Key Points
-
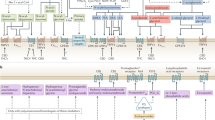
Cannabinoid receptor diversity and signalling in the immature brain are vastly different from those in the adult nervous system. These include the differential localization of, and signal transduction by, cannabinoid receptors and the enzymes that limit endocannabinoid bioavailability. CB1 and CB2 cannabinoid receptors signal neural progenitor proliferation in the developing brain, including gliogenesis, by coupling to mitogenic pathways such as PI3K and ERK.
-
Endocannabinoid hot spots can facilitate the directional migration of postmitotic neurons in the foetal nervous system, as well as of neuroblasts in neurogenic areas of the adult mammalian brain.
-
CB1 cannabinoid receptors, and probably CB2 and TRPV1 receptors, in growth cones signal to modulate steering decisions during directional axonal growth. In part, autocrine signalling by endocannabinoids mediates neurite elongation.
-
Diacylgycerol lipase-dependent endocannabainoid signalling regulates neurogenesis in the adult hippocampus and subventricular zone.
-
Δ9-THC affects neuronal development via CB1 cannabinoid receptor-mediated mechanisms, disrupting cytoskeletal integrity, axonal growth and synaptogenesis. Therefore, THC exposure is adverse in developmental contexts.
-
Preclinical evidence suggests that pharmacological activation of cannabinoid receptors might be exploited therapeutically to inhibit tumor growth in glioma patients.
Abstract
Among the many signalling lipids, endocannabinoids are increasingly recognized for their important roles in neuronal and glial development. Recent experimental evidence suggests that, during neuronal differentiation, endocannabinoid signalling undergoes a fundamental switch from the prenatal determination of cell fate to the homeostatic regulation of synaptic neurotransmission and bioenergetics in the mature nervous system. These studies also offer novel insights into neuropsychiatric disease mechanisms and contribute to the public debate about the benefits and the risks of cannabis use during pregnancy and in adolescence.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sang, N., Zhang, J., Marcheselli, V., Bazan, N. G. & Chen, C. Postsynaptically synthesized prostaglandin E2 (PGE2) modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor. J. Neurosci. 25, 9858–9870 (2005).
Abad-Rodriguez, J. & Robotti, A. Regulation of axonal development by plasma membrane gangliosides. J. Neurochem. 103 (Suppl. 1), 47–55 (2007).
Fyrst, H. & Saba, J. D. An update on sphingosine-1phosphate and other sphingolipid mediators. Nature Chem. Biol. 6, 489–497 (2010).
Piomelli, D. The molecular logic of endocannabinoid signalling. Nature Rev. Neurosci. 4, 873–884 (2003).
Ahn, K., McKinney, M. K. & Cravatt, B. F. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem. Rev. 108, 1687–1707 (2008).
Paria, B. C. et al. Dysregulated cannabinoid signaling disrupts uterine receptivity for embryo implantation. J. Biol. Chem. 276, 20523–20528 (2001).
Harkany, T., Mackie, K. & Doherty, P. Wiring and firing neuronal networks: endocannabinoids take center stage. Curr. Opin. Neurobiol. 18, 338–345 (2008).
Benard, G. et al. Mitochondrial CB1 receptors regulate neuronal energy metabolism. Nature Neurosci. 15, 558–564 (2012).
Kano, M., Ohno-Shosaku, T., Hashimotodani, Y., Uchigashima, M. & Watanabe, M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 89, 309–380 (2009).
Regehr, W. G., Carey, M. R. & Best, A. R. Activity-dependent regulation of synapses by retrograde messengers. Neuron 63, 154–170 (2009).
Goncalves, M. B. et al. A diacylglycerol lipase-CB2 cannabinoid pathway regulates adult subventricular zone neurogenesis in an age-dependent manner. Mol. Cell Neurosci. 38, 526–536 (2008). This paper shows that neural progenitor cells in the subventricular zone of the cerebral cortex express CB2Rs and use an autocrine DAGL–CB2R signalling arrangement to promote neurogenesis.
Jiang, W. et al. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J. Clin. Invest. 115, 3104–3116 (2005).
Pernia-Andrade, A. J. et al. Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization. Science 325, 760–764 (2009).
Bradshaw, H. B. & Walker, J. M. The expanding field of cannabimimetic and related lipid mediators. Br. J. Pharmacol. 144, 459–465 (2005).
Gao, Y. et al. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J. Neurosci. 30, 2017–2024 (2010). This paper reports the generation of DAGL-null mice and provides genetic evidence on the requirement of DAGL function for continued neurogenesis in the mouse forebrain during postnatal life.
Tanimura, A. et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase α mediates retrograde suppression of synaptic transmission. Neuron 65, 320–327 (2010).
Devane, W. A. et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258, 1946–1949 (1992). In this paper, the authors identify anandamide as the first endogenous ligand of CB1Rs in the brain. This landmark paper marks the advent of contemporary endocannabinoid research.
Pertwee, R. G. et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol. Rev. 62, 588–631 (2010).
Aguado, T. et al. The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J. Neurosci. 26, 1551–1561 (2006). This paper provides the molecular identity and describes the expression sites and signalling mechanisms of cannabinoid receptors and the key metabolic enzymes for endocannabinoids in astroglia. Moreover, it establishes the role of endocannabinoids in cell fate decisions and lineage commitment.
Aguado, T. et al. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 19, 1704–1706 (2005).
