Key Points
-
There is a growing appreciation that the gut microbiota plays a key role in maintaining homeostasis and that a disruption in its composition contributes to various disease states, including CNS disorders.
-
The concept of a microbiota–gut–brain axis, although debated, is emerging to capture the importance that the microbiota has on regulating bidirectional gut–brain communication pathways.
-
It is clear that stress, including stress in early life, can alter microbiota composition and this can have marked consequences on physiology in adulthood.
-
Studies in germ-free animals and in animals exposed to pathogenic bacterial infections, probiotic bacteria or antibiotic drugs suggest a role for the gut microbiota in the regulation of anxiety, mood, cognition and pain.
-
Although not as conceptually or empirically developed, the gut microbiota has also been implicated in obesity, autism and multiple sclerosis.
-
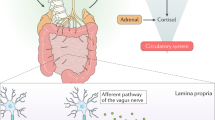
Mechanisms as to how the microbiota are affecting gut–brain signalling are only now being unravelled. These mechanisms may include alterations in microbial composition, immune activation, vagus nerve signalling, alterations in tryptophan metabolism, production of specific microbial neuroactive metabolites and bacterial cell wall sugars.
-
Harnessing such mechanisms may pave the way for microbial-based therapeutics for various CNS disorders.
Abstract
Recent years have witnessed the rise of the gut microbiota as a major topic of research interest in biology. Studies are revealing how variations and changes in the composition of the gut microbiota influence normal physiology and contribute to diseases ranging from inflammation to obesity. Accumulating data now indicate that the gut microbiota also communicates with the CNS — possibly through neural, endocrine and immune pathways — and thereby influences brain function and behaviour. Studies in germ-free animals and in animals exposed to pathogenic bacterial infections, probiotic bacteria or antibiotic drugs suggest a role for the gut microbiota in the regulation of anxiety, mood, cognition and pain. Thus, the emerging concept of a microbiota–gut–brain axis suggests that modulation of the gut microbiota may be a tractable strategy for developing novel therapeutics for complex CNS disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sekirov, I., Russell, S. L., Antunes, L. C. & Finlay, B. B. Gut microbiota in health and disease. Physiol. Rev. 90, 859–904 (2010).
Clemente, J. C., Ursell, L. K., Parfrey, L. W. & Knight, R. The impact of the gut microbiota on human health: an integrative view. Cell 148, 1258–1270 (2012).
Human Microbiome Project Consortium. A framework for human microbiome research. Nature 486, 215–221 (2012).
Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Banks, W. A. The blood–brain barrier: connecting the gut and the brain. Regul. Pept. 149, 11–14 (2008).
Mayer, E. A. Gut feelings: the emerging biology of gut–brain communication. Nature Rev. Neurosci. 12, 453–466 (2011). A comprehensive recent review of the underlying neurobiology and bidirectional nature of the gut–brain axis.
Aziz, Q. & Thompson, D. G. Brain–gut axis in health and disease. Gastroenterology 114, 559–578 (1998).
Tache, Y., Vale, W., Rivier, J. & Brown, M. Brain regulation of gastric secretion: influence of neuropeptides. Proc. Natl Acad. Sci. USA 77, 5515–5519 (1980).
Konturek, S. J., Konturek, J. W., Pawlik, T. & Brzozowki, T. Brain–gut axis and its role in the control of food intake. J. Physiol. Pharmacol. 55, 137–154 (2004).
Rhee, S. H., Pothoulakis, C. & Mayer, E. A. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nature Rev. Gastroenterol. Hepatol. 6, 306–314 (2009). One of the first papers to formalize the concept of a microbiota–gut–brain axis.
Reber, S. O. Stress and animal models of inflammatory bowel disease — an update on the role of the hypothalamo–pituitary–adrenal axis. Psychoneuroendocrinology 37, 1–19 (2012).
Gill, S. R. et al. Metagenomic analysis of the human distal gut microbiome. Science 312, 1355–1359 (2006).
Qin, J. et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 (2010).
O'Hara, A. M. & Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 7, 688–693 (2006).
Backhed, F., Ley, R. E., Sonnenburg, J. L., Peterson, D. A. & Gordon, J. I. Host–bacterial mutualism in the human intestine. Science 307, 1915–1920 (2005).
Round, J. L., O'Connell, R. M. & Mazmanian, S. K. Coordination of tolerogenic immune responses by the commensal microbiota. J. Autoimmun. 34, J220–J225 (2010).
Olszak, T. et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 336, 489–493 (2012).
