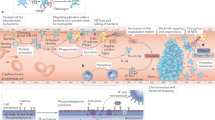
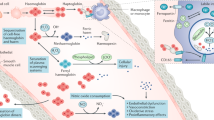
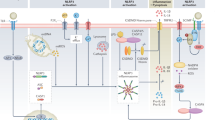
Key Points
-
Haemodialysis is a life-saving renal replacement modality for end-stage renal disease, but this therapy is associated with an increased risk of cardiovascular disease
-
The biomaterial surfaces that come into contact with blood in the extracorporeal circuit during haemodialysis trigger activation of the intravascular cascade systems
-
Generation of activation products from the intravascular cascade systems causes systemic inflammation involving blood cells and the vascular tree
-
The anaphylatoxins C3a and C5a, bradykinin, thrombin and factor Xa can induce activation of endothelial cells and the vascular wall
-
The thrombotic and inflammatory properties of these activation products lead to atherogenesis, which can progress to arteriosclerosis and cardiovascular disease
Abstract
Haemodialysis is a life-saving renal replacement modality for end-stage renal disease, but this therapy also represents a major challenge to the intravascular innate immune system, which is comprised of the complement, contact and coagulation systems. Chronic inflammation is strongly associated with cardiovascular disease (CVD) in patients on haemodialysis. Biomaterial-induced contact activation of proteins within the plasma cascade systems occurs during haemodialysis and initially leads to local generation of inflammatory mediators on the biomaterial surface. The inflammation is spread by soluble activation products and mediators that are generated during haemodialysis and transported in the extracorporeal circuit back into the patient together with activated leukocytes and platelets. The combined effect is activation of the endothelium of the cardiovascular system, which loses its anti-thrombotic and anti-inflammatory properties, leading to atherogenesis and arteriosclerosis. This concept suggests that maximum suppression of the intravascular innate immune system is needed to minimize the risk of CVD in patients on haemodialysis. A potential approach to achieve this goal is to treat patients with broad-specificity systemic drugs that target more than one of the intravascular cascade systems. Alternatively, 'stealth' biomaterials that cause minimal cascade system activation could be used in haemodialysis circuits.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Peters, S. A. et al. Haemodiafiltration and mortality in end-stage kidney disease patients: a pooled individual participant data analysis from four randomized controlled trials. Nephrol. Dial. Transplant. 31, 978–984 (2016).
Tonelli, M. et al. Chronic kidney disease and mortality risk: a systematic review. J. Am. Soc. Nephrol. 17, 2034–2047 (2006).
Rogacev, K. S. et al. Haemodialysis-induced transient CD16+ monocytopenia and cardiovascular outcome. Nephrol. Dial. Transplant. 24, 3480–3486 (2009).
Mares, J. et al. Proteomic profiling of blood-dialyzer interactome reveals involvement of lectin complement pathway in hemodialysis-induced inflammatory response. Proteomics Clin. Appl. 4, 829–838 (2010).
Daugirdas, J. T. & Bernardo, A. A. Hemodialysis effect on platelet count and function and hemodialysis-associated thrombocytopenia. Kidney Int. 82, 147–157 (2012).
Schoorl, M., Schoorl, M., Nubé, M. J. & Bartels, P. C. M. Platelet depletion, platelet activation and coagulation during treatment with hemodialysis. Scand. J. Clin. Lab. Invest. 71, 240–247 (2011).
Go, A. S., Chertow, G. M., Fan, D., McCulloch, C. E. & Hsu, C. Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351, 1296–1305 (2004).
Foley, R. N., Parfrey, P. S. & Sarnak, M. J. Epidemiology of cardiovascular disease in chronic renal disease. J. Am. Soc. Nephrol. 9, S16–S23 (1998).
Jha, V. et al. Chronic kidney disease: global dimension and perspectives. Lancet 382, 260–272 (2013).
Jardine, A. G., Gaston, R. S., Fellstrom, B. C. & Holdaas, H. Prevention of cardiovascular disease in adult recipients of kidney transplants. Lancet 378, 1419–1427 (2011).
Fellstrom, B. C. et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 360, 1395–1407 (2009).
Baigent, C. et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 377, 2181–2192 (2011).
Holdaas, H. et al. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet 361, 2024–2031 (2003).
