Daley, S. R., Thomas, P. G. & Rossjohn, J. Understanding the drivers of MHC restriction of T cell receptors. Nat. Rev. Immunol. 18, 467–478 (2018).
Bonneville, M., O’Brien, R. L. & Born, W. K. γδ T cell effector functions: a blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 10, 467–478 (2010).
Fichtner, A. S. et al. Alpaca (Vicugna pacos), the first nonprimate species with a phosphoantigen-reactive Vγ9Vδ2 T cell subset. Proc. Natl Acad. Sci. USA 117, 6697–6707 (2020).
Vermijlen, D., Gatti, D., Kouzeli, A., Rus, T. & Eberl, M. γδ T cell responses: how many ligands will it take till we know? Semin. Cell Dev. Biol. 84, 75–86 (2018).
Van Rhijn, I., Godfrey, D. I., Rossjohn, J. & Moody, D. B. Lipid and small-molecule display by CD1 and MR1. Nat. Rev. Immunol. 15, 643–654 (2015).
Hintz, M. et al. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli. FEBS Lett. 509, 317–322 (2001).
Corbett, A. J. et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature 509, 361–365 (2014). This study identifies simple adducts between microbial vitamin B2 precursors and small intracellular molecules such as methylglyoxal as the most potent ligands for MAIT cells that are stabilized by covalent binding to MR1.
Eberl, M. et al. Microbial isoprenoid biosynthesis and human γδ T cell activation. FEBS Lett. 544, 4–10 (2003).
Heuston, S., Begley, M., Gahan, C. G. M. & Hill, C. Isoprenoid biosynthesis in bacterial pathogens. Microbiology 158, 1389–1401 (2012).
Harly, C. et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood 120, 2269–2279 (2012).
Sandstrom, A. et al. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2 T cells. Immunity 40, 490–500 (2014).
Karunakaran, M. M. et al. Butyrophilin-2A1 directly binds germline-encoded regions of the Vγ9Vδ2 TCR and is essential for phosphoantigen sensing. Immunity 52, 487–498.e6 (2020). This is one of two seminal studies that identifies the butyrophilin BTN2A1 as the ligand for human Vγ9Vδ2 Tcells and proposes a model of how Vγ9Vδ2 T cells respond to phosphoantigens in the context of BTN2A1 and BTN3A1.
Rigau, M. et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science. 367, eaay5516 (2020). This is one of two seminal studies that identify the butyrophilin BTN2A1 as the ligand for human Vγ9Vδ2 Tcells and propose a model of how Vγ9Vδ2 T cells respond to phosphoantigens in the context of BTN2A1 and BTN3A1.
Sebestyen, Z., Prinz, I., Déchanet-Merville, J., Silva-Santos, B. & Kuball, J. Translating gammadelta (γδ) T cells and their receptors into cancer cell therapies. Nat. Rev. Drug Discov. 19, 169–184 (2020).
Vyborova, A. et al. γ9δ2T cell diversity and the receptor interface with tumor cells. J. Clin. Invest. 130, 4637–4651 (2020).
Liuzzi, A. R., McLaren, J. E., Price, D. A. & Eberl, M. Early innate responses to pathogens: pattern recognition by unconventional human T-cells. Curr. Opin. Immunol. 36, 31–37 (2015).
Davey, M. S. et al. Microbe-specific unconventional T cells induce human neutrophil differentiation into antigen cross-presenting cells. J. Immunol. 193, 3704–3716 (2014).
Howson, L. J. et al. Absence of mucosal-associated invariant T cells in a person with a homozygous point mutation in MR1. Sci. Immunol. 5, eabc9492 (2020).
Koay, H. F. et al. Diverse MR1-restricted T cells in mice and humans. Nat. Commun. 10, 2243 (2019).
Lepore, M., Lewinsohn, D. A. & Lewinsohn, D. M. T cell receptor diversity, specificity and promiscuity of functionally heterogeneous human MR1-restricted T cells. Mol. Immunol. 130, 64–68 (2021).
Kinjo, Y. et al. Functions of CD1d-restricted invariant natural killer T cells in antimicrobial immunity and potential applications for infection control. Front. Immunol. 9, 1266 (2018).
Morita, M. et al. Structure-activity relationship of α-galactosylceramides against B16-bearing mice. J. Med. Chem. 38, 2176–2187 (1995).
Mori, L., Lepore, M. & De Libero, G. The Immunology of CD1- and MR1-restricted T cells. Annu. Rev. Immunol. 34, 479–510 (2016).
Dhodapkar, M. V. & Kumar, V. Type II NKT cells and their emerging role in health and disease. J. Immunol. 198, 1015–1021 (2017).
Gras, S. et al. T cell receptor recognition of CD1b presenting a mycobacterial glycolipid. Nat. Commun. 7, 13257 (2016).
Roy, S. et al. Molecular basis of mycobacterial lipid antigen presentation by CD1c and its recognition by αβ T cells. Proc. Natl Acad. Sci. US A 111, E4648–E4657 (2014).
Kasmar, A. G. et al. Cutting edge: CD1a tetramers and dextramers identify human lipopeptide-specific T cells ex vivo. J. Immunol. 191, 4499–4503 (2013).
Déchanet, J. et al. Major expansion of γδ T lymphocytes following cytomegalovirus infection in kidney allograft recipients. J. Infect. Dis. 179, 1–8 (1999).
Déchanet, J. et al. Implication of γδ T cells in the human immune response to cytomegalovirus. J. Clin. Invest. 103, 1437–1449 (1999). The first study to show that human Vδ2neg γδ Tcells expand in the peripheral blood of kidney transplant recipients after CMV infection and have an important role in the immune response against this virus.