Berghuis, P. et al. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science 316, 1212–1216 (2007). In this paper, the authors show that axonal CB1Rs can sense extracellular ligand gradients and can induce growth cone repulsion or collapse. This mechanism is then implicated in disrupted synaptogenesis in the cortex of mice lacking CB1Rs in GABAergic cortical interneurons.
Vitalis, T. et al. The type 1 cannabinoid receptor is highly expressed in embryonic cortical projection neurons and negatively regulates neurite growth in vitro. Eur. J. Neurosci. 28, 1705–1718 (2008).
Leterrier, C. et al. Constitutive activation drives compartment-selective endocytosis and axonal targeting of type 1 cannabinoid receptors. J. Neurosci. 26, 3141–3153 (2006).
Oudin, M. J. et al. Endocannabinoids regulate the migration of subventricular zone-derived neuroblasts in the post-natal brain. J. Neurosci. 31, 4000–4011 (2011).
Elphick, M. R. & Egertova, M. The neurobiology and evolution of cannabinoid signalling. Phil. Trans. R. Soc. Lond. B 356, 381–408 (2001).
McPartland, J. M., Norris, R. W. & Kilpatrick, C. W. Coevolution between cannabinoid receptors and endocannabinoid ligands. Gene 397, 126–135 (2007).
Leung, H. T. et al. DAG lipase activity is necessary for TRP channel regulation in Drosophila photoreceptors. Neuron 58, 884–896 (2008).
Begbie, J., Doherty, P. & Graham, A. Cannabinoid receptor, CB1, expression follows neuronal differentiation in the early chick embryo. J. Anat. 205, 213–218 (2004). This paper reports that neuronal commitment coincides with the upregulation of CB1R in the earliest-born neurons of the chick embryo. This proposal led to subsequent mechanistic studies implicating CB1R functions in a broad range of developmental processes.
Watson, S., Chambers, D., Hobbs, C., Doherty, P. & Graham, A. The endocannabinoid receptor, CB1, is required for normal axonal growth and fasciculation. Mol. Cell Neurosci. 38, 89–97 (2008).
Li, L. et al. Endocannabinoid signaling is required for development and critical period plasticity of the whisker map in somatosensory cortex. Neuron 64, 537–549 (2009).
Argaw, A. et al. Concerted action of CB1 cannabinoid receptor and deleted in colorectal cancer in axon guidance. J. Neurosci. 31, 1489–1499 (2011).
Diaz-Alonso, J. et al. The CB1 cannabinoid receptor drives corticospinal motor neuron differentiation through the Ctip2/Satb2 transcriptional regulation axis. J. Neurosci. 32, 16651–16665 (2012).
Wu, C. S. et al. Requirement of cannabinoid CB1 receptors in cortical pyramidal neurons for appropriate development of corticothalamic and thalamocortical projections. Eur. J. Neurosci. 32, 693–706 (2010).
Dinieri, J. A. et al. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol. Psychiatry 70, 763–769 (2011).
Hurd, Y. L. et al. Marijuana impairs growth in mid-gestation fetuses. Neurotoxicol. Teratol. 27, 221–229 (2005). In this paper, the authors report on foetal growth retardation as a consequence of maternal cannabis smoking. The study is particularly important to show that many organ systems are sensitive to phytocannabinoid disruptors.
Wang, X., Dow-Edwards, D., Keller, E. & Hurd, Y. L. Preferential limbic expression of the cannabinoid receptor mRNA in the human fetal brain. Neuroscience 118, 681–694 (2003).
Wang, X., Dow-Edwards, D., Anderson, V., Minkoff, H. & Hurd, Y. L. In utero marijuana exposure associated with abnormal amygdala dopamine D2 gene expression in the human fetus. Biol. Psychiatry 56, 909–915 (2004).
Tortoriello, G. et al. Miswiring the brain: Δ9-tetrahydrocannabinol disrupts cortical development by inducing an SCG10/stathmin2 degradation pathway. EMBO J. 33, 668–685 (2014). This paper is the first molecular analysis of THC effects on neuronal differentiation, particularly axon guidance. By unbiased proteomics, SCG10 is identified as the first molecular effector of unwanted THC effects on neuronal morphology.
Marsicano, G. et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302, 84–88 (2003).
Foldy, C., Malenka, R. C. & Sudhof, T. C. Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling. Neuron 78, 498–509 (2013).
Minocci, D. et al. Genetic association between bipolar disorder and 524A>C (Leu133Ile) polymorphism of CNR2 gene, encoding for CB2 cannabinoid receptor. J. Affect. Disord. 134, 427–430 (2011).
Ishiguro, H. et al. Brain cannabinoid CB2 receptor in schizophrenia. Biol. Psychiatry 67, 974–982 (2010).
Ujike, H. et al. CNR1, central cannabinoid receptor gene, associated with susceptibility to hebephrenic schizophrenia. Mol. Psychiatry 7, 515–518 (2002).
Sipe, J. C., Chiang, K., Gerber, A. L., Beutler, E. & Cravatt, B. F. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc. Natl Acad. Sci. USA 99, 8394–8399 (2002).
Fiskerstrand, T. et al. Mutations in ABHD12 cause the neurodegenerative disease PHARC: an inborn error of endocannabinoid metabolism. Am. J. Hum. Genet. 87, 410–417 (2010).
Mulder, J. et al. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc. Natl Acad. Sci. USA 105, 8760–8765 (2008).
Pertwee, R. G. Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict. Biol. 13, 147–159 (2008).
Keimpema, E. et al. Differential subcellular recruitment of monoacylglycerol lipase generates spatial specificity of 2-arachidonoyl glycerol signaling during axonal pathfinding. J. Neurosci. 30, 13992–14007 (2010).