Wanner, C. et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N. Engl. J. Med. 353, 238–248 (2005).
Zhang, W. et al. Prognostic role of C-reactive protein and interleukin-6 in dialysis patients: a systematic review and meta-analysis. J. Nephrol. 26, 243–253 (2013).
Sun, J. et al. Biomarkers of cardiovascular disease and mortality risk in patients with advanced CKD. Clin. J. Am. Soc. Nephrol. 11, 1163–1172 (2016).
Tripepi, G., Mallamaci, F. & Zoccali, C. Inflammation markers, adhesion molecules, and all-cause and cardiovascular mortality in patients with ESRD: searching for the best risk marker by multivariate modeling. J. Am. Soc. Nephrol. 16 (Suppl. 1), S83–S88 (2005).
Cohen, S. D., Phillips, T. M., Khetpal, P. & Kimmel, P. L. Cytokine patterns and survival in haemodialysis patients. Nephrol. Dial. Transplant. 25, 1239–1243 (2010).
Zoccali, C., Tripepi, G. & Mallamaci, F. Dissecting inflammation in ESRD: do cytokines and C-reactive protein have a complementary prognostic value for mortality in dialysis patients? J. Am. Soc. Nephrol. 17, S169–S173 (2006).
Jofre, R., Rodriguez-Benitez, P., Lopez-Gomez, J. M. & Perez-Garcia, R. Inflammatory syndrome in patients on hemodialysis. J. Am. Soc. Nephrol. 17, S274–S280 (2006).
Akchurin, O. M. & Kaskel, F. Update on inflammation in chronic kidney disease. Blood Purif. 39, 84–92 (2015).
Cobo, G., Qureshi, A. R., Lindholm, B. & Stenvinkel, P. C-reactive protein: repeated measurements will improve dialysis patient care. Semin. Dial. 29, 7–14 (2016).
Betjes, M. G. Immune cell dysfunction and inflammation in end-stage renal disease. Nat. Rev. Nephrol. 9, 255–265 (2013).
Almasri, J. et al. Outcomes of vascular access for hemodialysis: a systematic review and meta-analysis. J. Vasc. Surg. 64, 236–243 (2016).
Carrero, J. J. & Stenvinkel, P. Persistent inflammation as a catalyst for other risk factors in chronic kidney disease: a hypothesis proposal. Clin. J. Am. Soc. Nephrol. 4 (Suppl. 1), S49–S55 (2009).
Ismail, G., Dumitriu, H. T., Dumitriu, A. S. & Ismail, F. B. Periodontal disease: a covert source of inflammation in chronic kidney disease patients. Int. J. Nephrol. 2013, 515796 (2013).
Gillespie, I. A. et al. Factors precipitating erythropoiesis-stimulating agent responsiveness in a European haemodialysis cohort: case-crossover study. Pharmacoepidemiol. Drug Saf. 24, 414–426 (2015).
Frank, R. D. et al. Role of contact system activation in hemodialyzer-induced thrombogenicity. Kidney Int. 60, 1972–1981 (2001).
Horl, W. H. Hemodialysis membranes: interleukins, biocompatibility, and middle molecules. J. Am. Soc. Nephrol. 13 (Suppl. 1), S62–S71 (2002).
Eckardt, K. U. et al. High cardiovascular event rates occur within the first weeks of starting hemodialysis. Kidney Int. 88, 1117–1125 (2015).
Friedrich, B. et al. Acute effects of hemodialysis on cytokine transcription profiles: evidence for C-reactive protein-dependency of mediator induction. Kidney Int. 70, 2124–2130 (2006).
Molnar, M. Z., Ojo, A. O., Bunnapradist, S., Kovesdy, C. P. & Kalantar-Zadeh, K. Timing of dialysis initiation in transplant-naive and failed transplant patients. Nat. Rev. Nephrol. 8, 284–292 (2012).
Merchant, A. A., Quinn, R. R. & Perl, J. Dialysis modality and survival: does the controversy live on? Curr. Opin. Nephrol. Hypertens. 24, 276–283 (2015).
Reis, E. S., Falcao, D. A. & Isaac, L. Clinical aspects and molecular basis of primary deficiencies of complement component C3 and its regulatory proteins factor I and factor H. Scand. J. Immunol. 63, 155–168 (2006).
Takemoto, Y., Naganuma, T. & Yoshimura, R. Biocompatibility of the dialysis membrane. Contrib. Nephrol. 168, 139–145 (2011).
Engberg, A. E. et al. Prediction of inflammatory responses induced by biomaterials in contact with human blood using protein fingerprint from plasma. Biomaterials 36, 55–65 (2015).
Huang, S. et al. Reciprocal relationship between the contact and the complement system activation on artificial polymers exposed to whole human blood. Biomaterials 77, 111–119 (2016).
Wanner, C., Amann, K. & Shoji, T. The heart and vascular system in dialysis. Lancet 388, 276–284 (2016).
Ghanavatian, S. et al. Subclinical atherosclerosis, endothelial function, and serum inflammatory markers in chronic kidney disease stages 3 to 4. Angiology 65, 443–449 (2014).
Annuk, M. et al. Oxidative stress markers in pre-uremic patients. Clin. Nephrol. 56, 308–314 (2001).
Annuk, M. et al. Endothelial function, CRP and oxidative stress in chronic kidney disease. J. Nephrol. 18, 721–726 (2005).
Liu, Y. et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA 291, 451–459 (2004).
Stenvinkel, P. et al. IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia — the good, the bad, and the ugly. Kidney Int. 67, 1216–1233 (2005).
Arici, M. & Walls, J. End-stage renal disease, atherosclerosis, and cardiovascular mortality: is C-reactive protein the missing link? Kidney Int. 59, 407–414 (2001).
Honda, H. et al. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am. J. Kidney Dis. 47, 139–148 (2006).
Annuk, M., Zilmer, M., Lind, L., Linde, T. & Fellstrom, B. Oxidative stress and endothelial function in chronic renal failure. J. Am. Soc. Nephrol. 12, 2747–2752 (2001).
Soveri, I. et al. Improvement in central arterial pressure waveform during hemodialysis is related to a reduction in asymmetric dimethylarginine (ADMA) levels. Nephron. Clin. Pract. 106, c180–c186 (2007).
Taal, M. W. Arterial stiffness in chronic kidney disease: an update. Curr. Opin. Nephrol. Hypertens. 23, 169–173 (2014).
Agharazii, M. et al. Inflammatory cytokines and reactive oxygen species as mediators of chronic kidney disease-related vascular calcification. Am. J. Hypertens. 28, 746–755 (2015).
Hung, A. M., Ellis, C. D., Shintani, A., Booker, C. & Ikizler, T. A. IL-1beta receptor antagonist reduces inflammation in hemodialysis patients. J. Am. Soc. Nephrol. 22, 437–442 (2011).
The Cholesterol Treatment Trialists' (CTT) Collaboration et al. Impact of renal function on the effects of reducing LDL cholesterol with statin-based regimens: meta-analysis of individual data from 28 randomised trials. Lancet Diabetes Endocrinol. 4, 829–839 (2016).
Woodruff, T. M., Nandakumar, K. S. & Tedesco, F. Inhibiting the C5-C5a receptor axis. Mol. Immunol. 48, 1631–1642 (2011).
Dinarello, C. A. An update on human interleukin-1: from molecular biology to clinical relevance. J. Clin. Immunol. 5, 287–297 (1985).
Varela, M. P. et al. Biocompatibility of hemodialysis membranes: interrelations between plasma complement and cytokine levels. Blood Purif. 19, 370–379 (2001).
Lhotta, K., Wurzner, R., Kronenberg, F., Oppermann, M. & Konig, P. Rapid activation of the complement system by cuprophane depends on complement component C4. Kidney Int. 53, 1044–1051 (1998).
Huang, Z., Gao, D., Letteri, J. J. & Clark, W. R. Blood-membrane interactions during dialysis. Semin. Dial. 22, 623–628 (2009).
Nilsson, B., Ekdahl, K. N., Mollnes, T. E. & Lambris, J. D. The role of complement in biomaterial-induced inflammation. Mol. Immunol. 44, 82–94 (2007).
Andersson, J., Ekdahl, K. N., Larsson, R., Nilsson, U. R. & Nilsson, B. C3 adsorbed to a polymer surface can form an initiating alternative pathway convertase. J. Immunol. 168, 5786–5791 (2002).
Tengvall, P., Askendal, A. & Lundström, I. Complement activation by IgG immobilized on methylated silicon. J. Biomed. Mater. Res. 31, 305–312 (1996).
Hulander, M. et al. Gradients in surface nanotopography used to study platelet adhesion and activation. Colloids Surf. B Biointerfaces 110, 261–269 (2013).
Andersson, J., Ekdahl, K. N., Lambris, J. D. & Nilsson, B. Binding of C3 fragments on top of adsorbed plasma proteins during complement activation on a model biomaterial surface. Biomaterials 26, 1477–1485 (2005).
Chenoweth, D. E., Cheung, A. K. & Henderson, L. W. Anaphylatoxin formation during hemodialysis: effects of different dialyzer membranes. Kidney Int. 24, 764–769 (1983).
Ekdahl, K. N., Nilsson, B., Pekna, M. & Nilsson, U. R. Generation of iC3 at the interface between blood and gas. Scand. J. Immunol. 35, 85–91 (1992).
Herzog, C. A., Mangrum, J. M. & Passman, R. Sudden cardiac death and dialysis patients. Semin. Dial. 21, 300–307 (2008).
Furebring, M., Hakansson, L., Venge, P. & Sjolin, J. C5a, interleukin-8 and tumour necrosis factor-alpha-induced changes in granulocyte and monocyte expression of complement receptors in whole blood and on isolated leukocytes. Scand. J. Immunol. 63, 208–216 (2006).
Ward, P. A. The harmful role of c5a on innate immunity in sepsis. J. Innate Immun. 2, 439–445 (2010).
Verschoor, A., Karsten, C. M., Broadley, S. P., Laumonnier, Y. & Kohl, J. Old dogs-new tricks: immunoregulatory properties of C3 and C5 cleavage fragments. Immunol. Rev. 274, 112–126 (2016).
Kourtzelis, I. et al. Complement anaphylatoxin C5a contributes to hemodialysis-associated thrombosis. Blood 116, 631–639 (2010).
Reis, E. S. et al. Therapeutic C3 inhibitor Cp40 abrogates complement activation induced by modern hemodialysis filters. Immunobiology 220, 476–482 (2015).
Niederbichler, A. D. et al. An essential role for complement C5a in the pathogenesis of septic cardiac dysfunction. J. Exp. Med. 203, 53–61 (2006).
Manthey, H. D. et al. Complement C5a inhibition reduces atherosclerosis in ApoE−/− mice. FASEB J. 25, 2447–2455 (2011).
Wezel, A. et al. Complement factor C5a induces atherosclerotic plaque disruptions. J. Cell. Mol. Med. 18, 2020–2030 (2014).
Koch, M. & Zernecke, A. The hemostatic system as a regulator of inflammation in atherosclerosis. IUBMB Life 66, 735–744 (2014).
Giri, S. & Jennings, L. K. The spectrum of thrombin in acute coronary syndromes. Thromb. Res. 135, 782–787 (2015).
Coughlin, S. R. Thrombin signalling and protease-activated receptors. Nature 407, 258–264 (2000).
Esmon, C. T. Targeting factor Xa and thrombin: impact on coagulation and beyond. Thromb. Haemost. 111, 625–633 (2014).
Hamad, O. A., Bäck, J., Nilsson, P. H., Nilsson, B. & Ekdahl, K. N. Platelets, complement, and contact activation: partners in inflammation and thrombosis. Adv. Exp. Med. Biol. 946, 185–205 (2012).
Terzuoli, E. et al. Antagonism of bradykinin B2 receptor prevents inflammatory responses in human endothelial cells by quenching the NF-kB pathway activation. PLoS ONE 9, e84358 (2014).
Marney, A. M., Ma, J., Luther, J. M., Ikizler, T. A. & Brown, N. J. Endogenous bradykinin contributes to increased plasminogen activator inhibitor 1 antigen following hemodialysis. J. Am. Soc. Nephrol. 20, 2246–2252 (2009).
Heitsch, H. The therapeutic potential of bradykinin B2 receptor agonists in the treatment of cardiovascular disease. Expert Opin. Investig. Drugs 12, 759–770 (2003).
