Abstract
Early detection is a key strategy to prevent kidney disease, its progression and related complications, but numerous studies show that awareness of kidney disease at the population level is low. Therefore, increasing knowledge and implementing sustainable solutions for early detection of kidney disease are public health priorities. Economic and epidemiological data underscore why kidney disease should be placed on the global public health agenda — kidney disease prevalence is increasing globally and it is now the seventh leading risk factor for mortality worldwide. Moreover, demographic trends, the obesity epidemic and the sequelae of climate change are all likely to increase kidney disease prevalence further, with serious implications for survival, quality of life and health care spending worldwide. Importantly, the burden of kidney disease is highest among historically disadvantaged populations that often have limited access to optimal kidney disease therapies, which greatly contributes to current socioeconomic disparities in health outcomes. This joint statement from the International Society of Nephrology, European Renal Association and American Society of Nephrology, supported by three other regional nephrology societies, advocates for the inclusion of kidney disease in the current WHO statement on major non-communicable disease drivers of premature mortality.
Similar content being viewed by others
Introduction
In 2015, United Nations member states agreed on the ambitious Sustainable Development Goals (SDGs), with the aim to end poverty and inequality, protect the planet and ensure that all people enjoy health, justice and prosperity. An important health-related target is the reduction of non-communicable disease (NCD)-related mortality by one-third by 2030 (ref. 1). The WHO) has listed heart disease, stroke, cancer, diabetes and chronic lung disease as the five major NCDs driving premature death and disability2. Notably absent from this list is kidney disease, either acute kidney injury (AKI) or chronic kidney disease (CKD); of note, AKI increases the risk of CKD and vice versa3,4,5,6. Additionally, kidney disease commonly occurs with, and enhances the risk of, major NCDs such as ischaemic heart disease, stroke and peripheral vascular disease, diabetes and cancer7,8.
Approximately 850 million people worldwide are estimated to have kidney disease, most of whom live in low-income and lower-middle-income countries (LICs and LMICs), and a large proportion of these individuals lack access to kidney disease diagnosis, prevention or treatment9. As many as 9 out of 10 individuals with CKD in resource-poor settings with weak primary care infrastructure are unaware that they have this condition and therefore do not seek treatment10,11,12. Ageing populations and population growth will translate to large increases in the prevalence of CKD in LICs and LMICs in the coming decades. In contrast to cardiovascular disease, stroke and respiratory disease, CKD mortality has been rising. Currently, kidney disease is the third fastest-growing cause of death globally and the only NCD to exhibit a continued rise in age-adjusted mortality13. By 2040, CKD is projected to be the 5th highest cause of years of life lost (YLL) globally14.
Population growth, ageing and the increasing burden of diabetes, heart disease and hypertension are the best-recognized drivers of CKD incidence, especially in regions with advanced economies. As many as 1 in 3 people with diabetes and 1 in 5 with hypertension in high-income countries (HICs) have CKD, which has led to the suggestion that focusing on the control of diabetes and cardiovascular disease will alleviate the growing burden of CKD15. This assumption is based on the premise that screening for CKD is part of the standard of care for these conditions and that no special interventions are required in those with kidney diseases. However, CKD and AKI have diverse causes, mediators and risk factors beyond diabetes and cardiovascular disease (Fig. 1), especially in LICs and LMICs, which account for two-thirds of the global burden of kidney disease16. For example, dehydration and infections are leading causes of AKI in LICs and LMICs17. Finally, the latest research shows that CKD and AKI require unique treatments and are not merely risk enhancers when they accompany other major NCDs.
Although chronic kidney disease (CKD) shares several risk factors with the non-communicable diseases (NCDs) recognized by the WHO as major drivers of premature mortality — heart disease, stroke, cancer, diabetes and chronic lung disease — several other major causes of CKD, including acute kidney injury (AKI), contribute to the relentless rise in the global burden of kidney disease. Consequently, addressing only the WHO-recognized NCDs will be insufficient to reduce the growing negative impact of CKD .
In this Consensus Statement, we discuss the unique environmental, social and medical drivers of kidney diseases, highlighting how tackling diabetes and heart disease alone will not target the core drivers of a large proportion of kidney diseases. We also discuss how such an approach worsens global inequities in access to the best attainable standards of health and hinders progress towards targets identified in the SDGs, making the absence of kidney disease from the global NCD health agenda morally indefensible and a substantial challenge to tackling the growing kidney disease burden.
Methods
The International Society of Nephrology (ISN), European Renal Association and the American Society of Nephrology convened a diverse core group of 19 experts representing HICs and LICs in the Americas, Africa, Europe and Asia–Pacific, across adult and paediatric nephrology. Over several meetings, the authorship team discussed current and future challenges, as well as strategies for increasing global awareness of CKD and decreasing its global impact, to develop this Consensus Statement and recommendations. The manuscript was reviewed by various regional representative bodies (ISN Regional Boards), and consensus was attained. The ISN has established a regional board in each of its 10 regions — Africa, Eastern and Central Europe, Latin America and the Caribbean, Middle East, New Independent States and Russia, North America and the Caribbean, North and East Asia, Oceania and Southeast Asia, South Asia and Western Europe. The Regional Boards have representation from all affiliated societies within the region and are a major link between the ISN and National Societies of Nephrology.
Endorsement was also provided by other major global and regional societies, namely the Asian Pacific Society of Nephrology, African Association of Nephrology, Latin American Society of Nephrology and Hypertension and the World Heart Federation.
Kidney disease is a growing global problem
Kidney disease is an increasing global problem that disproportionately affects poor, vulnerable and marginalized populations, and is associated with high individual, health care and societal costs. Approximately 700 million people are estimated to have CKD worldwide. To this must be added the global burden of AKI and kidney failure (including those receiving dialysis and kidney transplant recipients), which increases the global prevalence of kidney disease to ~850 million7,9, translating to a global prevalence >10%. Of note, this prevalence is probably an underestimate owing to the lack of early kidney disease detection and screening programmes in many parts of the world, which results in large-scale unawareness of the burden and prevalence of earlier stages of CKD11.
AKI affects 7–18% of hospitalized patients and 20–200 per million individuals annually in the community18. AKI is most common in LICs and LMICs, where 75% of cases are community acquired owing to infections, toxins (for example, from animal bites, herbs and medications) and pregnancy complications19,20. According to a systematic review, an estimated 13.3 million cases of AKI are recorded worldwide every year, with LICs and LMICs contributing 11.3 million21. Of the 1.7 million deaths per year from AKI globally, an estimated 1.4 million occur in LICs and LMICs18.
Furthermore, the burden of kidney disease is rising worldwide. According to the Global Burden of Disease (GBD) study7, the global prevalence of CKD increased by 33% between 1990 and 2017. Crucially, the greatest growth in the burden of CKD (prevalence and mortality) is concentrated outside of HICs, with almost one-third of all patients with CKD living in India and China alone7. Beyond population dynamics, numerous other social, environmental and economic threats increase the global risk of kidney disease (Fig. 1).
Given its increasing prevalence, if CKD remains largely undetected and is consequently not treated, the numbers of people developing kidney failure and requiring expensive kidney replacement therapy (KRT) will naturally increase. In 2010, ~2.6 million people received KRT, and this number is estimated to increase to 5.4 million by 2030 (ref. 22). Even in HICs, 15–20% of patients die within 12 months of starting dialysis23. Millions more develop kidney failure and require KRT but lack access to therapy and die prematurely24,25. Almost all of these people live in LICs and LMICs, which have only 7% of the global KRT population despite comprising 48% of the world population24. AKI and progressive CKD are also associated with high mortality even before the development of kidney failure, primarily owing to an increased risk of other major co-morbidities7,18. A greater number of people die of cardiovascular disease directly attributable to reduced kidney function than of kidney failure-related deaths26, with the GBD study attributing ~3.1 million deaths in 2019 to kidney dysfunction.
Population dynamics will increase the burden of kidney disease
Population dynamics are increasing the numbers of people at a high risk of kidney disease but with limited access to kidney care. This effect is driven both by population growth and an ageing population. Population growth is booming in LICs and LMICs (especially in Africa and India) — Central and Southern Asia, Eastern and South-Eastern Asia, and sub-Saharan Africa are expected to hold 70% of the world’s population by 2030 (refs. 27,28). The age-standardized rate of CKD has already increased by 3–5% between 1990 and 2017 in countries in the lowest three sociodemographic index quintiles7. The highest growth in the number of people requiring KRT is projected for Africa, where 23 of the 28 poorest countries in the world are located22. Policies aimed at decreasing the burden of other NCDs that do not target CKD in these countries will generate an additional CKD burden. The reasons for the increased risk of CKD in LICs and LMICs are explored further below.