Yoshida, T. et al. Localization of diacylglycerol lipase-α around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J. Neurosci. 26, 4740–4751 (2006).
Katona, I. & Freund, T. F. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nature Med. 14, 923–930 (2008).
Bisogno, T. et al. Cloning of the first sn-1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 163, 463–468 (2003). This paper identifies the DAGLs, shows their distribution in both the developing and adult nervous system and suggests a striking switch in DAGL expression sites during synaptogenesis. The substrate and product specificity of DAGL is also defined.
Dinh, T. P. et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl Acad. Sci. USA 99, 10819–10824 (2002).
Marrs, W. R. et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nature Neurosci. 13, 951–957 (2010).
Huang, G. Z. & Woolley, C. S. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron 74, 801–808 (2012).
Chavez, A. E., Chiu, C. Q. & Castillo, P. E. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nature Neurosci. 13, 1511–1518 (2010).
Grueter, B. A., Brasnjo, G. & Malenka, R. C. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nature Neurosci. 13, 1519–1525 (2010).
Gulyas, A. I. et al. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur. J. Neurosci. 20, 441–458 (2004).
Wilson, R. I. & Nicoll, R. A. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410, 588–592 (2001).
Kreitzer, A. C., Carter, A. G. & Regehr, W. G. Inhibition of interneuron firing extends the spread of endocannabinoid signaling in the cerebellum. Neuron 34, 787–796 (2002).
Walker, D. J., Suetterlin, P., Reisenberg, M., Williams, G. & Doherty, P. Down-regulation of diacylglycerol lipase-α during neural stem cell differentiation: Identification of elements that regulate transcription. J. Neurosci. Res. 88, 735–745 (2010).
Rimmerman, N. et al. Compartmentalization of endocannabinoids into lipid rafts in a dorsal root ganglion cell line. Br. J. Pharmacol. 153, 380–389 (2008).
Bari, M., Battista, N., Fezza, F., Finazzi-Agro, A. & Maccarrone, M. Lipid rafts control signaling of type-1 cannabinoid receptors in neuronal cells. Implications for anandamide-induced apoptosis. J. Biol. Chem. 280, 12212–12220 (2005).
Keimpema, E. et al. Diacylglycerol lipase α manipulation reveals developmental roles for intercellular endocannabinoid signaling. Sci. Rep. 3, 2093 (2013).
Duff, G. et al. Cannabinoid receptor CB2 modulates axon guidance. PLoS ONE. 8, e70849 (2013).
Morozov, Y. M., Torii, M. & Rakic, P. Origin, early commitment, migratory routes, and destination of cannabinoid type 1 receptor-containing interneurons. Cereb. Cortex 19, i78–i89 (2009).
Soderstrom, K. & Tian, Q. Developmental pattern of CB1 cannabinoid receptor immunoreactivity in brain regions important to zebra finch (Taeniopygia guttata) song learning and control. J. Comp. Neurol. 496, 739–758 (2006).
Gotz, M. & Huttner, W. B. The cell biology of neurogenesis. Nature Rev. Mol. Cell. Biol. 6, 777–788 (2005).
Keimpema, E. et al. Nerve growth factor scales endocannabinoid signaling by regulating monoacylglycerol lipase turnover in developing cholinergic neurons. Proc. Natl Acad. Sci. USA 110, 1935–1940 (2013).
Harkany, T. et al. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol. Sci. 28, 83–92 (2007).
Morozov, Y. M., Ben Ari, Y. & Freund, T. F. The spatial and temporal pattern of fatty acid amide hydrolase expression in rat hippocampus during postnatal development. Eur. J. Neurosci. 20, 459–466 (2004).
Johansson, C. B. et al. Identification of a neural stem cell in the adult mammalian central nervous system. Cell 96, 25–34 (1999).
Rakic, P. A century of progress in corticoneurogenesis: from silver impregnation to genetic engineering. Cereb. Cortex 16, i3–i17 (2006).
Metin, C., Vallee, R. B., Rakic, P. & Bhide, P. G. Modes and mishaps of neuronal migration in the mammalian brain. J. Neurosci. 28, 11746–11752 (2008).
Morozov, Y. M. & Freund, T. F. Post-natal development of type 1 cannabinoid receptor immunoreactivity in the rat hippocampus. Eur. J. Neurosci. 18, 1213–1222 (2003).
Stock, K. et al. Neural precursor cells induce cell death of high-grade astrocytomas through stimulation of TRPV1. Nature Med. 18, 1232–1238 (2012).
Pineiro, R., Maffucci, T. & Falasca, M. The putative cannabinoid receptor GPR55 defines a novel autocrine loop in cancer cell proliferation. Oncogene 30, 142–152 (2011).
Andradas, C. et al. The orphan G protein-coupled receptor GPR55 promotes cancer cell proliferation via ERK. Oncogene 30, 245–252 (2011).
Brown, I. et al. Cannabinoid receptor-dependent and -independent anti-proliferative effects of omega-3 ethanolamides in androgen receptor-positive and -negative prostate cancer cell lines. Carcinogenesis 31, 1584–1591 (2010).
De Petrocellis, L. et al. The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation. Proc. Natl Acad. Sci. USA 95, 8375–8380 (1998).
Goswami, C., Schmidt, H. & Hucho, F. TRPV1 at nerve endings regulates growth cone morphology and movement through cytoskeleton reorganization. FEBS J. 274, 760–772 (2007).