Vermijlen, D. et al. Human cytomegalovirus elicits fetal γδ T cell responses in utero. J. Exp. Med. 207, 807–821 (2010).
Willcox, C. R. et al. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat. Immunol. 13, 872–879 (2012).
Marlin, R. et al. Sensing of cell stress by human γδ TCR-dependent recognition of annexin A2. Proc. Natl Acad. Sci. USA 114, 3163–3168 (2017).
Silva-Santos, B., Schamel, W. W., Fisch, P. & Eberl, M. γδ T-cell conference 2012: close encounters for the fifth time. Eur. J. Immunol. 42, 3101–3105 (2012).
Bonneville, M. et al. Chicago 2014 – 30 years of γδ T cells. Cell Immunol. 296, 3–9 (2015).
Di Marco Barros, R. et al. Epithelia use butyrophilin-like molecules to shape organ-specific γδ T cell compartments. Cell 167, 203–218.e17 (2016).
Mayassi, T. et al. Chronic inflammation permanently reshapes tissue-resident immunity in celiac disease. Cell 176, 967–981.e19 (2019).
Tuffs, S. W., Haeryfar, S. M. M. & McCormick, J. K. Manipulation of innate and adaptive immunity by staphylococcal superantigens. Pathogens 7, 53 (2018).
Shepherd, F. R. & McLaren, J. E. T cell immunity to bacterial pathogens: mechanisms of immune control and bacterial evasion. Int. J. Mol. Sci. 21, 6144 (2020).
Simões, A. E., Di Lorenzo, B. & Silva-Santos, B. Molecular determinants of target cell recognition by human γδ T cells. Front. Immunol. 9, 929 (2018).
Rudak, P. T., Choi, J. & Haeryfar, S. M. M. MAIT cell-mediated cytotoxicity: roles in host defense and therapeutic potentials in infectious diseases and cancer. J. Leukoc. Biol. 104, 473–486 (2018).
Djaoud, Z. & Parham, P. HLAs, TCRs, and KIRs, a triumvirate of human cell-mediated immunity. Annu. Rev. Biochem. 89, 717–739 (2020).
Wesch, D., Peters, C., Oberg, H. H., Pietschmann, K. & Kabelitz, D. Modulation of γδ T cell responses by TLR ligands. Cell Mol. Life Sci. 68, 2357–2370 (2011).
Garner, L. C., Klenerman, P. & Provine, N. M. Insights into mucosal-associated invariant T cell biology from studies of invariant natural killer T cells. Front. Immunol. 9, 1478 (2018).
Provine, N. M. & Klenerman, P. MAIT cells in health and disease. Annu. Rev. Immunol. 38, 203–228 (2020).
Tyler, C. J., Doherty, D. G., Moser, B. & Eberl, M. Human Vγ9/Vδ2 T cells: innate adaptors of the immune system. Cell Immunol. 296, 10–21 (2015).
Papotto, P. H., Reinhardt, A., Prinz, I. & Silva-Santos, B. Innately versatile: γδ17 T cells in inflammatory and autoimmune diseases. J. Autoimmun. 87, 26–37 (2018).
Pisarska, M. M., Dunne, M. R., O’Shea, D. & Hogan, A. E. Interleukin-17 producing mucosal associated invariant T cells - emerging players in chronic inflammatory diseases? Eur. J. Immunol. 50, 1098–1108 (2020).
Houot, R., Kohrt, H. E., Marabelle, A. & Levy, R. Targeting immune effector cells to promote antibody-induced cytotoxicity in cancer immunotherapy. Trends Immunol. 32, 510–516 (2011).
Kyaw, T. et al. Cytotoxic lymphocytes and atherosclerosis: significance, mechanisms and therapeutic challenges. Br. J. Pharmacol. 174, 3956–3972 (2017).
Brandes, M., Willimann, K. & Moser, B. Professional antigen-presentation function by human γδ T cells. Science 309, 264–268 (2005).
Barisa, M. et al. E. coli promotes human Vγ9Vδ2 T cell transition from cytokine-producing bactericidal effectors to professional phagocytic killers in a TCR-dependent manner. Sci. Rep. 7, 2805 (2017).
Junqueira, C. et al. γδ T cells suppress Plasmodium falciparum blood-stage infection by direct killing and phagocytosis. Nat. Immunol. 22, 347–357 (2021).
Johnson, M. D., Witherden, D. A. & Havran, W. L. The role of tissue-resident T cells in stress surveillance and tissue maintenance. Cells 9, 686 (2020).
Kohlgruber, A. C. et al. γδ T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat. Immunol. 19, 464–474 (2018).
Sullivan, Z. A. et al. γδ T cells regulate the intestinal response to nutrient sensing. Science 371, eaba8310 (2021).
Carico, Z. & Krangel, M. S. Chromatin dynamics and the development of the TCRα and TCRδ repertoires. Adv. Immunol. 128, 307–361 (2015).
Pauza, C. D. & Cairo, C. Evolution and function of the TCR Vγ9 chain repertoire: it’s good to be public. Cell Immunol. 296, 22–30 (2015).
Davey, M. S. et al. The human Vδ2+ T-cell compartment comprises distinct innate-like Vγ9+ and adaptive Vγ9− subsets. Nat. Commun. 9, 1760 (2018).
Papadopoulou, M. et al. Fetal public Vγ9Vδ2 T cells expand and gain potent cytotoxic functions early after birth. Proc. Natl Acad. Sci. USA 117, 18638–18648 (2020).