An ageing population faces an inherently increased risk of kidney disease. Current predictions estimate that by 2035, as many as 1.1 billion people will be over 65 years of age, an increase of 60% from 2020, with the largest number of older individuals expected to be in China and India27. The controversies with regard to age-adjusted definition of CKD notwithstanding, the rising prevalence of CKD translates to an increased risk of adverse outcomes in individuals in all age groups29,30,31. The loss of kidney reserve with ageing also exacerbates the bidirectional interplay between AKI and progressive CKD risk32. Moreover, CKD is associated with inflammation and accelerated whole-body ageing, particularly of the cardiovascular system, thereby increasing the burden of ageing-associated health decline even in younger patients22,33.
Environmental and social threats to kidney health continue to rise
The burden of CKD risk factors that traditionally drive disease in HICs, such as diabetes, hypertension and obesity, is growing most rapidly in LICs and LMICs. Moreover, LICs and LMICs face a constellation of additional risks that translate to a greater kidney disease burden than that seen in HICs16. Risks related to environmental change, including global warming, environmental toxins, air pollution and declining biodiversity, are global. However, the lack of capacity and resources for mitigation and adaptation makes LIC and LMIC populations particularly vulnerable to such risks. These populations also continue to have high rates of infectious diseases, many of which affect the kidneys19,34,35. The lack of resources in these countries means that the contribution of local CKD risk factors is not as well-studied as those related to diabetes or cardiovascular disease that were first recognized in HICs, which leads to their continued under-recognition. Superimposed onto this background, limited public health architecture and high poverty levels create life-course threats to kidney health, with a particular impact on pregnancy and childhood.
Fragile and underfunded health systems struggle to cope with the burden of kidney disease, leading to high and increasing mortality. Regional variations in the distribution of risk factors and the ability to implement adaptation measures mean that, in some places, CKD is an even bigger threat than that observed at a global level. For example, in Central America, CKD is the second most common cause of death7. Even in HICs, social determinants of health and factors such as gender, race or ethnicity influence the risk of kidney disease and underscore many health outcome disparities36,37,38,39. Race, for example, is increasingly recognized as a social construct, highlighting that its impact on kidney health is potentially modifiable40.
Environmental factors and climate instability contribute to the increased risk of kidney disease globally41. The GBD team estimated that in 2019, as many as 8% of deaths due to CKD were attributable to non-optimal (high or low) ambient temperatures42. For example, persistent exposure to high temperatures, particularly for agricultural and outdoor workers in LICs and LMICs who lack access to adaptation interventions, increase heat stress, which exacerbates the risk of kidney disease43,44,45,46. Heat stress is a potential contributor to CKD of unknown cause (CKDu) in agricultural communities, which is increasingly recognized as a major global cause of CKD. This condition is characterized by the presence of a benign urinary sediment and tubulointerstitial changes on biopsy47. Proposed mechanisms include recurrent heat stress with repeated episodes of AKI and exposure to environmental toxins, including pesticides and heavy metals48,49,50,51. Also known as CKD of non-traditional cause, chronic interstitial nephritis in agricultural communities, Mesoamerican nephropathy and Uddanam Nephropathy, CKDu has been observed largely in the Global South, including India, Sri Lanka, parts of Africa, and Central and South America49,51,52. One study of people with CKDu in India found worsening of metabolic acidosis and hypertension in summer compared with winter, supporting the hypothesis that a warming planet also threatens kidney health50. Increasing salinity of drinking water in coastal areas of Bangladesh affected by rising sea levels has also been linked to increased rates of preeclampsia and gestational hypertension, in addition to hypertension and albuminuria in the general population, all of which are risk factors for subsequent kidney disease53,54,55. Furthermore, large population studies suggest that rises in fine particulate matter in the air are associated with an increased risk of AKI, as well as CKD prevalence and progression56,57,58.
Climate change will also affect water availability. Since 2000, the global frequency and duration of drought have increased by nearly 30%59. In the context of profound drought, current haemodialysis options are not environmentally sustainable, as the average haemodialysis treatment uses >500 l of water60. Emergencies such as extreme climate events (drought, snowstorms, floods and fires), natural disasters such as the earthquakes in Turkey and Syria, and floods in Pakistan, as well as man-made disasters such as wars and conflicts, all impact the ability of patients with kidney disease to access and receive life-saving treatment such as haemodialysis or kidney transplantation, thereby endangering lives61,62,63. Notably, extreme events can also directly cause kidney injury, such as mass rhabdomyolysis from crush injuries caused by earthquakes64.
Threats to kidney health vary across the lifespan. In low-resource and underprivileged settings, maternal factors such as malnutrition, poor health literacy and comorbidity burden contribute to an adverse uterine environment65. Mothers in low-resource settings are more likely to give birth to children who are small for gestational age, have low birthweight or are born prematurely than those in high-resource settings66. Importantly, numerous large population studies show that small for gestational age, low birthweight and prematurity increase the risk that the infant might develop proteinuria, hypertension, CKD and kidney failure in later life67,68,69,70,71,72,73. This risk seems to be mediated through multiple mechanisms, with low nephron endowment owing to suboptimal growth in utero increasing susceptibility to kidney injury later in life74. Poor maternal nutrition, which, despite improvements, remains substantially prevalent in large parts of the world, also increases the risk of gestational diabetes and hypertension, both of which are known risk factors for CKD65. Poor infant and childhood nutrition, as well as childhood AKI events related to infections, superimpose additional layers of risk to kidney health across the lifespan65.
Although kidney diseases are commonly grouped with other NCDs, infections are also important causes of AKI in LICs and LMICs, either through direct kidney involvement (for example, in cases of leptospirosis or HIV infection) or indirectly through haemodynamic mechanisms, systemic inflammatory responses or infection-related glomerulonephritis19,35,75. The adverse kidney effects of infections in LICs and LMICs are exacerbated by decreased access to specialized care, especially in areas endemic for diseases such as malaria, leptospirosis, scrub typhus, haemorrhagic fevers or dengue, and in cases of severe gastrointestinal fluid loss76,77. AKI is associated with increased morbidity and mortality and affects all age groups, from neonates to older individuals. Continued high mortality due to childhood AKI in the absence of dialysis prompted the ISN to introduce the Saving Young Lives programme in collaboration with the International Society of Peritoneal Dialysis, European Peritoneal Dialysis, and the International Paediatric Nephrology Association to promote peritoneal dialysis in Africa, later expanded to Asia and Latin America78. However, this programme is just scratching the surface with regard to addressing the KRT needs of people with AKI in these regions.
Climate change and loss of global biodiversity are also increasing the risk of infectious diseases that predispose to AKI and CKD outside of current tropical areas as the climate becomes more conducive to the survival of parasites (for example, those causing malaria or schistosomiasis) and/or their vectors (for example, mosquitoes or ticks)41. Of note, although the association between certain infections and AKI is well known, the role of infections in the development and/or progression of CKD is not well studied. However, emerging data suggest that leptospirosis might contribute to the development of CKDu or increase susceptibility to triggers such as heat stress79,80,81.
Globally, multifactorial social determinants of health influence kidney health profoundly. Indigenous populations, people living in rural areas, migrants, older individuals or those affected by poverty, homelessness and food insecurity are more likely to be affected by kidney disease and develop its worst manifestations36,37,38,82,83. For example, underprivileged people with CKD are more likely to experience rapid progression of the disease84. Importantly, progressive CKD can also exacerbate poverty (see later discussion). Sex and gender further influence the causes of CKD, the profile of comorbidities and disease evolution over time39. These differences are probably driven by complex biological, social and system-level factors. For example, women are 29% more likely to have CKD than men, but men are more likely to die from CKD than women7,85. Notably, despite the increased competing risk of death, men are 47% more likely than women to access dialysis or have a kidney transplant7. In older general population cohorts, women had a lower baseline glomerular filtration rate (GFR), although men had a steeper rate of GFR decline over time86,87. This complex interplay of age, and sex or gender needs to be better understood to allow the development of appropriate health system-level responses.
Lack of health system response to kidney disease and global health
Despite being the third fastest-growing cause of death13, kidney disease has not received the attention it deserves from governments, multilateral organizations such as the WHO, the lay press or health systems. Failure to diagnose CKD is driven by the silent nature of the disease, as well as lack of awareness of the devastating consequences of opportunities missed owing to a lack of timely detection (including through coordinated screening programmes), referral to nephrologists and adequate management. Even when treatment is sought, the quality of care might be poor. Information asymmetry is evident in the lay press for kidney disease. In an analysis of US newspapers, kidney disease was 11-fold under-represented in the media as a discussed versus actual cause of death88. Diagnosis is further hampered by the limited availability or lack of tests needed to assess kidney function in many LICs or LMICs89.
Globally, health systems and governments have failed to create robust systems for generating data on the burden of kidney disease and its drivers. Data registries are vital for understanding disease epidemiology, tracking progress and developing cost-effective intervention targets. Kidney disease registries are sparse in LICs and LMICs, which is where they are most needed90. A 2022 review of dialysis registries found none in large Asian countries91. The African Association of Nephrology registry has been established in Africa, but so far only involves seven countries (Botswana, Burundi, Ghana, Kenya, Nigeria, South Africa and Zambia)92. Latin America is served by the Latin America Dialysis and Transplant Registry93. Of note, CKD registries are rare in both HICs and LICs.
Data from the 2023 Global Kidney Health Atlas revealed that the availability of national strategies to address CKD correlated positively with income level — LIC 11%, LMIC 23%, upper-middle-income countries 22% and HIC 33%94. Despite the immense health care costs, only 48% of national governments recognize CKD and/or its treatment and prevention as a health priority94. In 2016, 50% of countries had no national health system oversight of kidney care95. Even in HICs, coordinated multi-agency approaches are lacking96. For example, the 2022–2027 European Commission “Healthier Together — European Union Non-Communicable Diseases Initiative” does not address CKD97,98.
Targeting current WHO major NCDs will not solve the growing global kidney disease problem
CKD is often caused by pre-existing major NCDs that have been acknowledged by the WHO as priority conditions, such as diabetes, or by risk factors common to heart disease and stroke, such as hypertension. The WHO further reports that one-third of kidney mortality is caused by diabetic kidney disease99. However, glomerulonephritis, infection, malnutrition, environmental stressors and other toxins, pollution, climate change and obstetric catastrophes are all major causes of AKI and CKD that are not addressed in the current list of major NCDs. CKD of causes other than diabetes and hypertension already accounts for the highest global age-standardized rate of disability-adjusted life years (DALYs)7. Crucially, a common cause of kidney failure, even in HICs, is ‘kidney failure where the cause cannot be ascertained’. In a recent report of the European Renal Association, the cause of kidney failure was unknown in 28% of participants100. This finding emphasizes the need for research that can advance understanding of underlying causes of kidney disease and enable the development of cause-specific therapies. Failing to address kidney disease risk factors and mediators will fail to curb its devastating health, economic and psychosocial consequences.
Kidney diseases have multiple adverse consequences
Kidney disease causes premature mortality, disability, reduced quality of life and other psychosocial harms, and incurs high costs to governments, health care systems, and patients and their families (Box 1). The burden of this harm disproportionately affects those living in LICs and LMICs. Progressive CKD is a systemic disease and contributes to the evolution and progression of other major NCDs, most notably cardiovascular disease101.
Kidney disease carries high morbidity and mortality
The overall global age-standardized DALY rate declined sharply from 1990 to 2019 (ref. 102). In particular, the age-standardized DALY rate for WHO-recognized major NCDs such as ischaemic heart disease and stroke decreased by 28% and 35%, respectively102. By contrast, the age-standardized DALY rate for CKD increased by 6% (with an absolute increase of 62%) over the last 30 years, causing CKD to rise from the 29th to the 18th leading cause of global disability102,103. In 2017 alone, CKD resulted in 36 million DALYs7. Nearly 75% of DALYs occurred in countries within the lowest three sociodemographic index quintiles7. Current population demographic trajectories and the increase in kidney disease risk factors mean that, without urgent action, CKD will continue to rise through the league table of global causes of death and disability.
The symptom burden of CKD is profound, and patients with kidney failure experience a similar or greater symptom burden (including fatigue, itch and pain) than those with terminal malignancies104. Kidney disease has multiple adverse psychosocial consequences, including reduced quality of life, poor life participation and mental illness. A 2022 meta-analysis of nearly 200,000 patients demonstrated that CKD reduced quality of life, especially for those on dialysis, for whom the pooled 36-item Short Form Health Survey (SF-36) physical component summary score was 36 out of 100 (ref. 105). Even among patients who were not on KRT (that is, dialysis or transplantation), many reported a high symptom burden. At least 45% reported fatigue, poor mobility, bone and/or joint pain, drowsiness, insomnia and/or poor sleep, anxiety, pain, sexual dysfunction, muscle cramps, gastrointestinal distress, dyspnoea, itching, heartburn or oedema105. Children with CKD also have worse quality of life reported than those with type 1 diabetes or survivors of childhood cancer106. In older individuals, who typically have lower access to KRT than the younger adults, quality of life decreases and symptom burden increases for years before starting KRT and, in those starting KRT, symptom burden might stabilize but does not improve107,108,109. Of note, in advanced CKD, quality of life is worse in women than in men107. Moreover, having a person with CKD in the family adversely impacts the mental health of caregivers, 30–50% of whom report symptoms of anxiety or depression110,111,112,113.
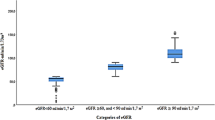
The burden from kidney disease naturally extends to mortality. The global mortality from all kidney diseases likely ranges between 5 million and 11 million per year114 and kidney dysfunction is currently the seventh leading risk factor for death99. AKI contributes to an estimated additional 1.7 million deaths per year21. Deaths due to kidney disease increased by 50% from 2000 to 2019, and even mild CKD increases the risk of morbidity and mortality115,116. In 2019, CKD in LICs led to ~600 YLL per age-standardized 100,000 population and around ~560 YLL per 100,000 in LMICs, compared with 200 YLL per 100,000 in HICs102. Increasing prevalence and the relatively young age at death mean that overall deaths and YLL due to kidney disease are predicted to escalate dramatically at a global level (Figs. 2 and 3). In 2040, kidney disease is predicted to cause 52 million YLL, moving from the 16th most common cause of YLL (in 2016) to the fifth, surpassing other major NCD drivers of early mortality listed by the WHO such as diabetes14 (Fig. 3). In 2040, CKD is expected to account for 5% of YLL14,18 (Fig. 4).
Modelling of Global Burden of Disease data reveals the global trend for an increase in predicted deaths due to chronic kidney disease (CKD) between 1990 and 2040. Data shown refers to CKD as cause of death for all ages and both sexes (deaths per 100,000). Data obtained from the Global Burden of Disease Foresight Visualization tool from the Institute for Health Metrics and Evaluation, University of Washington. Reprinted with permission from the Institute for Health Metrics and Evaluation.
Modelling of Global Burden of Disease data reveals the global trend for an increase in predicted years of life (YLL) lost because of chronic kidney disease (CKD) between 1990 and 2040. Data shown refer to chronic kidney disease (CKD) as cause of YLL for all ages and both sexes (YLL per 100,000). Data obtained from the Global Burden of Disease Foresight Visualization tool from the Institute for Health Metrics and Evaluation, University of Washington. Reprinted with permission from the Institute for Health Metrics and Evaluation.
Compared with data from 2016, the years of life lost (YLL) because of WHO-recognized major non-communicable diseases such as stroke and heart disease are predicted to have decreased by 2040. By contrast, YLL due to chronic kidney disease (CKD) are predicted to continue to increase and CKD is expected to surpass diabetes as a cause of YLL by 2040. Graph created using data from ref. 14.
CKD also increases the risk of developing severe acute and chronic infections (such as COVID-19 and tuberculosis), which are major causes of death in LICs and LMICs117,118. Hence, decreasing the incidence and severity of CKD will have beneficial effects on other NCDs and communicable diseases.
Kidney disease increases the risk of other major NCDs
Beyond the directly attributable toll of kidney disease, CKD contributes to and exacerbates other major NCDs. Uraemic toxins and systemic inflammation have profound effects on other organ systems, and extensive kidney–heart, kidney–brain, and kidney–lung interactions underscore the high co-morbidity burden of CKD119,120,121. In 2017, the GBD study estimated that 1.4 million cardiovascular disease-related deaths and 25 million cardiovascular DALYs were lost owing to kidney disease7. Accordingly, the 2021 European Society of Cardiology cardiovascular disease prevention guidelines suggest screening people with CKD for atherosclerotic cardiovascular disease122. These guidelines also advocate for albuminuria screening in those with high cholesterol or diabetes, acknowledging the importance of CKD as a risk factor for cardiovascular disease123. Recognition of the role of CKD in increasing the risk of other major NCDs is essential to reducing overall NCD burden.
Kidney disease imposes unacceptably high costs on economies, health care systems and individuals
The direct health care costs of kidney disease are relevant at the global, country, health system and individual levels. Patients with CKD are complex to manage and account for a disproportionately large amount of national economic expenditure.