Backhed, F. et al. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl Acad. Sci. USA 101, 15718–15723 (2004).
Bercik, P., Collins, S. M. & Verdu, E. F. Microbes and the gut–brain axis. Neurogastroenterol. Motil. 24, 405–413 (2012).
Fraher, M. H., O'Toole, P. W. & Quigley, E. M. Techniques used to characterize the gut microbiota: a guide for the clinician. Nature Rev. Gastroenterol. Hepatol. 9, 312–322 (2012).
Ley, R. E., Peterson, D. A. & Gordon, J. I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124, 837–848 (2006).
Eckburg, P. B. et al. Diversity of the human intestinal microbial flora. Science 308, 1635–1638 (2005).
Grenham, S., Clarke, G., Cryan, J. & Dinan, T. G. Brain–gut–microbe communication in health and disease. Front. Physiol. 2, 94 (2011).
Mackie, R. I., Sghir, A. & Gaskins, H. R. Developmental microbial ecology of the neonatal gastrointestinal tract. Am. J. Clin. Nutr. 69, 1035S–1045S (1999).
Palmer, C., Bik, E. M., DiGiulio, D. B., Relman, D. A. & Brown, P. O. Development of the human infant intestinal microbiota. PLoS Biol. 5, e177 (2007).
Gulati, A. S. et al. Mouse background strain profoundly influences Paneth cell function and intestinal microbial composition. PLoS ONE 7, e32403 (2012).
Arumugam, M. et al. Enterotypes of the human gut microbiome. Nature 473, 174–180 (2011).
Cryan, J. F. & O'Mahony, S. M. The microbiome–gut–brain axis: from bowel to behavior. Neurogastroenterol. Motil. 23, 187–192 (2011).
Wu, S. V. & Hui, H. Treat your bug right. Front. Physiol. 2, 9 (2011).
Forsythe, P., Sudo, N., Dinan, T., Taylor, V. H. & Bienenstock, J. Mood and gut feelings. Brain Behav. Immun. 24, 9–16 (2010).
Claesson, M. J. et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl Acad. Sci. USA 108, 4586–4591 (2011).
Claesson, M. J. et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 488, 178–184 (2012).
Collins, S. M. & Bercik, P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology 136, 2003–2014 (2009).
Tannock, G. W. & Savage, D. C. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect. Immun. 9, 591–598 (1974).
Dinan, T. G. & Cryan, J. F. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology 37, 1369–1378 (2012).
O'Mahony, S. M., Hyland, N. P., Dinan, T. G. & Cryan, J. F. Maternal separation as a model of brain–gut axis dysfunction. Psychopharmacology 214, 71–88 (2011).
Bailey, M. T. & Coe, C. L. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev. Psychobiol. 35, 146–155 (1999).
O'Mahony, S. M. et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 65, 263–267 (2009). An important study demonstrating that stress early in life alters brain–gut axis function and also modifies the relative diversity of the gut microbiota.
Bailey, M. T. et al. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav. Immun. 25, 397–407 (2011). This study is one of the first to show that stress in adulthood modifies the composition of the gut microbiota.
Santos, J., Yang, P. C., Soderholm, J. D., Benjamin, M. & Perdue, M. H. Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut 48, 630–636 (2001).
Soderholm, J. D. & Perdue, M. H. Stress and gastrointestinal tract. II. Stress and intestinal barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G7–G13 (2001).
Zareie, M. et al. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut 55, 1553–1560 (2006).
Ait-Belgnaoui, A. et al. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 25 April 2012 (doi:10.1016/j.psyneuen.2012.03.02).
Maes, M., Kubera, M., Leunis, J. C. & Berk, M. Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J. Affect. Disord. 141, 55–62 (2012).
Gems, D. & Partridge, L. Stress-response hormesis and aging: “that which does not kill us makes us stronger”. Cell. Metab. 7, 200–203 (2008).
Franceschi, C. et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. NY Acad. Sci. 908, 244–254 (2000).
Sudo, N. et al. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J. Physiol. 558, 263–275 (2004). A landmark study showing that germ-free mice have altered HPA axis function, which can be reversed by colonization with specific bacterial strains early in life.
Clarke, G. et al. The microbiome–gut–brain axis during early-life regulates the hippocampal serotonergic system in a gender-dependent manner. Mol. Psychiatry 12 Jun 2012 (doi:10.1038/mp.2012.77).
Heijtz, R. D. et al. Normal gut microbiota modulates brain development and behavior. Proc. Natl Acad. