Advertisement
-
-
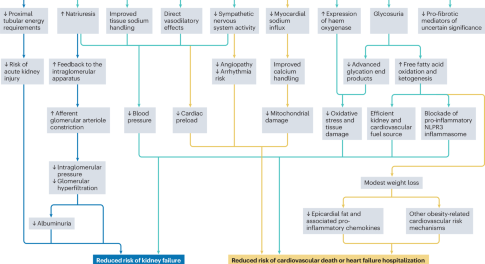
Applications of SGLT2 inhibitors beyond glycaemic control
Here, the authors discuss the beneficial effects of sodium–glucose cotransporter 2 (SGLT2) inhibitors for a range of clinical outcomes beyond glucose lowering, including kidney and cardiovascular protection. They also discuss the need for implementation and adherence initiatives to help translate the benefits of these agents into real-world clinical outcomes.
-
-
Trending - Altmetric
-
Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup
-
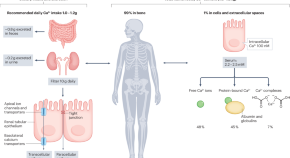
Gut microbiome studies in CKD: opportunities, pitfalls and therapeutic potential
-
Applications of SGLT2 inhibitors beyond glycaemic control
-
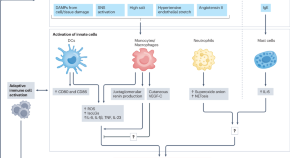
Immune mechanisms in the pathophysiology of hypertension