Most countries use a mix of public and private funding to provide kidney care. In 2016, only 19% of countries had completely publicly funded kidney care95. In the USA, kidney failure qualifies adults for Medicare benefits regardless of age. Of note, although <1% of Medicare beneficiaries have kidney failure, expenditures for kidney failure accounted for over 6% of Medicare spending in 2020, exceeding $50 billion23. US federal costs for people with CKD are >$85.4 billion annually, representing 23.5% of Medicare fee-for-service spending23. Similarly, in the UK, half of the National Health Service budget spent on CKD care went to those with kidney failure, who comprise only 2% of patients with CKD stages 3–5 (ref. 124). In a range of other HICs and MICs, 2–4% of the health care budget is spent on the 0.1–0.2% of the population with kidney failure125,126. Moreover, the costs of CKD care are rising given the increased prevalence of CKD and the complexity of patients with CKD. Inflation-adjusted spending in the USA on people with kidney failure in 2021 had increased 20% over the preceding decade23. Notably, US expenditure on people with CKD rose faster than that for the general population or even patients with diabetes or heart failure23. A large Canadian general population study also found that patients with kidney disease had the highest comorbidity burden, number of medications, death rate and need for placement in long-term care facilities127. In Europe, aggregated annual health care costs of CKD are estimated to be higher than those of cancer or diabetes mellitus128. The average length of hospital stay in the UK is 35% longer for people with CKD than for people without CKD124. Higher health care costs are also incurred from the increased rates of diseases such as cardiovascular disease in people with CKD. In the UK in 2010, a cost of £174 million was incurred from excess stroke and myocardial infarction in people with CKD124.
Health systems in LICs and LMICs will need increased funding to manage the rising burden of kidney disease. The prohibitive cost of dialysis likely explains the nearly 30-fold difference in the reported rates of kidney failure treated with dialysis between the country with the highest rate (Taiwan) and the country with the lowest rate (Bangladesh)125. In 2016, 40% of LIC and 22% of LMIC reported poor to extremely poor health care infrastructure for CKD care95. In Africa, where AKI is more common than in the rest of the world, 50% of countries reported poor to extremely poor AKI care infrastructure95. The negative impact of this lack of infrastructure is exacerbated by poor population coverage by nephrologists (0.2 nephrologists per million population in LICs, compared with 23 per million in HICs)129. For children, the situation is even more dire. In the 2018 Global Health Kidney Atlas, nearly 40% of LICs and LMICs reported absent or extremely limited access to a paediatric nephrologist130.
At the individual level, the costs of kidney care can be staggering. In LICs and LMICs the burden of meeting health care costs is largely placed on the individual. Only 13% of LICs and 19% of LMICs cover the cost of KRT for adults95. A World Bank report highlighted that out of all the disease groups, CKD is responsible for the largest number of people (188 million annually in LICs and LMICS) suffering catastrophic health care expenditures worldwide131. Even in the USA, where Medicare covers people with kidney failure, younger adults with CKD are not covered unless they have private health insurance, leading to massive out-of-pocket costs.
Kidney disease also has many indirect economic costs. The individual patient faces decreased earning potential, and educational and vocational outcomes are compromised in children with kidney disease132,133. In the USA, >75% of patients initiating dialysis were unemployed134. Caregivers face similar lost earning opportunities, and the state receives less taxation revenue.
With increasing prevalence, the overall global cost of providing kidney care is likely to rise. Moreover, with an ageing global population, the global tax base to fund health care will shrink over the next 30 years, emphasizing the crucial need to prevent kidney failure and its associated high health care costs (such as KRT costs)135. The recognition of kidney disease as a major global driver of mortality is, therefore, essential to focus efforts on improving kidney health and decreasing the massive health care costs associated with kidney disease.
The moral case for kidney health prioritization
It is unacceptable that the only NCD consistently witnessing an increase in the number of deaths year-on-year is not identified as a priority for policy action. Kidney diseases disproportionately affect the poor and disadvantaged, globally and within each country. Moreover, kidney disease not only has a profound negative impact on patients owing to its debilitating symptom burden but it also increases their risk of developing other major NCDs, restricts their ability to work and care for family members, and is cripplingly expensive for individuals, families, health systems and governments.
The changing population dynamics predicted over the next 20 years will translate to an increase in the number of people with kidney disease in LICs and LMICs, who are the least able to access kidney care. Arguments have been made that prioritizing kidney disease is not necessary in health systems without the resources to pay for the care of people with kidney disease. This approach will perpetuate and exacerbate the current global inequities in the care of patients with kidney disease, represents a pressing moral quandary to the world and is contrary to the Sustainable Development Agenda of leaving no one behind. Acceptance of such a situation by using the framing of cost-effectiveness as the primary metric further deprioritizes these patients, leading to the outright denial of care. The status quo perpetuates this injustice.
When kidney health care costs cannot be met, people die
Most people access care in community or secondary health care settings in LICs and LMICs. However, less than a third of LIC or LMIC community health care settings can access essential diagnostics such as those measuring kidney function (for example, estimated GFR and/or albuminuria testing)136. Medicines that can decrease albuminuria, or treat glomerulonephritis and the complications of CKD (such as anaemia or CKD–mineral and bone disorder) are crucial to slow disease progression and limit the burden of its complications (for example, cardiovascular disease)137,138. However, in a recent survey of the nephrology workforce in LICs and LMICs, only a third of respondents reported that essential kidney medicines (such as angiotensin-converting enzyme (ACE) inhibitors, anti-hypertensives, medications to treat acid–base and electrolyte disturbances or for CKD–mineral and bone disorder) were mostly available in community settings139. This finding highlights the avoidable progression to kidney failure and death faced by many patients in low-resource settings. Newer, paradigm-shifting medications, such as sodium–glucose cotransporter 2 (SGLT2) inhibitors and mineralocorticoid receptor antagonists are even less accessible.
Globally, <50% of all people requiring KRT can access it, with vast discrepancies in access between HICs and LICs22,24,140,141. Up to 98% of people with kidney failure in LICs do not receive KRT, compared with up to 30% in HICs. Of those patients unable to access KRT, 88% reside in Africa or Asia. The great disparity in wealth and availability of nephrologists greatly impact KRT funding and outcomes142. Approximately 93% of the world population receiving KRT lives in HICs or upper-middle-income countries, who comprise only 52% of the world population. Women, children, socially marginalized groups, migrants, and refugees are particularly disadvantaged140,143. Even those who access KRT often cease treatment quickly owing to cost constraints. In sub-Saharan Africa, only ~10% of adults and 35% of children who managed to access KRT were still on therapy by 3 months144. In a report from India that evaluated a state-funded dialysis programme, the number of patients accessing the service increased over time, but about two-thirds of patients discontinued dialysis in less than 1 year and likely died because they could not afford the substantial out-of-pocket payments needed to meet the indirect costs of care126.
Access to dialysis for AKI is similarly poor in LICs. Up to 85% of people with AKI live in the Global South21. In the ISN 0by25 global snapshot of AKI, nearly 50% of people who required dialysis in LICs and LMICs were unable to receive it owing to resource constraints or inability to pay17. A systematic review of AKI outcomes in sub-Saharan Africa found that only 64% of children and 33% of adults could access dialysis when needed19. In those unable to access dialysis, mortality was ~80%19.
Expected impact of placing kidney disease in the WHO list of NCD drivers of early death
The ISN, American Society of Nephrology and European Renal Association and nephrology communities worldwide unite in calling for kidney health to become a core part of the global health agenda. A crucial first step is the official recognition by the WHO that kidney disease is a major NCD driver of early mortality. The significance of prioritizing CKD by an important multilateral organization such as the WHO in strengthening the fight against CKD cannot be overstated. Specifically, prioritization by the WHO will help to raise awareness and demand for care, develop and implement guidelines and standards, improve implementation of locally appropriate surveillance and monitoring mechanisms, coordinate international efforts, and allocate resources more efficiently. In addition to enhanced efforts to prevent the development and progression of kidney disease, prioritization will foster investment towards the development of sorely needed new therapies (Box 2).
First, placing kidney disease on the WHO list of major NCD causes of premature mortality will enable a cohesive and targeted global campaign to decrease the harm caused by kidney disease, especially in emerging economies. Failure to spotlight kidney disease will undo or substantially slow progress towards the 2015 United Nations Sustainable Development Goal 3.4 of reducing premature mortality from NCDs by a third by 2030. Combating kidney disease will also contribute to action on many other SDGs, including SDG 1 (no poverty), 2 (gender equity), 6 (water security), 8 (work and economic growth), 10 (inequalities), and 13 (climate action). Adding kidney disease to the WHO major NCD list will translate to better health outcomes across the world and enhance the ability to address pervasive inequities that place disadvantaged populations at an increased risk of kidney disease.
Previous successful collaborations between the Latin American Society of Nephrology and Hypertension and the Pan American Health Organization, which is a specialized agency of the United Nations, exemplify the progress that can be made by multilateral organizational collaboration. This joint initiative has been pivotal in implementing and developing dialysis and transplantation registries, increasing knowledge of CKD and AKI among primary care health personnel, establishing a clinical and epidemiological definition of CKD of non-traditional causes, prioritizing individuals with kidney disease for COVID-19 vaccination, and establishing a direct line of action with local ministries of health145. The ongoing evolution of digital health technologies that can be leveraged to improve the detection and monitoring of kidney disease will accelerate these programmes and improve their implementation. Recognition of kidney disease as a major NCD driver of mortality is crucial to translating these gains to a global stage.
Early disease detection and a life course approach are cornerstones for reducing CKD-related morbidity and mortality worldwide. CKD meets the WHO principles for screening as the disease is asymptomatic in its early stages and effective early interventions are available98,146. In 2021, the Kidney Disease Improving Global Outcomes global multidisciplinary expert panel recommended screening high-risk groups (for example, individuals with diabetes or hypertension) for CKD98.
One of the main historical arguments against CKD screening has been the lack of effective therapies to slow disease progression, but this landscape has changed dramatically in the past 5 years. The advent of new therapeutic agents such as SGLT2 inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, endothelin receptor antagonists, selective mineralocorticoid receptor antagonists, and new glomerulonephritis-targeted therapies, means that early recognition of disease can translate into massive health improvements. For example, a 2021 meta-analysis revealed that SGLT2 inhibitor use decreased the risk of CKD progression by 37% in people with and without diabetes128. Consensus-based expert opinion recommends case detection in individuals with known risk factors such as diabetes, hypertension and cardiovascular disease98. As discussed above, the list of relevant risk factors is likely longer and poorly studied in LIC and LMICs16,147. Prioritization by the WHO will spur studies to more accurately document disease burden and risk factors in these geographic areas. Failure to identify people at a high risk of kidney disease development and/or progression is a missed opportunity to intervene and prevent kidney failure and its stratospheric health, economic and psychosocial costs138.
Prioritizing kidney disease will also facilitate the development and expansion of kidney disease registries. Accurate registry data are crucial to understanding trends and risk factors and to informing cost-effective and equitable resource delivery. The ISN, through its Share-RR program, is providing support for the setting up of registries, but such initiatives are unlikely to be sustainable without embedding in local health systems148. Registries are already highlighting global hotspots of kidney failure, enabling investigation of disease causes and improving the understanding of new disease entities such as CKDu93,149.
Greater attention to kidney health and disease will also translate into increased investment by governments, the private sector and research funders, which will spur innovation and new therapies. Despite spending disproportionate amounts of money on funding KRT, governments worldwide have not prioritized innovation in these areas. Professional societies have been trying to fill this gap, with exciting results. The Affordable Dialysis Prize co-funded by the ISN, the Asian Pacific Society of Nephrology, the Farrell Family Foundation and the George Institute for Global Health and has led to the development of a prototype of a low-cost dialysis machine. The KidneyX project in the USA is a public–private partnership that has raised tens of millions of dollars to foster innovations in dialysis care, including an artificial kidney prize .
New therapies that focus on the prevention of kidney disease development and progression will result in major health and socio-economic benefits. Finally, highlighting kidney disease will enable kidney health societies to expand advocacy for appropriate access to care for patients with kidney disease, increasing access to diagnosis and treatment and including medications needed to treat kidney disease in the WHO essential medicines list.
Shining a spotlight on kidney disease will not only result in decreased numbers of people needing resource-intensive kidney treatment but also empower programmes to reduce waste in dialysis. More sustainable dialysis modalities and technologies are urgently needed. Haemodialysis, the most common form of KRT, uses hundreds of litres of water per session60. In drought-affected areas, this demand poses a profound challenge. Decreasing the amount of people on dialysis by disease prevention will improve water security and reduce waste. Dialysis also produces >900,000 tonnes of plastic waste a year, rendering it one of the highest emitters of carbon emissions in health care150. In the USA, annual emissions per haemodialysis facility are estimated at nearly 770,000 kg of CO2 equivalents151. Nearly 38,000,000 kg of recyclable plastic waste is generated annually from peritoneal dialysis globally, but limited recycling options exist152. Encouragingly, programmes to monitor electricity and water use in dialysis facilities have resulted in 30–50% savings, despite increasing patient numbers in France153.
Grand challenges for kidney health
Several major unmet policy, advocacy and implementation needs (Box 3) must be tackled to alleviate the global burden of kidney disease. We call for the global health community to address the following urgent public health needs to meet the needs of those at risk of, and with kidney diseases:
-
1.
Improved access to care: Many people with CKD do not have access to adequate diagnostic and treatment (including preventive) services, particularly in LICs and LMICs. Similarly, >1 million people with potentially reversible AKI die yearly owing to lack of access to timely therapies, including dialysis. These gaps must be addressed by increasing the availability of affordable and accessible health care services.
-
2.
Better prevention: More effective strategies are needed to prevent the development of CKD and AKI. Kidney disease risk factors need to be better understood, especially in LICs and LMICs, through appropriately designed studies using a multidisciplinary approach, interpreting the results in the context of the study population and its limitations, and considering their implications for the community and public health. In a recent White Paper, an ISN Working Group suggested points that countries should consider before developing a CKD case finding and management program, and put forward an evidence-based, resource-sensitive framework that can be adjusted to suit the local contexts. (139)
-
3.
Developing, testing and scaling up novel balanced models of care: Implementing affordable, scalable and sustainable models of care requires co-development with stakeholder communities. Balanced models should also outline a systematic but flexible approach to planning treatment and care within the overall context of strengthened primary health care services. In low-resource settings, this approach might include a combination of task-sharing between physicians and non-physician health care workers in a locally appropriate way for diagnosis and follow-up care (facilitated by digital mobile technology and the use of clinical decision support systems with regional supervision), the use of online platforms to deliver competency training and facilitate supervision, and the use of peers for quality assurance. Potential risks of such approaches should be recognized and addressed by implementing policies that ensure the equitable delivery of safe, effective and high-quality care. In high-resource settings, care delivery needs to be refined across all levels of health care with the addition of an extended range of services in terms of coverage and degree of specialization.
-
4.
Greater awareness and education: Many people with CKD are not aware that they have the disease, and many more are not aware of the steps that they can take to slow its progression. Similarly, a large proportion of the primary care community does not fully appreciate the adverse consequences of early-stage CKD. Greater awareness and education campaigns are needed to help people to understand the importance of early diagnosis and management of CKD.
-
5.
Addressing social determinants of kidney health: As kidney diseases disproportionately affect impoverished and marginalized communities, addressing social determinants of health, such as poverty, poor housing and a lack of access to healthy food and clean water, is essential to address the burden of kidney diseases.
-
6.
Increased funding for research and development: More funding is needed to support the development of new treatments and therapies for kidney diseases, and to improve understanding of these diseases and their underlying causes in different parts of the world.
-
7.
International cooperation and coordination: To promote the development and implementation of effective policies and programmes for the prevention, early detection and management of kidney diseases, and to share knowledge and best practices, international cooperation and coordination are needed.
-
8.
Greater engagement with patient communities: The demand for meaningful participation by community members in shaping health policies and in planning, delivering, quality assurance and evaluation of services has increased steadily. Greater engagement with patient communities is needed to ensure that policies and programmes address the needs and priorities of people living with kidney diseases effectively. Community involvement can range from consultation and collaboration to leadership.
Conclusion
The 2015 United Nations Sustainable Development Goal 3.4 is aimed at reducing premature mortality from NCDs by a third by 2030. To tackle this goal, the WHO has recognized cancer, heart disease, stroke, chronic lung disease and diabetes as the major NCD drivers of early mortality. Failure to include kidney disease in this initiative misses the opportunity to address a major contributor to premature and preventable mortality. Changing population dynamics and evolving risk accumulation mean that the global burden of kidney disease is increasing relentlessly to become the fifth most common NCD driver of mortality by 2040. Kidney disease increases the risk of mortality, morbidity and disability, decreases quality of life and has profound individual- and health-system-level economic consequences, as well as dire environmental impacts. Kidney disease is under-recognized and under-resourced. Recognizing kidney disease as a major driver of NCD-related mortality will translate into coordinated global efforts to minimize the burden of kidney disease and will save lives.
References
World Health Organization. SDG target 3.4 non-communicable diseases and mental health. https://www.who.int/data/gho/data/themes/topics/sdg-target-3_4-noncommunicable-diseases-and-mental-health (2024).
World Health Organization. Non-communicable diseases. https://www.who.int/health-topics/noncommunicable-diseases#tab=tab_1 (2022).
Hsu, C. Y. et al. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 74, 101–107 (2008).
Coca, S. G., Singanamala, S. & Parikh, C. R. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 81, 442–448 (2012).
James, M. T. et al. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet 376, 2096–2103 (2010).
James, M. T. et al. A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am. J. Kidney Dis. 66, 602–612 (2015).
GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395, 709–733 (2020).
Bikbov, B. Core global metrics of chronic kidney disease (CKD) mortality in the GBD 2019 study. Zenodo https://doi.org/10.5281/zenodo.8312881 (2023).
Jager, K. J. et al. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol. Dial. Transplant. 34, 1803–1805 (2019).
Bosi, A. et al. Use of nephrotoxic medications in adults with chronic kidney disease in Swedish and US routine care. Clin. Kidney J. 15, 442–451 (2022).
Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States, 2021 (US Department of Health and Human Services, 2021).
Gummidi, B. et al. A systematic study of the prevalence and risk factors of CKD in Uddanam, India. Kidney Int. Rep. 5, 2246–2255 (2020).
GBD. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1459–1544 (2016).
Foreman, K. J. et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 392, 2052–2090 (2018).
US National Institute of Diabetes and Digestive and Kidney Diseases. Kidney disease statistics for the United States. https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease (2023).
Stanifer, J. W., Muiru, A., Jafar, T. H. & Patel, U. D. Chronic kidney disease in low- and middle-income countries. Nephrol. Dial. Transplant. 31, 868–874 (2016).
Mehta, R. L. et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet 387, 2017–2025 (2016).
Lewington, A. J., Cerdá, J. & Mehta, R. L. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int. 84, 457–467 (2013).
Olowu, W. A. et al. Outcomes of acute kidney injury in children and adults in sub-Saharan Africa: a systematic review. Lancet Glob. Health 4, e242–e250 (2016).
Jha, V. & Parameswaran, S. Community-acquired acute kidney injury in tropical countries. Nat. Rev. Nephrol. 9, 278–290 (2013).
Mehta, R. L. et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet 385, 2616–2643 (2015).
Liyanage, T. et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 385, 1975–1982 (2015).
United States Renal Data System. 2022 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States (National Institute of Diabetes and Digestive and Kidney Diseases, 2022).
Bello, A. K. et al. Status of care for end stage kidney disease in countries and regions worldwide: international cross sectional survey. BMJ 367, l5873 (2019).
Anand, S., Bitton, A. & Gaziano, T. The gap between estimated incidence of end-stage renal disease and use of therapy. PLoS One 8, e72860 (2013).
Thomas, B. et al. Global cardiovascular and renal outcomes of reduced GFR. J. Am. Soc. Nephrol. 28, 2167–2179 (2017).
United Nations Department of Economic and Social Affairs, Population Division. World population prospects 2019: highlights. https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf (2019).
WDA Global Longevity Council. Living longer around the world: opportunities and challenges. https://www.wdaforum.org/fileadmin/ablage/wda_global_longevity_council/wda_paper_living__longer_around_the__world.pdf (2022).
Levey, A. S., Inker, L. A. & Coresh, J. Should the definition of CKD be changed to include age-adapted GFR criteria?”: con: the evaluation and management of CKD, not the definition, should be age-adapted. Kidney Int. 97, 37–40 (2020).
Hallan, S. I. et al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA 308, 2349–2360, (2012).
Ravani, P. et al. Association of age with risk of kidney failure in adults with stage IV chronic kidney disease in Canada. JAMA Netw. Open 3, e2017150–e2017150 (2020).
O’Sullivan, E. D., Hughes, J. & Ferenbach, D. A. Renal aging: causes and consequences. J. Am. Soc. Nephrol. 28, 407–420 (2017).
Ebert, T. et al. Inflammation and premature ageing in chronic kidney disease. Toxins 12, 227, (2020).
Kataria, A., Trasande, L. & Trachtman, H. The effects of environmental chemicals on renal function. Nat. Rev. Nephrol. 11, 610–625 (2015).
Wearne, N. & Okpechi, I. G. HIV-associated renal disease — an overview. Clin. Nephrol. 86, 41–47 (2016).
Hossain, M. P., Goyder, E. C., Rigby, J. E. & El Nahas, M. CKD and poverty: a growing global challenge. Am. J. Kidney Dis. 53, 166–174 (2009).
Shoham, D. A. et al. Kidney disease and the cumulative burden of life course socioeconomic conditions: the Atherosclerosis Risk in Communities (ARIC) study. Soc. Sci. Med 67, 1311–1320 (2008).
Akrawi, D. S., Li, X., Sundquist, J., Sundquist, K. & Zöller, B. End stage renal disease risk and neighbourhood deprivation: a nationwide cohort study in Sweden. Eur. J. Intern. Med. 25, 853–859 (2014).
Wyld, M. L. R. et al. Sex-based differences in risk factors and complications of chronic kidney disease. Semin. Nephrol. 42, 153–169 (2022).
Eneanya, N. D. et al. Health inequities and the inappropriate use of race in nephrology. Nat. Rev. Nephrol. 18, 84–94 (2022).
Barraclough, K. A., Blashki, G. A., Holt, S. G. & Agar, J. W. M. Climate change and kidney disease-threats and opportunities. Kidney Int. 92, 526–530 (2017).
Burkart, K. G. et al. Estimating the cause-specific relative risks of non-optimal temperature on daily mortality: a two-part modelling approach applied to the Global Burden of Disease Study. Lancet 398, 685–697 (2021).
Ebi, K. L. et al. Hot weather and heat extremes: health risks. Lancet 398, 698–708 (2021).
Rosinger, A. Y. et al. Drinking water salinity is associated with hypertension and hyperdilute urine among Daasanach pastoralists in Northern Kenya. Sci. Total. Environ. 770, 144667 (2021).
Wan, E. T., Darssan, D., Karatela, S., Reid, S. A. & Osborne, N. J. Association of pesticides and kidney function among adults in the US population 2001–2010. Int. J. Environ. Res. Public Health 18, 10249 (2021).
Gao, Z. et al. Toxic nephropathy secondary to chronic mercury poisoning: clinical characteristics and outcomes. Kidney Int. Rep. 7, 1189–1197 (2022).
Johnson, R. J., Wesseling, C. & Newman, L. S. Chronic kidney disease of unknown cause in agricultural communities. N. Engl. J. Med. 380, 1843–1852 (2019).
John, O. et al. Chronic kidney disease of unknown etiology in India: what do we know and where we need to go. Kidney Int. Rep. 6, 2743–2751 (2021).
Parameswaran, S. et al. A newly recognized endemic region of CKD of undetermined etiology (CKDu) in South India — “Tondaimandalam nephropathy”. Kidney Int. Rep. 5, 2066–2073 (2020).
Arora, S., Ps, P., Sahoo, J., Vairappan, B. & Parameswaran, S. Seasonal changes in kidney function in CKD of uncertain etiology. Kidney Int. Rep. 6, 2918–2921 (2021).
Wijewickrama, E. S. et al. Prevalence of CKD of unknown etiology and its potential risk factors in a rural population in Sri Lanka. Kidney Int. Rep. 7, 2303–2307 (2022).
Cabrera, J. W. et al. Chronic interstitial nephritis in agricultural communities: a patient in Paraguay. Kidney Int. Rep. 7, 1131–1135 (2022).
Talukder, M. R. R., Rutherford, S., Phung, D., Islam, M. Z. & Chu, C. The effect of drinking water salinity on blood pressure in young adults of coastal Bangladesh. Environ. Pollut. 214, 248–254 (2016).
Khan, J. R., Awan, N., Archie, R. J., Sultana, N. & Muurlink, O. The association between drinking water salinity and hypertension in coastal Bangladesh. Glob. Health J. 4, 153–158 (2020).
Khan, A. E. et al. Salinity in drinking water and the risk of (pre)eclampsia and gestational hypertension in coastal Bangladesh: a case-control study. PLoS One 9, e108715 (2014).
Blum, M. F. et al. Particulate matter and albuminuria, glomerular filtration rate, and incident CKD. Clin. J. Am. Soc. Nephrol. 15, 311–319 (2020).
Bowe, B. et al. Estimates of the 2016 global burden of kidney disease attributable to ambient fine particulate matter air pollution. BMJ Open 9, e022450 (2019).
He, P. et al. Higher ambient nitrogen dioxide is associated with an elevated risk of hospital-acquired acute kidney injury. Clin. Kidney J. 15, 95–100 (2021).
United Nations Convention to Combat Desertification. Drought in numbers 2022 — restoration for readiness and resilience. https://www.unccd.int/sites/default/files/2022-05/Drought%20in%20Numbers.pdf (2022).
Agar, J. W. M. & Barraclough, K. A. Water use in dialysis: environmental considerations. Nat. Rev. Nephrol. 16, 556–557 (2020).
Sekkarie, M., Hiracham, P., Soudan, K. & Rifai, A. O. Hemodialysis machines capable of performing isolated ultrafiltration in the absence of adequate water supply are needed during disasters. Kidney Int. Rep. 6, 1480–1481 (2021).
Isreb, M. A. et al. The effect of war on Syrian refugees with end-stage renal disease. Kidney Int. Rep. 2, 960–963 (2017).
Koubar, S. H., Hajj Nasan, K. & Sekkarie, M. A. K. Nephrology workforce and education in conflict zones. Kidney Int. Rep. 7, 129–132 (2022).
Sever, M. et al. The Marmara earthquake: epidemiological analysis of the victims with nephrological problems. Kidney Int. 60, 1114–1123 (2001).
Luyckx, V. A. & Brenner, B. M. Birth weight, malnutrition and kidney-associated outcomes — a global concern. Nat. Rev. Nephrol. 11, 135–149 (2015).
Wilding, S. et al. Are socioeconomic inequalities in the incidence of small-for-gestational-age birth narrowing? Findings from a population-based cohort in the South of England. BMJ Open 9, e026998 (2019).
Hoy, W. E., Swanson, C. E. & Mott, S. A. Birthweight and the prevalence, progression, and incidence of CKD in a multideterminant model in a high-risk Australian aboriginal community. Kidney Int. Rep. 6, 2782–2793 (2021).
Gjerde, A., Skrunes, R., Reisæter, A. V., Marti, H.-P. & Vikse, B. E. Familial contributions to the association between low birth weight and risk of CKD in adult life. Kidney Int. Rep. 6, 2151–2158 (2021).
Lillås, B. S., Qvale, T. H., Richter, B. K. & Vikse, B. E. Birth weight is associated with kidney size in middle-aged women. Kidney Int. Rep. 6, 2794–2802 (2021).
Hsu, C. W., Yamamoto, K. T., Henry, R. K., De Roos, A. J. & Flynn, J. T. Prenatal risk factors for childhood CKD. J. Am. Soc. Nephrol. 25, 2105–2111 (2014).
Crump, C., Sundquist, J., Winkleby, M. A. & Sundquist, K. Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: national cohort study. BMJ 365, l1346 (2019).
Sanderson, K. R. et al. Albuminuria, hypertension, and reduced kidney volumes in adolescents born extremely premature. Front Pediatr. 8, 230 (2020).
Hingorani, S. et al. Prevalence and risk factors for kidney disease and elevated BP in 2-year-old children born extremely premature. Clin. J. Am. Soc. Nephrol. 17, 1129–1138 (2022).
Luyckx, V. A. et al. A developmental approach to the prevention of hypertension and kidney disease: a report from the Low Birth Weight and Nephron Number Working Group. Lancet 390, 424–428 (2017).
Guo, Q., Wu, S., Xu, C., Wang, J. & Chen, J. Global disease burden from acute glomerulonephritis 1990–2019. Kidney Int. Rep. 6, 2212–2217 (2021).
Cerdá, J. et al. Acute kidney injury recognition in low- and middle-income countries. Kidney Int. Rep. 2, 530–543 (2017).
Kashani, K. et al. Acute kidney injury risk assessment: differences and similarities between resource-limited and resource-rich countries. Kidney Int. Rep. 2, 519–529 (2017).
Smoyer, W. E. et al. Saving young lives with acute kidney injury: the challenge of acute dialysis in low-resource settings. Kidney Int. 89, 254–256 (2016).
Yang, C. W. Leptospirosis renal disease: emerging culprit of chronic kidney disease unknown etiology. Nephron 138, 129–136 (2018).
Phannajit, J. et al. Long-term kidney outcomes after leptospirosis: a prospective multicenter cohort study in Thailand. Nephrol. Dial. Transplant. 38, 2182–2191 (2023).
Riefkohl, A. et al. Leptospira seropositivity as a risk factor for Mesoamerican nephropathy. Int. J. Occup. Environ. Health 23, 1–10 (2017).
Martins, D. et al. The association of poverty with the prevalence of albuminuria: data from the Third National Health and Nutrition Examination Survey (NHANES III). Am. J. Kidney Dis. 47, 965–971 (2006).
Drey, N., Roderick, P., Mullee, M. & Rogerson, M. A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am. J. Kidney Dis. 42, 677–684 (2003).
Morton, R. L. et al. Impact of CKD on household income. Kidney Int. Rep. 3, 610–618 (2018).
Chesnaye, N. C., Carrero, J. J., Hecking, M. & Jager, K. J. Differences in the epidemiology, management and outcomes of kidney disease in men and women. Nat. Rev. Nephrol. 20, 7–20 (2024).
Melsom, T. et al. Sex differences in age-related loss of kidney function. J. Am. Soc. Nephrol. 33, 1891–1902 (2022).
van der Burgh, A. C., Rizopoulos, D., Ikram, M. A., Hoorn, E. J. & Chaker, L. Determinants of the evolution of kidney function with age. Kidney Int. Rep. 6, 3054–3063 (2021).
Ritchie, H. Causes of death. Our World in Data https://ourworldindata.org/does-the-news-reflect-what-we-die-from (2019).
Tummalapalli, S. L. et al. Availability and affordability of kidney health laboratory tests around the globe. Am. J. Nephrol. 51, 959–965 (2020).
Talbot, B., Athavale, A., Jha, V. & Gallagher, M. Data challenges in addressing chronic kidney disease in low- and lower-middle-income countries. Kidney Int. Rep. 6, 1503–1512 (2021).
Ng, M. S. Y., Charu, V., Johnson, D. W., O’Shaughnessy, M. M. & Mallett, A. J. National and international kidney failure registries: characteristics, commonalities, and contrasts. Kidney Int. 101, 23–35 (2022).
Davids, M. R. et al. A renal registry for Africa: first steps. Clin. Kidney J. 9, 162–167 (2015).
Luxardo, R., Ceretta, L., González-Bedat, M., Ferreiro, A. & Rosa-Diez, G. The Latin American Dialysis and Renal Transplantation Registry: report 2019. Clin. Kidney J. 15, 425–431 (2021).
Bello, A.K. et al. ISN — global kidney health atlas: a report by the International Society of Nephrology: an assessment of global kidney health care status focussing on capacity, availability, accessibility, affordability and outcomes of kidney disease. (International Society of Nephrology, 2023).
Bello, A. K. et al. Global overview of health systems oversight and financing for kidney care. Kidney Int. Suppl. 8, 41–51 (2018).
Narva, A. S. et al. Toward a more collaborative federal response to chronic kidney disease. Adv. Chronic kidney Dis. 17, 282–288 (2010).
European Commission. Healthier together — EU non-communicable diseases initiative. https://health.ec.europa.eu/system/files/2022-06/eu-ncd-initiative_publication_en_0.pdf (2022).
Shlipak, M. G. et al. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 99, 34–47 (2021).
World Health Organization. The top 10 causes of death. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (2020).
Boenink, R. et al. The ERA registry annual report 2019: summary and age comparisons. Clin. Kidney J. 15, 452–472 (2022).
Zoccali, C. et al. The systemic nature of CKD. Nat. Rev. Nephrol. 13, 344–358 (2017).
Vos, T. et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1204–1222 (2020).
Xie, Y. et al. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 94, 567–581 (2018).
Kalantar-Zadeh, K. et al. Patient-centred approaches for the management of unpleasant symptoms in kidney disease. Nat. Rev. Nephrol. 18, 185–198 (2022).
Fletcher, B. R. et al. Symptom burden and health-related quality of life in chronic kidney disease: a global systematic review and meta-analysis. PLOS Med. 19, e1003954 (2022).
Francis, A. et al. Quality of life of children and adolescents with chronic kidney disease: a cross-sectional study. Arch. Dis. Child 104, 134–140 (2019).
Chesnaye, N. C. et al. Health-related quality-of-life trajectories over time in older men and women with advanced chronic kidney disease. Clin. J. Am. Soc. Nephrol. 17, 205–214 (2022).
de Rooij, E. N. M. et al. Quality of life before and after the start of dialysis in older patients. Clin. J. Am. Soc. Nephrol. 17, 1159–1167 (2022).
de Rooij, E. N. M. et al. Symptom burden before and after dialysis initiation in older patients. Clin. J. Am. Soc. Nephrol. 17, 1719–1729 (2022).
Mahmoud, D. A. M., Saad, A., Abdelhamid, Y. H. & El Hawary, Y. Depression and psychosocial burden among caregivers of children with chronic kidney disease. Middle East Curr. Psychiatry 28, 12 (2021).
Pereira, Bd. S. et al. Beyond quality of life: a cross sectional study on the mental health of patients with chronic kidney disease undergoing dialysis and their caregivers. Health Qual. Life Outcomes 15, 74 (2017).
Shukri, M., Mustofai, M. A., Md Yasin, M. A. S. & Tuan Hadi, T. S. Burden, quality of life, anxiety, and depressive symptoms among caregivers of hemodialysis patients: the role of social support. Int. J. Psychiatry Med. 55, 397–407 (2020).
Adejumo, O. A., Iyawe, I. O., Akinbodewa, A. A., Abolarin, O. S. & Alli, E. O. Burden, psychological well-being and quality of life of caregivers of end stage renal disease patients. Ghana Med. J. 53, 190–196, (2019).
Luyckx, V. A., Tonelli, M. & Stanifer, J. W. The global burden of kidney disease and the sustainable development goals. Bull. World Health Organ. 96, 414–422 (2018).