Ravens, S. et al. Microbial exposure drives polyclonal expansion of innate γδ T cells immediately after birth. Proc. Natl Acad. Sci. USA 117, 18649–18660 (2020).
Davey, M. S. et al. Clonal selection in the human Vδ1 T cell repertoire indicates γδ TCR-dependent adaptive immune surveillance. Nat. Commun. 8, 14760 (2017).
Ravens, S. et al. Human γδ T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nat. Immunol. 18, 393–401 (2017). The first study to show rapid expansion of CMV-reactive γδ TCR clonotypes in patients receiving haematopoietic stem cell transplants, which is strong evidence of an adaptive γδ T cell response.
Kumar, V. & Delovitch, T. L. Different subsets of natural killer T cells may vary in their roles in health and disease. Immunology 142, 321–336 (2014).
Kezić, A., Stajic, N. & Thaiss, F. Innate immune response in kidney ischemia/reperfusion injury: potential target for therapy. J. Immunol. Res. 2017, 6305439 (2017).
Göcze, I. et al. Postoperative cellular stress in the kidney is associated with an early systemic γδ T-cell immune cell response. Crit. Care 22, 168 (2018).
Zhu, C. et al. Kidney injury in response to crystallization of calcium oxalate leads to rearrangement of the intrarenal T cell receptor delta immune repertoire. J. Transl. Med. 17, 278 (2019).
Savransky, V. et al. Role of the T-cell receptor in kidney ischemia-reperfusion injury. Kidney Int. 69, 233–238 (2006). This study suggests that murine γδ T cells can facilitate and amplify kidney lesions after IRI by recruiting adaptive αβ T cells.
Hochegger, K. et al. Role of α/β and γ/δ T cells in renal ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol 293, F741–F747 (2007).
Bauerle, J. D., Grenz, A., Kim, J. H., Lee, H. T. & Eltzschig, H. K. Adenosine generation and signaling during acute kidney injury. J. Am. Soc. Nephrol. 22, 14–20 (2011).
Lappas, C. M., Day, Y. J., Marshall, M. A., Engelhard, V. H. & Linden, J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J. Exp. Med. 203, 2639–2648 (2006).
Zhang, J. et al. Hypoxia-inducible factor-2α limits natural killer T cell cytotoxicity in fenal ischemia/reperfusion injury. J. Am. Soc. Nephrol. 27, 92–106 (2016). This study shows that HIF2α is induced in iNKT cells during hypoxia and enhances the protective effect of adenosine by suppressing iNKT cell activation during IRI.
Li, L. et al. Dendritic cells tolerized with adenosine A2AR agonist attenuate acute kidney injury. J. Clin. Invest. 122, 3931–3942 (2012). This study shows that during ischaemia–reperfusion, DCs loaded with α-GalCer and tolerized by adenosine receptor agonists inhibit IFNγ production by iNKT, and thereby limit IRI in the kidney.
Yang, S. H. et al. Sulfatide-reactive natural killer T cells abrogate ischemia-reperfusion injury. J. Am. Soc. Nephrol. 22, 1305–1314 (2011). This study shows that type II NKT cells protect mouse kidneys from experimental IRI lesions and attenuated the apoptosis of kidney tubule cells via IL-10 in vitro.
Zhang, C. et al. Rapamycin protects kidney against ischemia reperfusion injury through recruitment of NKT cells. J. Transl. Med. 12, 224 (2014).
Yu, A., et al. Brenner and Rector’s The Kidney, 2-Volume Set 11th edn (Elsevier, 2019).
Wyatt, R. J. & Julian, B. A. IgA nephropathy. N. Engl. J. Med. 368, 2402–2414 (2013).
Olive, C. et al. Expression of the mucosal γδ T cell receptor V region repertoire in patients with IgA nephropathy. Kidney Int. 52, 1047–1053 (1997).
Buck, K. S. et al. Expression of T cell receptor variable region families by bone marrow γδ T cells in patients with IgA nephropathy. Clin. Exp. Immunol. 127, 527–532 (2002).
Toyabe, S., Harada, W. & Uchiyama, M. Oligoclonally expanding γδ T lymphocytes induce IgA switching in IgA nephropathy. Clin. Exp. Immunol. 124, 110–117 (2001).
Wu, H., Clarkson, A. R. & Knight, J. F. Restricted γδ T-cell receptor repertoire in IgA nephropathy renal biopsies. Kidney Int. 60, 1324–1331 (2001).
Nakazawa, D., Masuda, S., Tomaru, U. & Ishizu, A. Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nat. Rev. Rheumatol. 15, 91–101 (2019).
Paust, H. J. et al. The IL-23/Th17 axis contributes to renal injury in experimental glomerulonephritis. J. Am. Soc. Nephrol. 20, 969–979 (2009).
Fazekas, B. et al. Alterations in circulating lymphoid cell populations in systemic small vessel vasculitis are non-specific manifestations of renal injury. Clin. Exp. Immunol. 191, 180–188 (2018).
Holmén, C. et al. Anti endothelial cell autoantibodies selectively activate SAPK/JNK signalling in Wegener’s granulomatosis. J. Am. Soc. Nephrol. 18, 2497–2508 (2007).
Rosenkranz, A. R. et al. Regulatory interactions of αβ and γδ T cells in glomerulonephritis. Kidney Int. 58, 1055–1066 (2000). This pioneering study shows that γδ T cell-deficient mice display fewer CD8+ T cells and macrophages in the kidneys than wild-type mice, suggesting that γδ T cells have the ability to recruit these cells into the kidney interstitium.