Goswami, C. & Hucho, T. TRPV1 expression-dependent initiation and regulation of filopodia. J. Neurochem. 103, 1319–1333 (2007).
Antypa, M., Faux, C., Eichele, G., Parnavelas, J. G., & Andrews, W. D. Differential gene expression in migratory streams of cortical interneurons. Eur. J. Neurosci. 34, 1584–1594 (2011).
Herkenham, M. et al. Cannabinoid receptor localization in brain. Proc. Natl Acad. Sci. USA 87, 1932–1936 (1990).
Howlett, A. C. et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 54, 161–202 (2002).
Galve-Roperh, I. et al. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog. Lipid Res. 52, 633–650 (2013).
Bromberg, K. D., Ma'ayan, A., Neves, S. R. & Iyengar, R. Design logic of a cannabinoid receptor signaling network that triggers neurite outgrowth. Science 320, 903–909 (2008). In this paper, the authors define a minimal transcription factor network downstream from CB1Rs that is required for neurite outgrowth. They also implicate BRCA1 in cannabinoid signalling, which was shown as a candidate to control MAGL stability by Keimpema in 2013.
Diaz-Alonso, J. et al. CB1 cannabinoid receptor-dependent activation of mTORC1/PAX6 signaling drives TBR2 expression and basal progenitor expansion in the developing mouse cortex. Cereb. Cortex 7 Mar 2014 [Epub ahead of print].
Vasquez, C. & Lewis, D. L. The CB1 cannabinoid receptor can sequester G-proteins, making them unavailable to couple to other receptors. J. Neurosci. 19, 9271–9280 (1999).
Kearn, C. S., Blake-Palmer, K., Daniel, E., Mackie, K. & Glass, M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: A mechanism for receptor cross-talk? Mol. Pharmacol. 67, 1697–1704 (2005).
Hudson, B. D., Hebert, T. E. & Kelly, M. E. Ligand- and heterodimer-directed signaling of the CB1 cannabinoid receptor. Mol. Pharmacol. 77, 1–9 (2010).
Dalton, G. D. & Howlett, A. C. Cannabinoid CB1 receptors transactivate multiple receptor tyrosine kinases and regulate serine/threonine kinases to activate ERK in neuronal cells. Br. J. Pharmacol. 165, 2497–2511 (2012).
Berghuis, P. et al. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TRKB receptor. Proc. Natl Acad. Sci. USA 102, 19115–19120 (2005).
Ahn, K. H., Mahmoud, M. M., Shim, J. Y. & Kendall, D. A. Distinct roles of β-arrestin 1 and β-arrestin 2 in ORG27569-induced biased signaling and internalization of the cannabinoid receptor 1 (CB1). J. Biol. Chem. 288, 9790–9800 (2013).
Maison, P., Walker, D. J., Walsh, F. S., Williams, G. & Doherty, P. BDNF regulates neuronal sensitivity to endocannabinoids. Neurosci. Lett. 467, 90–94 (2009).
Williams, E. J., Walsh, F. S. & Doherty, P. The FGF receptor uses the endocannabinoid signaling system to couple to an axonal growth response. J. Cell Biol. 160, 481–486 (2003). In this paper, the authors suggest that endocannabinoid signalling is regulated by FGFRs and that this coupling underpins FGF-induced neurite outgrowth.
Brittis, P. A., Silver, J., Walsh, F. S. & Doherty, P. Fibroblast growth factor receptor function is required for the orderly projection of ganglion cell axons in the developing mammalian retina. Mol. Cell Neurosci. 8, 120–128 (1996).
Ross, R. A. Anandamide and vanilloid TRPV1 receptors. Br. J. Pharmacol. 140, 790–801 (2003).
Puente, N. et al. The transient receptor potential vanilloid-1 is localized at excitatory synapses in the mouse dentate gyrus. Brain Struct. Funct. http:dx.doi.org/10.1007/s00429-014-0711-2 (2014).
Maccarrone, M. et al. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nature Neurosci. 11, 152–159 (2008). This paper describes the interplay between AEA action at TRPV1 and the metabolic control and CB1R engagement of 2-AG, which allow AEA to control the 'endogenous tone' and physiological action of 2-AG in the striatum.
Berrendero, F. et al. Localization of mRNA expression and activation of signal transduction mechanisms for cannabinoid receptor in rat brain during fetal development. Development 125, 3179–3188 (1998).
Berrendero, F., Sepe, N., Ramos, J. A., Di Marzo, V. & Fernandez-Ruiz, J. J. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse 33, 181–191 (1999). This paper describes the distribution of CB1Rs, 2-AG and AEA at successive developmental periods in foetal rodent brains. The authors suggest a temporal association between receptor expression and ligand bioavailability.
Palazuelos, J., Ortega, Z., Diaz-Alonso, J., Guzman, M. & Galve-Roperh, I. CB2 cannabinoid receptors promote neural progenitor cell proliferation via mTORC1 signaling. J. Biol. Chem. 287, 1198–1209 (2012).
Palazuelos, J. et al. Non-psychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation. FASEB J. 20, 2405–2407 (2006).
Fishell, G. & Hanashima, C. Pyramidal neurons grow up and change their mind. Neuron 57, 333–338 (2008).
Jin, M. et al. Ca2+-dependent regulation of rho GTPases triggers turning of nerve growth cones. J. Neurosci. 25, 2338–2347 (2005).
Ishii, I. & Chun, J. Anandamide-induced neuroblastoma cell rounding via the CB1 cannabinoid receptors. Neuroreport 13, 593–596 (2002).
Ford, L. A. et al. A role for Lα-lysophosphatidylinositol and GPR55 in the modulation of migration, orientation and polarization of human breast cancer cells. Br. J. Pharmacol. 160, 762–771 (2010).
Lauckner, J. E. et al. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc. Natl Acad. Sci. USA 105, 2699–2704 (2008).
Zhou, D. & Song, Z. H. CB1 cannabinoid receptor-mediated tyrosine phosphorylation of focal adhesion kinase-related non-kinase. FEBS Lett. 525, 164–168 (2002).
Derkinderen, P. et al. Regulation of a neuronal form of focal adhesion kinase by anandamide. Science 273, 1719–1722 (1996).
Mato, S., Del Olmo, E. & Pazos, A. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur. J. Neurosci. 17, 1747–1754 (2003).
Coutts, A. A. et al. Agonist-induced internalization and trafficking of cannabinoid CB1 receptors in hippocampal neurons. J. Neurosci. 21, 2425–2433 (2001).
He, J. C. et al. The G αo/i-coupled cannabinoid receptor-mediated neurite outgrowth involves RAP regulation of SRC and STAT3. J. Biol. Chem. 280, 33426–33434 (2005).
Ade, K. K. & Lovinger, D. M. Anandamide regulates postnatal development of long-term synaptic plasticity in the rat dorsolateral striatum. J. Neurosci. 27, 2403–2409 (2007).
Zhu, P. J. & Lovinger, D. M. Developmental alteration of endocannabinoid retrograde signaling in the hippocampus. J. Neurophysiol. 103, 1123–1129 (2010).
Yasuda, H., Huang, Y. & Tsumoto, T. Regulation of excitability and plasticity by endocannabinoids and PKA in developing hippocampus. Proc. Natl Acad. Sci. USA 105, 3106–3111 (2008).
Arevalo-Martin, A. et al. Cannabinoids modulate OLIG2 and polysialylated neural cell adhesion molecule expression in the subventricular zone of post-natal rats through cannabinoid receptor 1 and cannabinoid receptor 2. Eur. J. Neurosci. 26, 1548–1559 (2007).
Gomez, O. et al. The constitutive production of the endocannabinoid 2-arachidonoylglycerol participates in oligodendrocyte differentiation. Glia 58, 1913–1927 (2010).
Molina-Holgado, E. et al. Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/AKT signaling. J. Neurosci. 22, 9742–9753 (2002).
Molina-Holgado, F. et al. CB2 cannabinoid receptors promote mouse neural stem cell proliferation. Eur. J. Neurosci. 25, 629–634 (2007).
Sun, J. et al. WIN55, 212–212 promotes differentiation of oligodendrocyte precursor cells and improve remyelination through regulation of the phosphorylation level of the ERK 1/2 via cannabinoid receptor 1 after stroke-induced demyelination. Brain Res. 1491, 225–235 (2013).
Soltys, J., Yushak, M. & Mao-Draayer, Y. Regulation of neural progenitor cell fate by anandamide. Biochem. Biophys. Res. Commun. 400, 21–26 (2010).
Shinjyo, N., Piscitelli, F., Verde, R. & Di, M. V. Impact of ω6 polyunsaturated fatty acid supplementation and γ-aminobutyric acid on astrogliogenesis through the endocannabinoid system. J. Neurosci. Res. 91, 943–953 (2013).
Gomez Del Pulgar, T., de Ceballos, M. L., Guzman, M. & Velasco, G. Cannabinoids protect astrocytes from ceramide-induced apoptosis through the phosphatidylinositol-3 kinase/protein kinase B pathway. J. Biol. Chem. 277, 36527–36533 (2002).
Gomez, O. et al. Cannabinoid receptor agonists modulate oligodendrocyte differentiation by activating PI3K/AKT and the mammalian target of rapamycin (mTOR) pathways. Br. J. Pharmacol. 163, 1520–1532 (2011).
Fernandez-Lopez, D. et al. The cannabinoid WIN55,212-2 promotes neural repair after neonatal hypoxia-ischemia. Stroke 41, 2956–2964 (2010).
Solbrig, M. V., Fan, Y., Hermanowicz, N., Morgese, M. G. & Giuffrida, A. A synthetic cannabinoid agonist promotes oligodendrogliogenesis during viral encephalitis in rats. Exp. Neurol. 226, 231–241 (2010).
Compagnucci, C. et al. Type1 (CB1) cannabinoid receptor promotes neuronal differentiation and maturation of neural stem cells. PLoS ONE. 8, e54271 (2013).
Xapelli, S. et al. Activation of type 1 cannabinoid receptor (CB1R) promotes neurogenesis in murine subventricular zone cell cultures. PLoS ONE. 8, e63529 (2013).
Xapelli, S. et al. Modulation of subventricular zone oligodendrogenesis: a role for hemopressin? Front. Cell Neurosci. 8, 59 (2014).
Zimmer, A., Zimmer, A. M., Hohmann, A. G., Herkenham, M. & Bonner, T. I. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc. Natl Acad. Sci. USA 96, 5780–5785 (1999). This paper describes the initial characterization of CB1R-null mice, showing their premature death during aging. However, this study failed to identify developmental abnormalities.
Saez, T. M., Aronne, M. P., Caltana, L. & Brusco, A. H. Prenatal exposure to the CB and CB cannabinoid receptor agonist WIN 55, 212–212 alters migration of early-born glutamatergic neurons and GABAergic interneurons in the rat cerebral cortex. J. Neurochem. 129, 637–648 (2013).
Antonelli, T. et al. Long-term effects on cortical glutamate release induced by prenatal exposure to the cannabinoid receptor agonist (R)-(+)-[2,3dihydro-5methyl3-(4morpholinyl-methyl)pyrrolo[1,2,3de]-1, 4benzoxazin-6yl]-1naphthalenylmethanone: an in vivo microdialysis study in the awake rat. Neuroscience 124, 367–375 (2004).
Mereu, G. et al. Prenatal exposure to a cannabinoid agonist produces memory deficits linked to dysfunction in hippocampal long-term potentiation and glutamate release. Proc. Natl Acad. Sci. USA 100, 4915–4920 (2003).
Antonelli, T. et al. Prenatal exposure to the CB1 receptor agonist WIN 55, 212-2 causes learning disruption associated with impaired cortical NMDA receptor function and emotional reactivity changes in rat offspring. Cereb. Cortex 15, 2013–2020 (2005).
Schlosburg, J. E. et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nature Neurosci. 13, 1113–1119 (2010).
Wu, C. S. et al. Long-term consequences of perinatal fatty acid amino hydrolase inhibition. Br. J. Pharmacol. 171, 1420–1434 (2014).
Wu, C. S. et al. GPR55, a G-protein coupled receptor for lysophosphatidylinositol, plays a role in motor coordination. PLoS ONE. 8, e60314 (2013).
Huizink, A. C. & Mulder, E. J. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci. Biobehav. Rev. 30, 24–41 (2006).
Goldschmidt, L., Richardson, G. A., Cornelius, M. D. & Day, N. L. Prenatal marijuana and alcohol exposure and academic achievement at age 10. Neurotoxicol. Teratol. 26, 521–532 (2004).
Castaldo, P. et al. Altered regulation of glutamate release and decreased functional activity and expression of GLT1 and GLAST glutamate transporters in the hippocampus of adolescent rats perinatally exposed to Δ9-THC. Pharmacol. Res. 61, 334–341 (2010).
Spano, M. S., Ellgren, M., Wang, X. & Hurd, Y. L. Prenatal cannabis exposure increases heroin seeking with allostatic changes in limbic enkephalin systems in adulthood. Biol. Psychiatry 61, 554–563 (2007).
Szutorisz, H. et al. Parental THC exposure leads to compulsive heroin-seeking and altered striatal synaptic plasticity in the subsequent generation. Neuropsychopharmacology 39, 1315–1323 (2014).
Wang, X., Dow-Edwards, D., Anderson, V., Minkoff, H. & Hurd, Y. L. Discrete opioid gene expression impairment in the human fetal brain associated with maternal marijuana use. Pharmacogenomics J. 6, 255–264 (2006).
Jourdain, L., Curmi, P., Sobel, A., Pantaloni, D. & Carlier, M. F. Stathmin: a tubulin-sequestering protein which forms a ternary T2S complex with two tubulin molecules. Biochemistry 36, 10817–10821 (1997).
Shin, J. E. et al. SCG10 is a JNK target in the axonal degeneration pathway. Proc. Natl Acad. Sci. USA 109, E3696–E3705 (2012).
Fride, E. et al. Inhibition of milk ingestion and growth after administration of a neutral cannabinoid CB1 receptor antagonist on the first postnatal day in the mouse. Pediatr. Res. 62, 533–536 (2007).
O'Shea, M. & Mallet, P. E. Impaired learning in adulthood following neonatal Δ9-THC exposure. Behav. Pharmacol. 16, 455–461 (2005).
Rubino, T. et al. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus 19, 763–772 (2009).
Zamberletti, E. et al. Alterations of prefrontal cortex GABAergic transmission in the complex psychotic-like phenotype induced by adolescent Δ9-tetrahydrocannabinol exposure in rats. Neurobiol. Dis. 63, 35–47 (2014).
Eggan, S. M., Stoyak, S. R., Verrico, C. D. & Lewis, D. A. Cannabinoid CB1 receptor immunoreactivity in the prefrontal cortex: comparison of schizophrenia and major depressive disorder. Neuropsychopharmacology 35, 2060–2071 (2010).
Lewis, D. A., Cruz, D., Eggan, S. & Erickson, S. Postnatal development of prefrontal inhibitory circuits and the pathophysiology of cognitive dysfunction in schizophrenia. Ann. NY Acad. Sci. 1021, 64–76 (2004).
Jung, K. M. et al. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nature Commun. 3, 1080 (2012).
Lie, D. C., Song, H., Colamarino, S. A., Ming, G. L. & Gage, F. H. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu. Rev. Pharmacol. Toxicol. 44, 399–421 (2004).
Deng, W., Aimone, J. B. & Gage, F. H. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nature Rev. Neurosci. 11, 339–350 (2010).
Lois, C. & Alvarez-Buylla, A. Long-distance neuronal migration in the adult mammalian brain. Science 264, 1145–1148 (1994).
Sanai, N. et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 478, 382–386 (2011).
Ernst, A. et al. Neurogenesis in the striatum of the adult human brain. Cell 156, 1072–1083 (2014).
Arvidsson, A., Collin, T., Kirik, D., Kokaia, Z. & Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nature Med. 8, 963–970 (2002).
Butti, E. et al. Subventricular zone neural progenitors protect striatal neurons from glutamatergic excitotoxicity. Brain 135, 3320–3335 (2012).
Jin, K. et al. Neurogenesis and aging: FGF2 and HBEGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell 2, 175–183 (2003).