Feng, W. et al. Increased age-related cardiac dysfunction in bradykinin B2 receptor-deficient mice. J. Gerontol. A Biol. Sci. Med. Sci. 71, 178–187 (2016).
Leeb-Lundberg, L. M., Kang, D. S., Lamb, M. E. & Fathy, D. B. The human B1 bradykinin receptor exhibits high ligand-independent, constitutive activity. Roles of residues in the fourth intracellular and third transmembrane domains. J. Biol. Chem. 276, 8785–8792 (2001).
Duchene, J. & Ahluwalia, A. The kinin B1 receptor and inflammation: new therapeutic target for cardiovascular disease. Curr. Opin. Pharmacol. 9, 125–131 (2009).
Lizama, A. J. et al. Expression and bioregulation of the kallikrein-related peptidases family in the human neutrophil. Innate Immun. 21, 575–586 (2015).
Figueroa, C. D. et al. Kinin B1 receptor regulates interactions between neutrophils and endothelial cells by modulating the levels of Mac-1, LFA-1 and intercellular adhesion molecule-1. Innate Immun. 21, 289–304 (2015).
Papageorgiou, P. C., Chan, C. T., Yeo, E. L., Backx, P. H. & Floras, J. S. Coagulation factor XIIa-kinin-mediated contribution to hypertension of chronic kidney disease. J. Hypertens. 32, 1523–1533 (2014).
Ceravolo, G. S. et al. An interaction of renin-angiotensin and kallikrein-kinin systems contributes to vascular hypertrophy in angiotensin II-induced hypertension: in vivo and in vitro studies. PLoS ONE 9, e111117 (2014).
Ghebrehiwet, B., Kaplan, A. P., Joseph, K. & Peerschke, E. I. The complement and contact activation systems: partnership in pathogenesis beyond angioedema. Immunol. Rev. 274, 281–289 (2016).
Bjork, I. & Lindahl, U. Mechanism of the anticoagulant action of heparin. Mol. Cell. Biochem. 48, 161–182 (1982).
Dhondt, A. et al. Where and when to inject low molecular weight heparin in hemodiafiltration? A cross over randomised trial. PLoS ONE 10, e0128634 (2015).
Hemker, H. C. A century of heparin: past, present and future. J. Thromb. Haemost. 14, 2329–2338 (2016).
Gong, J. et al. Tubing loops as a model for cardiopulmonary bypass circuits: both the biomaterial and the blood-gas phase interfaces induce complement activation in an in vitro model. J. Clin. Immunol. 16, 222–229 (1996).
Larsson, M. et al. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci. Transl Med. 6, 222ra17 (2014).
Olsson, C. et al. Heparin-coated cardiopulmonary bypass circuits reduce circulating complement factors and interleukin-6 in paediatric heart surgery. Scand. Cardiovasc. J. 34, 33–40 (2000).
François, K. et al. Avoidance of systemic anticoagulation during intermittent haemodialysis with heparin-grafted polyacrilonitrile membrane and citrate-enriched dialysate: a retrospective cohort study. BMC Nephrol. 15, 104 (2014).
Kessler, M. et al. Heparin-grafted dialysis membrane allows minimal systemic anticoagulation in regular hemodialysis patients: a prospective proof-of-concept study. Hemodial. Int. 17, 282–293 (2013).
Cheng, Y.-L. et al. Anticoagulation during haemodialysis using a citrate-enriched dialysate: a feasibility study. Nephrol. Dial. Transplant. 26, 641–646 (2011).
Persona, P. et al. Anticoagulation with citrate for hemodiafiltration in an acute bleeding trauma. Int. J. Artif. Organs 38, 343–344 (2015).
Ishihara, K. & Takai, M. Bioinspired interface for nanobiodevices based on phospholipid polymer chemistry. J. R. Soc. Interface 6 (Suppl. 3), S279–S291 (2009).
Sakai, K. & Matsuda, M. in High-Performance Membrane Dialyzers. Contributions to Nephrology (eds Saito, A., Kawanishi, H., Yamashita, A. & Mineshima, M.) 11–22 (Karger, 2011).
Zweigert, C., Neubauer, M. & Storr, M. in Comprehensive Membrane Science and Engineering (eds Drioli, E. & Giorno, L.) 351–387 (Elsevier, 2013).
Krummel, T. & Hannedouche, T. Clinical potentials of adsorptive dialysis membranes. Blood Purif. 35 (Suppl. 2), 1–4 (2013).
Saito, A., Kawanishi, H., Yamashita, A. & Mineshima, M. (eds) in High-Performance Membrane Dialyzers. Contributions to Nephrology 137–147 (Karger, 2011).
Nakano, A. in High-Performance Membrane Dialyzers. Contributions to Nephrology (eds Saito, A., Kawanishi, H., Yamashita, A. & Mineshima, M) 164–171 (Karger, 2011).
Bonomini, M. et al. Proteomics characterization of protein adsorption onto hemodialysis membranes. J. Proteome Res. 5, 2666–2674 (2006).
Kolár˘ová, H., Ambruzová, B., Svihálková Šindlerová, L., Klinke, A. & Kubala, L. Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediators Inflamm. 2014, 694312 (2014).
Yang, G. et al. Novel mechanisms of endothelial dysfunction in diabetes. J. Cardiovasc. Dis. Res. 1, 59–63 (2010).
Ekdahl, K. N. et al. Dangerous liaisons: complement, coagulation, and kallikrein/kinin cross-talk act as a linchpin in the events leading to thromboinflammation. Immunol. Rev. 274, 245–269 (2016).
Ricklin, D., Hajishengallis, G., Yang, K. & Lambris, J. D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 11, 785–797 (2010).
Trouw, L. A. & Daha, M. R. Role of complement in innate immunity and host defense. Immunol. Lett. 138, 35–37 (2011).
Bäck, J. et al. Distinctive regulation of contact activation by antithrombin and C1-inhibitor on activated platelets and material surfaces. Biomaterials 30, 6573–6580 (2009).
Janssen, B. J. et al. Structures of complement component C3 provide insights into the function and evolution of immunity. Nature 437, 505–511 (2005).
Acknowledgements
The authors thank Deborah McClellan for editing the manuscript before submission and Osama Hamad (Uppsala University, Sweden) for excellent help with drafting the figures. The authors' work was supported by grant 2013-65X-05647-34-4 from the Swedish Research Council (VR), the European Community's Seventh Framework Programme under the grant agreement n°602699 (DIREKT), and faculty grants from the Linnæus University, Sweden.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, discussed the content, wrote the text and reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Leukocytes
-
White blood cells that participate in immune defence.
- Anaphylatoxins
-
C3a and C5a generated during complement activation. They promote inflammation by binding to receptors on phagocytes and mast cells.
- Atherosclerotic events
-
Clinical events that occur as a result of atheromatous plaque.
- Thromboembolic events
-
Clinical events caused by thrombi or emboli.
- Autonomic neuropathy
-
Damage to nerves that control involuntary bodily functions.
- Kallikrein/kinin system
-
A plasma protein system that generates the potent proinflammatory and angiogenic mediator bradykinin. The contact system is the initiation point for both the kallikrein/kinin system and the intrinsic branch of the coagulation system.
- Polymorphonuclear leukocytes
-
Phagocytic leukocytes; the major cell type that mediates acute inflammation
- Monocytes
-
Leukocytes that are recruited to inflammatory sites and differentiate into macrophages.
Rights and permissions
About this article
Cite this article
Ekdahl, K., Soveri, I., Hilborn, J. et al. Cardiovascular disease in haemodialysis: role of the intravascular innate immune system. Nat Rev Nephrol 13, 285–296 (2017). https://doi.org/10.1038/nrneph.2017.17
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2017.17
This article is cited by
-
A guide to complement biology, pathology and therapeutic opportunity
Nature Reviews Immunology (2024)
-
Self-anticoagulant sponge for whole blood auto-transfusion and its mechanism of coagulation factor inactivation
Nature Communications (2023)
-
Plasma C4 level was associated with mortality, cardiovascular and cerebrovascular complications in hemodialysis patients
BMC Nephrology (2022)
-
Blood compatibility of widely used central venous catheters; an experimental study
Scientific Reports (2022)
-
Does delivering more dialysis improve clinical outcomes? What randomized controlled trials have shown
Journal of Nephrology (2022)