Krebs, C. F. et al. Autoimmune renal disease is exacerbated by S1P-receptor-1-dependent intestinal Th17 cell migration to the kidney. Immunity 45, 1078–1092 (2016).
Gan, P. Y. et al. Pathogenic role for γδ T cells in autoimmune anti-myeloperoxidase glomerulonephritis. J. Immunol. 199, 3042–3050 (2017).
Yang, S. H. et al. NKT cells inhibit the development of experimental crescentic glomerulonephritis. J. Am. Soc. Nephrol. 19, 1663–1671 (2008). This study shows that murine type II NKT cells ameliorate glomerular lesions in experimental crescentic glomerulonephritis, and inhibit lipopolysaccharide-induced proliferation of mesangial cells in vitro.
Mesnard, L. et al. Invariant natural killer T cells and TGF-β attenuate anti-GBM glomerulonephritis. J. Am. Soc. Nephrol. 20, 1282–1292 (2009).
Riedel, J. H. et al. Immature renal dendritic cells recruit regulatory CXCR6+ invariant natural killer T cells to attenuate crescentic GN. J. Am. Soc. Nephrol. 23, 1987–2000 (2012).
Gatto, M. et al. Emerging and critical issues in the pathogenesis of lupus. Autoimmun. Rev. 12, 523–536 (2013).
Wang, L. et al. Downregulation of CD94/NKG2A inhibitory receptor on decreased γδ T cells in patients with systemic lupus erythematosus. Scand. J. Immunol. 76, 62–69 (2012).
Li, X. et al. Generation of human regulatory γδ T cells by TCRγδ stimulation in the presence of TGF-β and their involvement in the pathogenesis of systemic lupus erythematosus. J. Immunol. 186, 6693–6700 (2011).
Peng, S. L., Madaio, M. P., Hayday, A. C. & Craft, J. Propagation and regulation of systemic autoimmunity by γδ T cells. J. Immunol. 157, 5689–5698 (1996).
Rajagopalan, S., Zordan, T., Tsokos, G. C. & Datta, S. K. Pathogenic anti-DNA autoantibody-inducing T helper cell lines from patients with active lupus nephritis: isolation of CD4-8- T helper cell lines that express the γδ T-cell antigen receptor. Proc. Natl Acad. Sci. USA 87, 7020–7024 (1990).
Rezende, R. M. et al. γδ T cells control humoral immune response by inducing T follicular helper cell differentiation. Nat. Commun. 9, 3151 (2018). In this study, CXCR5+ murine γδ T cells are shown to induce TFH cell differentiation, enhance autoantibody production and thereby promote the development of lupus nephritis.
Riedel, J. H. et al. IL-17F promotes tissue injury in autoimmune kidney diseases. J. Am. Soc. Nephrol. 27, 3666–3677 (2016).
Yang, J. Q., Kim, P. J. & Singh, R. R. Brief treatment with iNKT cell ligand α-galactosylceramide confers a long-term protection against lupus. J. Clin. Immunol. 32, 106–113 (2012).
Yang, J. Q. et al. Examining the role of CD1d and natural killer T cells in the development of nephritis in a genetically susceptible lupus model. Arthritis Rheum. 56, 1219–1233 (2007).
Esteban, L. M. et al. Genetic control of NKT cell numbers maps to major diabetes and lupus loci. J. Immunol. 171, 2873–2878 (2003).
Ando, T. et al. Infiltration of canonical Vγ4/Vδ1 γδ T cells in an adriamycin-induced progressive renal failure model. J. Immunol. 167, 3740–3745 (2001).
Wu, H. et al. Depletion of γδ T cells exacerbates murine adriamycin nephropathy. J. Am. Soc. Nephrol. 18, 1180–1189 (2007).
Koenecke, C. et al. In vivo application of mAb directed against the γδ TCR does not deplete but generates “invisible” γδ T cells. Eur. J. Immunol. 39, 372–379 (2009).
Wu, H., Knight, J. F. & Alexander, S. I. Regulatory γδ T cells in Heymann nephritis express an invariant Vγ6/Vδ1 with a canonical CDR3 sequence. Eur. J. Immunol. 34, 2322–2330 (2004).
Kawakami, T., Mimura, I., Shoji, K., Tanaka, T. & Nangaku, M. Hypoxia and fibrosis in chronic kidney disease: crossing at pericytes. Kidney Int. Suppl. 4, 107–112 (2014).
Peng, X. et al. IL-17A produced by both γδ T and Th17 cells promotes renal fibrosis via RANTES-mediated leukocyte infiltration after renal obstruction. J. Pathol. 235, 79–89 (2015).
Law, B. M. et al. Effector γδ T cells in human renal fibrosis and chronic kidney disease. Nephrol. Dial. Transpl. 34, 40–48 (2019).
Law, B. M. P. et al. Human tissue-resident mucosal-associated invariant T (MAIT) cells in renal fibrosis and CKD. J. Am. Soc. Nephrol. 30, 1322–1335 (2019). This study shows that human MAIT cells infiltrate kidney tissue with tubulo-interstitial fibrosis and induce proximal tubule epithelial cell necrosis via perforin and granzyme B in vitro.
Ullah, M. M. & Basile, D. P. Role of renal hypoxia in the progression from acute kidney injury to chronic kidney disease. Semin. Nephrol. 39, 567–580 (2019).
Locatelli, F., Carfagna, F., Del Vecchio, L. & La Milia, V. Haemodialysis or haemodiafiltration: that is the question. Nephrol. Dial. Transpl. 33, 1896–1904 (2018).
Kumbar, L. & Yee, J. Current concepts in hemodialysis vascular access infections. Adv. Chronic Kidney Dis. 26, 16–22 (2019).
Crépin, T. et al. Uraemia-induced immune senescence and clinical outcomes in chronic kidney disease patients. Nephrol. Dial. Transpl. 35, 624–632 (2020).
Goldblum, S. E. & Reed, W. P. Host defenses and immunologic alterations associated with chronic hemodialysis. Ann. Intern. Med. 93, 597–613 (1980).
Yoon, J. W., Gollapudi, S., Pahl, M. V. & Vaziri, N. D. Naïve and central memory T-cell lymphopenia in end-stage renal disease. Kidney Int. 70, 371–376 (2006).
Matsumoto, Y. et al. Peripheral deletion of γδ T cells in haemodialysis patients. Nephrol. Dial. Transpl. 13, 2861–2866 (1998).
Szczepánska, M., Szprynger, K., Mazur, B. & Szczepánski, T. αβ and γδ T cell subsets in chronic renal failure in children on dialysis treatment. Pediatr. Int. 44, 32–36 (2002).
Juno, J. A. et al. γδ T-cell function is inhibited in end-stage renal disease and impacted by latent tuberculosis infection. Kidney Int. 92, 1003–1014 (2017).
Baron, M. et al. Innate-like and conventional T cell populations from hemodialyzed and kidney transplanted patients are equally compromised. PLoS ONE 9, e105422 (2014).
Juno, J. A. et al. Mucosal-associated invariant T cells are depleted and exhibit altered chemokine receptor expression and elevated granulocyte macrophage-colony stimulating factor production during end-stage renal disease. Front. Immunol. 9, 1076 (2018).
Peukert, K. et al. Invariant natural killer T cells are depleted in renal impairment and recover after kidney transplantation. Nephrol. Dial. Transpl. 29, 1020–1028 (2014).
Schaefer, F. & Warady, B. A. Peritoneal dialysis in children with end-stage renal disease. Nat. Rev. Nephrol. 7, 659–668 (2011).
Li, P. K. et al. Changes in the worldwide epidemiology of peritoneal dialysis. Nat. Rev. Nephrol. 13, 90–103 (2017).
Aufricht, C. et al. Biomarker research to improve clinical outcomes of peritoneal dialysis: consensus of the European Training and Research in Peritoneal Dialysis (EuTRiPD) network. Kidney Int. 92, 824–835 (2017).
Betjes, M. G. et al. Intraperitoneal interleukin-8 and neutrophil influx in the initial phase of a CAPD peritonitis. Perit. Dial. Int. 16, 385–392 (1996).
Liuzzi, A. R. et al. Unconventional human T cells accumulate at the site of infection in response to microbial ligands and induce local tissue remodeling. J. Immunol. 197, 2195–2207 (2016).
Liao, C. T. et al. Peritoneal macrophage heterogeneity is associated with different peritoneal dialysis outcomes. Kidney Int. 91, 1088–1103 (2017).
Burton, R. J., Ahmed, R., Cuff, S. M., Artemiou, A. & Eberl, M. CytoPy: an autonomous cytometry analysis framework. PLoS Comput. Biol. 17, e1009071 (2021).
Fricke, H. et al. Continuous ambulatory peritoneal dialysis impairs T lymphocyte selection in the peritoneum. Kidney Int. 49, 1386–1395 (1996).
Eberl, M. et al. A rapid crosstalk of human γδ T cells and monocytes drives the acute inflammation in bacterial infections. PLoS Pathog. 5, e1000308 (2009).
Davey, M. S. et al. Human neutrophil clearance of bacterial pathogens triggers anti-microbial γδ T cell responses in early infection. PLoS Pathog. 7, e1002040 (2011).
Rodrigues-Díez, R. et al. IL-17A is a novel player in dialysis-induced peritoneal damage. Kidney Int. 86, 303–315 (2014).
Ibidapo-Obe, O. et al. Mucosal-associated invariant T cells redistribute to the peritoneal cavity during spontaneous bacterial peritonitis and contribute to peritoneal inflammation. Cell Mol. Gastroenterol. Hepatol. 9, 661–677 (2020).
Niehaus, C. E. et al. MAIT cells are enriched and highly functional in ascites of patients with decompensated liver cirrhosis. Hepatology 72, 1378–1393 (2020).
Lin, C. Y., Kift-Morgan, A., Moser, B., Topley, N. & Eberl, M. Suppression of pro-inflammatory T-cell responses by human mesothelial cells. Nephrol. Dial. Transpl. 28, 1743–1750 (2013).
Wolfe, R. A. et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 341, 1725–1730 (1999).
Sellarés, J. et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am. J. Transpl. 12, 388–399 (2012).
Humar, A. et al. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am. J. Transpl. 10, 1228–1237 (2010).
Witzke, O. et al. Valganciclovir prophylaxis versus preemptive therapy in cytomegalovirus-positive renal allograft recipients: 1-year results of a randomized clinical trial. Transplantation 93, 61–68 (2012).
Crough, T. & Khanna, R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin. Microbiol. Rev. 22, 76–98 (2009).
Couzi, L. et al. Common features of γδ T cells and CD8+ αβ T cells responding to human cytomegalovirus infection in kidney transplant recipients. J. Infect. Dis. 200, 1415–1424 (2009).
Scheper, W. et al. γδT cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia 27, 1328–1338 (2013).
Couzi, L. et al. Gamma-delta T cell expansion is closely associated with cytomegalovirus infection in all solid organ transplant recipients. Transpl. Int. 24, e40–e42 (2011).
Pitard, V. et al. Long-term expansion of effector/memory Vδ2−γδ T cells is a specific blood signature of CMV infection. Blood 112, 1317–1324 (2008).
Kaminski, H. et al. Characterization of a unique γδ T cell subset as a specific marker of CMV infection severity. J. Infect. Dis. 5, jiaa400 (2020).
Pistillo, M. et al. The effects of age and viral serology on γδ T-cell numbers and exercise responsiveness in humans. Cell Immunol. 284, 91–97 (2013).
Couzi, L. et al. Antibody-dependent anti-cytomegalovirus activity of human γδ T cells expressing CD16 (FcγRIIIa). Blood 119, 1418–1427 (2012). This study identifies a new antiviral function for CMV-induced CD16+ human γδ T cells through cooperation with anti-CMV IgG that contributes to surveillance of CMV reactivation in solid organ transplant recipients.
Halary, F. et al. Shared reactivity of Vδ2neg γδ T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J. Exp. Med. 201, 1567–1578 (2005).
Khairallah, C. et al. γδ T cells confer protection against murine cytomegalovirus (MCMV). PLoS Pathog. 11, e1004702 (2015).
Sell, S. et al. Control of murine cytomegalovirus infection by γδ T cells. PLoS Pathog. 11, e1004481 (2015).
Kaminski, H. et al. Surveillance of γδ T cells predicts cytomegalovirus infection resolution in kidney transplants. J. Am. Soc. Nephrol. 27, 637–645 (2016).
Renneson, J. et al. IL-12 and type I IFN response of neonatal myeloid DC to human CMV infection. Eur. J. Immunol. 39, 2789–2799 (2009).
Guerville, F. et al. TCR-dependent sensitization of human γδ T cells to non-myeloid IL-18 in cytomegalovirus and tumor stress surveillance. Oncoimmunology 4, e1003011 (2015).
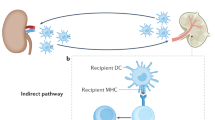
Marino, J., Paster, J. & Benichou, G. Allorecognition by T lymphocytes and allograft rejection. Front. Immunol. 7, 582 (2016).
Chen, C. C. et al. T cell help is mandatory for naive and memory donor-specific antibody responses: Impact of therapeutic immunosuppression. Front. Immunol. 9, 275 (2018).
Zhang, X. & Reed, E. F. Effect of antibodies on endothelium. Am. J. Transpl. 9, 2459–2465 (2009).
Sicard, A. et al. Detection of C3d-binding donor-specific anti-HLA antibodies at diagnosis of humoral rejection predicts renal graft loss. J. Am. Soc. Nephrol. 26, 457–467 (2015).
Hidalgo, L. G. et al. NK cell transcripts and NK cells in kidney biopsies from patients with donor-specific antibodies: evidence for NK cell involvement in antibody-mediated rejection. Am. J. Transpl. 10, 1812–1822 (2010).
Drobyski, W. R., Majewski, D. & Hanson, G. Graft-facilitating doses of ex vivo activated γδ T cells do not cause lethal murine graft-vs.-host disease. Biol. Blood Marrow Transpl. 5, 222–230 (1999).
Zhang, Z. et al. Identifying 4 Novel lncRNAs as potential biomarkers for acute rejection and graft loss of renal allograft. J. Immunol. Res. 2020, 2415374 (2020).
Benveniste, P. M. et al. Generation and molecular recognition of melanoma-associated antigen-specific human γδ T cells. Sci. Immunol. 3, eaav4036 (2018).
Itoh, S. et al. Interleukin-17 accelerates allograft rejection by suppressing regulatory T cell expansion. Circulation 124, S187–S196 (2011). The first study to show that in the mouse, γδ T cells are a potential source of IL-17, which is crucial for promoting acute allograft rejection by inhibiting the expansion of Treg cells.
Li, Y. et al. Vγ4 γδ T cells provide an early source of IL-17a and accelerate skin graft rejection. J. Invest. Dermatol. 137, 2513–2522 (2017).
Rahimpour, A. et al. γδ T cells augment rejection of skin grafts by enhancing cross-priming of CD8 T cells to skin-derived antigen. J. Invest. Dermatol. 132, 1656–1664 (2012).
Loupy, A. et al. The Banff 2019 Kidney Meeting Report (I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am. J. Transpl. 20, 2318–2331 (2020).
Bachelet, T. et al. Cytomegalovirus-responsive γδ T cells: novel effector cells in antibody-mediated kidney allograft microcirculation lesions. J. Am. Soc. Nephrol. 25, 2471–2482 (2014). An important study showing that CMV-induced CD16+ human γδ T cells can perform antibody-dependent cellular cytotoxicity against donor cells coated with donor-specific antibodies, supporting the notion that these cells are involved in antibody-mediated injury of kidney transplants.
Martínez-Llordella, M. et al. Multiparameter immune profiling of operational tolerance in liver transplantation. Am. J. Transpl. 7, 309–319 (2007).
Zhao, X. et al. Intragraft Vδ1 γδ T cells with a unique T-cell receptor are closely associated with pediatric semiallogeneic liver transplant tolerance. Transplantation 95, 192–202 (2013).
Puig-Pey, I. et al. Characterization of γδ T cell subsets in organ transplantation. Transpl. Int. 23, 1045–1055 (2010).