World Health Organization. Global health estimates: leading causes of death. Cause-specific mortality, 2000–2019. Global Health Observatory https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (2020).
Tonelli, M. et al. Chronic kidney disease and mortality risk: a systematic review. J. Am. Soc. Nephrol. 17, 2034–2047 (2006).
Francis, A., Baigent, C., Ikizler, T. A., Cockwell, P. & Jha, V. The urgent need to vaccinate dialysis patients against severe acute respiratory syndrome coronavirus 2: a call to action. Kidney Int. 99, 791–793 (2021).
McDonald, H. I., Thomas, S. L. & Nitsch, D. Chronic kidney disease as a risk factor for acute community-acquired infections in high-income countries: a systematic review. BMJ Open 4, e004100 (2014).
Tanaka, S. & Okusa, M. D. Crosstalk between the nervous system and the kidney. Kidney Int. 97, 466–476 (2020).
Rangaswami, J. et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation 139, e840–e878 (2019).
Husain-Syed, F., Slutsky, A. S. & Ronco, C. Lung-kidney cross-talk in the critically ill patient. Am. J. Respir. Crit. Care Med. 194, 402–414 (2016).
Visseren, F. L. J. et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: developed by the task force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 42, 3227–3337 (2021).
Ortiz, A., Wanner, C. & Gansevoort, R. Chronic kidney disease as cardiovascular risk factor in routine clinical practice: a position statement by the Council of the European Renal Association. Eur. J. Prev. Cardiol. 29, 2211–2215 (2022).
Kerr, M., Bray, B., Medcalf, J., O’Donoghue, D. J. & Matthews, B. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol. Dial. Transplant. 27 (Suppl. 3), 73–80 (2012).
Vanholder, R. et al. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat. Rev. Nephrol. 13, 393–409 (2017).
Shaikh, M. et al. Utilization, costs, and outcomes for patients receiving publicly funded hemodialysis in India. Kidney Int. 94, 440–445 (2018).
Tonelli, M. et al. Comparison of the complexity of patients seen by different medical subspecialists in a universal health care system. JAMA Netw. Open 1, e184852–e184852 (2018).
Vanholder, R. et al. Fighting the unbearable lightness of neglecting kidney health: the decade of the kidney. Clin. Kidney J. 14, 1719–1730 (2021).
Riaz, P. et al. Workforce capacity for the care of patients with kidney failure across world countries and regions. BMJ Glob. Health 6, e004014 (2021).
Lalji, R. et al. Disparities in end-stage kidney disease care for children: a global survey. Kidney Int. 98, 527–532 (2020).
Essue, B. M. et al. In Disease Control Priorities: Improving Health and Reducing Poverty (eds Jamison, D. T. et al.) (The International Bank for Reconstruction and Development/The World Bank, 2017).
Kim, S. et al. Cognitive and academic outcomes in children with chronic kidney disease. Pediatr. Nephrol. 37, 2715–2724 (2022).
Khalid, R. et al. Association between socioeconomic status and academic performance in children and adolescents with chronic kidney disease. Pediatr. Nephrol. 37, 3195–3204 (2022).
Erickson, K. F., Zhao, B., Ho, V. & Winkelmayer, W. C. Employment among patients starting dialysis in the United States. Clin. J. Am. Soc. Nephrol. 13, 265–273 (2018).
Vollset, S. E. et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet 396, 1285–1306 (2020).
Htay, H. et al. Global access of patients with kidney disease to health technologies and medications: findings from the Global Kidney Health Atlas project. Kidney Int. Suppl. 8, 64–73 (2018).
Levin A, S. P. et al. Kidney disease: improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 3, 1–150 (2013).
Li, P. K.-T. et al. Kidney health for everyone everywhere — from prevention to detection and equitable access to care. Kidney Int. Rep. 5, 245–251 (2020).
Francis, A. et al. Barriers to accessing essential medicines for kidney disease in low and low middle income countries. Kidney Int. 5, 969–973 (2022).
Thurlow, J. S. et al. Global epidemiology of end-stage kidney disease and disparities in kidney replacement therapy. Am. J. Nephrol. 52, 98–107 (2021).
Alencar de Pinho, N. et al. Understanding international variations in kidney failure incidence and initiation of replacement therapy. Kidney Int. Rep. 7, 2364–2375 (2022).
Tang, S. C. W. et al. Dialysis care and dialysis funding in Asia. Am. J. Kidney Dis. 75, 772–781 (2020).
Lalji, R., Francis, A., Johnson, D. W. & McCulloch, M. Health disparities in access to kidney replacement therapy amongst children and adolescents with end-stage kidney disease in low- and lower-middle-income countries. Kidney Int. 97, 463–465 (2020).
Ashuntantang, G. et al. Outcomes in adults and children with end-stage kidney disease requiring dialysis in sub-Saharan Africa: a systematic review. Lancet Glob. Health 5, e408–e417 (2017).
Gonzalez-Bedat, M. C. et al. Burden of disease: closing the gaps in the burden of end-stage kidney disease in Latin America. Clin. Nephrol. 93, 55–59 (2020).
Baigent, C. et al. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet 400, 1788–1801 (2022).
Stanifer, J. W. et al. The epidemiology of chronic kidney disease in sub-Saharan Africa: aasystematic review and meta-analysis. Lancet Glob. Health 2, e174–e181 (2014).
Hole, B. D. et al. International collaborative efforts to establish kidney health surveillance systems. Kidney Int. 98, 812–816 (2020).
Gutierrez-Peña, M. et al. High prevalence of end-stage renal disease of unknown origin in Aguascalientes Mexico: role of the registry of chronic kidney disease and renal biopsy in its approach and future directions. Clin. Kidney J. 14, 1197–1206 (2021).
Salas, R. N., Maibach, E., Pencheon, D., Watts, N. & Frumkin, H. A pathway to net zero emissions for healthcare. BMJ 371, m3785 (2020).
Sehgal, A. R., Slutzman, J. E. & Huml, A. M. Sources of variation in the carbon footprint of hemodialysis treatment. J. Am. Soc. Nephrol. 33, 1790–1795 (2022).
Rao, N., Rajan, T. & Stigant, C. Quantification of recyclable peritoneal dialysis plastics in a home dialysis program — an opportunity for resource stewardship. Kidney Int. Rep. 8, 365–367 (2020).
Bendine, G. et al. Haemodialysis therapy and sustainable growth: a corporate experience in France. Nephrol. Dial. Transplant. 35, 2154–2160 (2020).
Acknowledgements
The authors thank representatives of the African Association of Nephrology (Abdou Niang and Hany Hafez), Asian Pacific Society of Nephrology (Sydney Tang), the International Diabetes Federation (Akthar Hussain), the International Society of Nephrology’s regional board chairs (Fatiu Arogundade, Muhammad Rafiqul Alam, Alejandro Ferreiro-Fuentes, Talerngsak Kanjanabuch, Kirill Komissarov, Jolanta Malyszko, Narayan Prasad, Larisa Prikhodina, Bassam Saeed, Maria José Soler Romeo, Carmen Tzanno-Martins, and Angela Wang), the Latin American Society of Nephrology and Hypertension (Guillermo Alvarez) and the World Heart Federation (Fausto Pinto) for reviewing and approving the manuscript before submission.
Author information
Authors and Affiliations
Consortia
Contributions
A.F. and V.J. researched data for the article. A.C.M.O., S.L.T., A.O., A.B.F., D.F., P.R.-C., M.F., M.N., C.W., C.M. L.S., I.U., and V.J. made substantial contributions to discussions of the content. A.F., M.N.H. and V.J. wrote the manuscript. A.H., D.A., S.B., A.C., L.S. and I.U. reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
V.J. has received grant funding from GSK, Baxter Healthcare, and Biocon and honoraria from Bayer, AstraZeneca, Boehringer Ingelheim, NephroPlus and Zydus Cadilla, under the policy of all honoraria being paid to the organization unrelated to the submitted work. S.L.T. reports research funding from Scanwell Health and SAIGroup (paid to institution), unrelated to the submitted work. M.N.H. reports consulting fees from Nephria Bio, unrelated to the submitted work. The other authors declare no competing interests.
Peer review
Peer review information
Nat. Rev. Nephrol. thanks Urmila Anandh, Etienne Macedo, Ravindra Mehta and Katherine Tuttle for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
KidneyX project: http://www.kidneyx.org/
Prototype of a low-cost dialysis machine: https://www.ellenmedical.com/
Sustainable Development Goal 3.4: https://sdgs.un.org/goals/goal3#targets_and_indicators
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Francis, A., Harhay, M.N., Ong, A.C.M. et al. Chronic kidney disease and the global public health agenda: an international consensus. Nat Rev Nephrol (2024). https://doi.org/10.1038/s41581-024-00820-6
Accepted:
Published:
DOI: https://doi.org/10.1038/s41581-024-00820-6