Kim, J. et al. GDF11 controls the timing of progenitor cell competence in developing retina. Science 308, 1927–1930 (2005).
Wu, H. H. et al. Autoregulation of neurogenesis by GDF11. Neuron 37, 197–207 (2003).
Williams, G. et al. Transcriptional basis for the inhibition of neural stem cell proliferation and migration by the TGFβ-family member GDF11. PLoS ONE. 8, e78478 (2013).
Goncalves, M. B. et al. The COX2 inhibitors, meloxicam and nimesulide, suppress neurogenesis in the adult mouse brain. Br. J. Pharmacol. 159, 1118–1125 (2010).
Shigemoto-Mogami, Y., Hoshikawa, K., Goldman, J. E., Sekino, Y. & Sato, K. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J. Neurosci. 34, 2231–2243 (2014).
Sutterlin, P. et al. The molecular basis of the cooperation between EGF, FGF and eCB receptors in the regulation of neural stem cell function. Mol. Cell Neurosci. 52, 20–30 (2013).
Zhao, C., Deng, W. & Gage, F. H. Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660 (2008).
Santarelli, L. et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809 (2003).
Li, G. L. et al. Assessment of the pharmacology and tolerability of PF04457845, an irreversible inhibitor of fatty acid amide hydrolase-1, in healthy subjects. Br. J. Clin. Pharmacol. 73, 706–716 (2012).
Kitambi, S. S. et al. Vulnerability of glioblastoma cells to catastrophic vacuolization and death induced by a small molecule. Cell http://dx.doi.org/10.1016/j.cell.2014.02.021 (2014).
Velasco, G., Sanchez, C. & Guzman, M. Towards the use of cannabinoids as antitumour agents. Nature Rev. Cancer 12, 436–444 (2012).
Blazquez, C. et al. Inhibition of tumor angiogenesis by cannabinoids. FASEB J. 17, 529–531 (2003).
Ramer, R. & Hinz, B. Inhibition of cancer cell invasion by cannabinoids via increased expression of tissue inhibitor of matrix metalloproteinases-1. J. Natl Cancer Inst. 100, 59–69 (2008).
Blazquez, C. et al. Cannabinoids inhibit glioma cell invasion by down-regulating matrix metalloproteinase-2 expression. Cancer Res. 68, 1945–1952 (2008).
Torres, S. et al. A combined preclinical therapy of cannabinoids and temozolomide against glioma. Mol. Cancer Ther. 10, 90–103 (2011).
Guzman, M. et al. A pilot clinical study of Δ9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br. J. Cancer 95, 197–203 (2006).
Sanchez, C. et al. Inhibition of glioma growth in vivo by selective activation of the CB2 cannabinoid receptor. Cancer Res. 61, 5784–5789 (2001).
Carracedo, A. et al. The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell 9, 301–312 (2006). This paper defines transcriptional elements by which cannabinoids can trigger glioma cell death. It also highlights the relevance and the efficacy of phytocannabinoids to cancer therapy.
Salazar, M. et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Invest. 119, 1359–1372 (2009).
Pasquariello, N. et al. Characterization of the endocannabinoid system in human neuronal cells and proteomic analysis of anandamide-induced apoptosis. J. Biol. Chem. 284, 29413–29426 (2009).
Galve-Roperh, I. et al. Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nature Med. 6, 313–319 (2000).
Lorente, M. et al. Stimulation of the midkine/ALK axis renders glioma cells resistant to cannabinoid antitumoral action. Cell Death. Differ. 18, 959–973 (2011).
Cudaback, E., Marrs, W., Moeller, T. & Stella, N. The expression level of CB1 and CB2 receptors determines their efficacy at inducing apoptosis in astrocytomas. PLoS ONE. 5, e8702 (2010).
Volkow, N. D., Baler, R. D., Compton, W. M. & Weiss, S. R. Adverse health effects of marijuana use. N. Engl. J. Med. 370, 2219–2227 (2014).
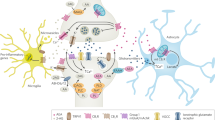
Han, J. et al. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 148, 1039–1050 (2012).
Navarrete, M. & Araque, A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68, 113–126 (2010).
Morozov, Y. M. et al. Antibodies to cannabinoid type 1 receptor coreact with stomatin-like protein 2 in mouse brain mitochondria. Eur. J. Neurosci. 38, 2341–2348 (2013).
Eggan, S. M., Hashimoto, T. & Lewis, D. A. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch. Gen. Psychiatry 65, 772–784 (2008).
Veen, N. D. et al. Cannabis use and age at onset of schizophrenia. Am. J. Psychiatry 161, 501–506 (2004).
Monteleone, P. et al. Investigation of CNR1 and FAAH endocannabinoid gene polymorphisms in bipolar disorder and major depression. Pharmacol. Res. 61, 400–404 (2010).
Krakowiak, P. et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 129, e1121–e1128 (2012).
Di Marzo, V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia 51, 1356–1367 (2008).
Cristino, L. et al. Obesity-driven synaptic remodeling affects endocannabinoid control of orexinergic neurons. Proc. Natl Acad. Sci. USA 110, E2229–E2238 (2013).
Reisenberg, M., Singh, P. K., Williams, G. & Doherty, P. The diacylglycerol lipases: structure, regulation and roles in and beyond endocannabinoid signalling. Phil. Trans. R. Soc. B 367, 3264–3275 (2012).
Kaczocha, M., Glaser, S. T. & Deutsch, D. G. Identification of intracellular carriers for the endocannabinoid anandamide. Proc. Natl Acad. Sci. USA 106, 6375–6380 (2009).