Pillai, A. B., George, T. I., Dutt, S. & Strober, S. Host natural killer T cells induce an interleukin-4-dependent expansion of donor CD4+CD25+Foxp3+ T regulatory cells that protects against graft-versus-host disease. Blood 113, 4458–4467 (2009).
Hongo, D., Tang, X., Dutt, S., Nador, R. G. & Strober, S. Interactions between NKT cells and Tregs are required for tolerance to combined bone marrow and organ transplants. Blood 119, 1581–1589 (2012).
Hongo, D., Tang, X., Zhang, X., Engleman, E. G. & Strober, S. Tolerogenic interactions between CD8+ dendritic cells and NKT cells prevent rejection of bone marrow and organ grafts. Blood 129, 1718–1728 (2017).
Vajdic, C. M. et al. Cancer incidence before and after kidney transplantation. JAMA 296, 2823–2831 (2006).
Matinfar, M., Shahidi, S. & Feizi, A. Incidence of nonmelanoma skin cancer in renal transplant recipients: a systematic review and meta-analysis. J. Res. Med. Sci. 23, 14 (2018).
Fisher, J. P., Heuijerjans, J., Yan, M., Gustafsson, K. & Anderson, J. γδ T cells for cancer immunotherapy: a systematic review of clinical trials. Oncoimmunology 3, e27572 (2014).
Billon, E. et al. Soluble BTN2A1 is a potential prognosis biomarker in pre-treated advanced renal cell carcinoma. Front. Immunol. 12, 670827 (2021).
Krone, B. et al. Impact of vaccinations and infectious diseases on the risk of melanoma–evaluation of an EORTC case-control study. Eur. J. Cancer 39, 2372–2378 (2003).
Devaud, C. et al. Antitumor activity of γδ T cells reactive against cytomegalovirus-infected cells in a mouse xenograft tumor model. Cancer Res. 69, 3971–3978 (2009).
Couzi, L. et al. Cytomegalovirus-induced γδ T cells associate with reduced cancer risk after kidney transplantation. J. Am. Soc. Nephrol. 21, 181–188 (2010).
Godder, K. T. et al. Long term disease-free survival in acute leukemia patients recovering with increased γδ T cells after partially mismatched related donor bone marrow transplantation. Bone Marrow Transpl. 39, 751–757 (2007).
Elmaagacli, A. H. et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood 118, 1402–1412 (2011).
Lin, C. Y. et al. Pathogen-specific local immune fingerprints diagnose bacterial infection in peritoneal dialysis patients. J. Am. Soc. Nephrol. 24, 2002–2009 (2013). The first study to demonstrate that different types of bacterial peritonitis can be distinguished based on local pathogen-specific biomarker signatures on the day of presentation with acute symptoms, implicating human Vγ9Vδ2 T cells in the response.
Zhang, J. et al. Machine-learning algorithms define pathogen-specific local immune fingerprints in peritoneal dialysis patients with bacterial infections. Kidney Int. 92, 179–191 (2017).
US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03339661 (2017).
Gold, M. C. et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 8, e1000407 (2010).
Eberl, M., Oldfield, E. & Herrmann, T. Immuno-antibiotics: targeting microbial metabolic pathways sensed by unconventional T cells. Immunother. Adv. 1, ltab005 (2021).
Singh, K. S. et al. IspH inhibitors kill Gram-negative bacteria and mobilize immune clearance. Nature 589, 597–602 (2020). A study demonstrating the dual action of inhibitors of HMB-PP reductase as ‘immuno-antibiotics’ with direct antimicrobial activity against multidrug-resistant bacteria and at the same time harnessing Vγ9Vδ2 T cells against those bacteria.
Blazquez, J. L., Benyamine, A., Pasero, C. & Olive, D. New insights into the regulation of γδ T cells by BTN3A and other BTN/BTNL in tumor immunity. Front. Immunol. 9, 1601 (2018).
Raffray, L., Burton, R. J., Baker, S. E., Morgan, M. P. & Eberl, M. Zoledronate rescues immunosuppressed monocytes in sepsis patients. Immunology 159, 88–95 (2020).
Smith, C. et al. Autologous adoptive T-cell therapy for recurrent or drug-resistant cytomegalovirus complications in solid organ transplant recipients: a single-arm open-label phase I clinical trial. Clin. Infect. Dis. 68, 632–640 (2019).
Almeida, A. R. et al. Delta One T cells for immunotherapy of chronic lymphocytic leukemia: clinical-grade expansion/differentiation and preclinical proof of concept. Clin. Cancer Res. 22, 5795–5804 (2016). A pioneering study that develops a robust and highly reproducible clinical grade method for selective and large-scale expansion and differentiation of cytotoxic human Vδ1+ T cells, ready to use for adoptive immunotherapy.
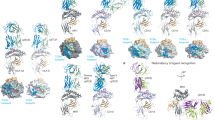
Gadola, S. D. et al. Structure and binding kinetics of three different human CD1d-α-galactosylceramide-specific T cell receptors. J. Exp. Med. 203, 699–710 (2006).
Le Nours, J. et al. Atypical natural killer T-cell receptor recognition of CD1d-lipid antigens. Nat. Commun. 7, 10570 (2016).
Van Rhijn, I. et al. A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nat. Immunol. 14, 706–713 (2013).
Harriff, M. J. et al. MR1 displays the microbial metabolome driving selective MR1-restricted T cell receptor usage. Sci. Immunol. 3, eaao2556 (2018).
Cotton, R. N. et al. Human skin is colonized by T cells that recognize CD1a independently of lipid. J. Clin. Invest. 131, 140706 (2021).
Reijneveld, J. F. et al. Human γδ T cells recognize CD1b by two distinct mechanisms. Proc. Natl Acad. Sci. USA 117, 22944–22952 (2020).
Wun, K. S. et al. T cell autoreactivity directed toward CD1c itself rather than toward carried self lipids. Nat. Immunol. 19, 397–406 (2018).
Lafarge, X. et al. Cytomegalovirus infection in transplant recipients resolves when circulating γδ T lymphocytes expand, suggesting a protective antiviral role. J. Infect. Dis. 184, 533–541 (2001).
Acknowledgements
The authors apologize to all colleagues in the field whose work was not cited owing to space limitations or unintended oversight. H.K. and L.C. were supported by Fondation pour la Recherche Médicale, Fondation du Rein and Fondation Bordeaux Université; M.E. was supported by the Medical Research Council, Kidney Research UK, the National Institute for Health Research and the Welsh European Funding Office’s Accelerate programme. The authors thank the members of their research teams and J. McLaren for critical comments on the manuscript before submission. H.K. and L.C. are deeply grateful to J. Déchanet-Merville and P. Merville for their thoughtful advice, mentoring and unwavering support.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made substantial contributions to discussions of the content, wrote, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Nephrology thanks D. Doherty, M. Dunne, I. Prinz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Memory T cells
-
Long-lived antigen-specific T cells that remain in the body after the initial immune response has resolved and confer protection against a subsequent challenge with the same stimulus; effector memory T cells and tissue-resident memory T cells mount rapid recall responses at local sites, whereas central memory T cells patrol secondary lymphoid tissues.
- Adaptive immunity
-
Selective clonal expansion of individual T and B cells that are specific for non-self (for example, microbes, viruses or allergens) or self-antigens (autoimmunity or tumour antigens) and mount highly antigen-specific cellular and/or antibody responses. The expansion and differentiation of these antigen-specific cells increases the speed and efficiency of future memory responses to the same antigen.
- TCR repertoires
-
Summary of unique genetic rearrangements of the T cell receptor (TCR) in each T cell within an anatomical or functional compartment, which for classical T cells are typically polyclonal and ‘private’, whereas unconventional T cells are often oligoclonal and can carry invariant, ‘public’ TCR sequences shared between people.
- Cytomegalovirus
-
(CMV). A virus that causes an infection that is almost asymptomatic in immunocompetent individuals but is associated with considerable morbidity and mortality in immunocompromised individuals; despite prevention strategies based on the use of antivirals, CMV-seronegative recipients who receive an organ from CMV-seropositive donors have the highest risk (20%) of developing CMV disease.
- Innate immunity
-
Nonspecific defence mechanism that is deployed within hours of encountering non-self structures (for example, a pathogen or a foreign object) or danger signals (triggered, for example, by tissue injury or stress); mediated by innate immune cells such as natural killer cells, mast cells, granulocytes (eosinophils, basophils or neutrophils), monocytes, macrophages and dendritic cells.
- Antigen presentation
-
Cellular process whereby antigenic epitopes are displayed on the surface of a cell for recognition by the T cell receptor (TCR) of a neighbouring T cell, typically as short peptides in the context of major histocompatibility complex (MHC) class I and class II molecules in the case of classical CD8+ and CD4+ T cells, respectively, or as non-peptide antigens in association with MHC-related molecules such as CD1 or MR1 in the case of unconventional T cells.
- Antibody-dependent cellular cytotoxicity
-
(ADCC). Immune mechanism through which effector cells carrying receptors for the crystallizable fragment (Fc) region of antibodies can recognize and lyse antibody-coated (that is, opsonized) target cells.
- Ischaemia–reperfusion injury
-
(IRI). Tissue damage that occurs after a period of oxygen deprivation due to ischaemia (that is, disrupted blood supply), which can be caused by sepsis, thrombosis, organ transplantation or trauma, and leads to inflammation, oxidative stress and necrosis following restoration of the normal blood supply.
- IgA nephropathy
-
The most prevalent form of glomerulonephritis in the world and a common cause of kidney failure; it seems to be a systemic disease in which the kidneys are the targets of galactose-deficient IgA1, which stimulates mesangial cells to proliferate, secrete pro-inflammatory and profibrotic cytokines, components of the extracellular matrix and growth factors, activate complement and release reactive oxygen species.
- Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis
-
A severe autoimmune disease that mainly affects small vessels in various organs (including the kidney in up to 80% of patients) and is characterized by the presence of ANCA antibodies in serum, excessive neutrophil activation and release of pro-inflammatory cytokines, reactive oxygen species and lytic enzymes.
- T cell-mediated rejection
-
(TCMR). Recognition of mismatched donor antigens (mainly represented by highly polymorphic major histocompatibility complex molecules), which results in priming of effector T cells against these alloantigens and ultimately leads to allograft rejection.
- Antibody-mediated rejection
-
(ABMR). Allograft rejection due to the recognition of mismatched donor major histocompatibility complex molecules by recipient B cells; effective treatments that can halt donor-specific antibody-mediated rejection are currently not available and this type of graft rejection is thus associated with poor prognosis.
Rights and permissions
About this article
Cite this article
Kaminski, H., Couzi, L. & Eberl, M. Unconventional T cells and kidney disease. Nat Rev Nephrol 17, 795–813 (2021). https://doi.org/10.1038/s41581-021-00466-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-021-00466-8
This article is cited by
-
Mucosal T-cell responses to chronic viral infections: Implications for vaccine design
Cellular & Molecular Immunology (2024)