Oddi, S. et al. Molecular identification of albumin and HSP70 as cytosolic anandamide-binding proteins. Chem. Biol. 16, 624–632 (2009).
Fu, J. et al. A catalytically silent FAAH1 variant drives anandamide transport in neurons. Nature Neurosci. 15, 64–69 (2012).
Oddi, S. et al. Evidence for the intracellular accumulation of anandamide in adiposomes. Cell. Mol. Life Sci. 65, 840–850 (2008).
Viscomi, M. T. et al. Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/AKT pathway. J. Neurosci. 29, 4564–4570 (2009).
Alpar, A. et al. Endocannabinoids modulate cortical development by configuring SLIT2/ROBO1 signalling. Nature Commun. 5, 4421 (2014).
Katona, I. et al. Molecular composition of the endocannabinoid system at glutamatergic synapses. J. Neurosci. 26, 5628–5637 (2006).
Tamamaki, N. et al. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67–GFP knockin mouse. J. Comp. Neurol. 467, 60–79 (2003).
Goebbels, S. et al. Genetic targeting of principal neurons in neocortex and hippocampus of NEX–Cre mice. Genesis. 44, 611–621 (2006).
Trazzi, S., Steger, M., Mitrugno, V. M., Bartesaghi, R. & Ciani, E. CB1 cannabinoid receptors increase neuronal precursor proliferation through AKT/glycogen synthase kinase3β/β-catenin signaling. J. Biol. Chem. 285, 10098–10109 (2010).
Cravatt, B. F. et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc. Natl Acad. Sci. USA 98, 9371–9376 (2001).
Jin, K. et al. Defective adult neurogenesis in CB1 cannabinoid receptor knockout mice. Mol. Pharmacol. 66, 204–208 (2004).
Acknowledgements
This work was supported by the Ministero dell'Istruzione, dell'Università e della Ricerca (PRIN 2010–2011 grant to M.M.), Swedish Medical Research Council (to T.H.), Novo Nordisk Foundation (to T.H.), Petrus and Augusta Hedlunds' Foundation (to T.H.), the Wellcome Trust (to P.D.), the Spanish Ministry of Economy and Competitiveness (grant SAF201235759 to M.G.), the European Commission (FP7 'PAINCAGE' integrated project to T.H.) and the National Institutes of Health (grant DA023214 to T.H., and grants DA011322 and DA021696 to K.M.). The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health. M.M. dedicates this Review to his late father, Giuseppe.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Morphogenetic signals
-
Gradients of molecules that determine the position of specialized cellular subtypes and instruct their communication and functional role during histogenesis.
- Sphingolipids
-
Molecules that contain the organic aliphatic amino alcohol sphingosine or a structurally similar molecule as a backbone.
- Endocannabinoids
-
Endogenous compounds that bind to cannabinoid 1 receptors (CB1Rs) and/or CB2Rs with high affinity, and are able to evoke a Δ9-tetrahydrocannabinol-like behavioural tetrad.
- sn-1-diacylglycerol lipase-α
-
(DAGLα). Two isoforms of DAGL, DAGLα and DAGLβ, which probably evolved evolutionarily through gene duplication, are chiefly responsible for 2-AG synthesis.
- Tripartite synapse
-
A concept of synaptic anatomy that includes the presynaptic terminal, postsynaptic specialization and astroglial end-feet that isolate the synaptic cleft as a discrete entity.
- Subventricular zone
-
A progenitor cell-rich part of the cerebral cortex that lines the dorsolateral surface of the lateral ventricle and retains neurogenic capacity throughout life.
- Corticothalamic–thalamocortical handshake
-
Where corticothalamic and thalamocortical axons with opposite growth trajectories cross one another.
- Cytoskeletal instability
-
The dynamic reorganization of the cytoskeleton by differentially altering the elongation and shortening of the '+' and '−' ends of mictotubules.
- Fasciculation
-
The process by which developing axons follow a primary pioneer axon, thus maintaining strict directionality towards a distal target.
- Neuroblast
-
A postmitotic cell in an undifferentiated form migrating towards its final position, where it becomes a neuron.
- Functional antagonist
-
A drug that is pharmacologically classified as an agonist; it induces a phenotype similar to that provoked by an antagonist, often by downregulation of signalling.
- Psychoactive component
-
The molecular component of a plant extract that can provoke acute changes in perception of behaviour.
- Ceramide
-
A molecule that consists of sphingosine and a fatty acyl chain; it is therefore one of the structurally simplest forms of sphingolipids.
Rights and permissions
About this article
Cite this article
Maccarrone, M., Guzmán, M., Mackie, K. et al. Programming of neural cells by (endo)cannabinoids: from physiological rules to emerging therapies. Nat Rev Neurosci 15, 786–801 (2014). https://doi.org/10.1038/nrn3846
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3846
This article is cited by
-
Cannabisgebrauch bei Jugendlichen
neuropsychiatrie (2023)
-
The Role of Cannabinoids in CNS Development: Focus on Proliferation and Cell Death
Cellular and Molecular Neurobiology (2023)
-
Deciphering lipid dysregulation in ALS: from mechanisms to translational medicine
Translational Neurodegeneration (2022)
-
A GPCR-based yeast biosensor for biomedical, biotechnological, and point-of-use cannabinoid determination
Nature Communications (2022)
-
Chronic adolescent exposure to cannabis in mice leads to sex-biased changes in gene expression networks across brain regions
Neuropsychopharmacology (2022)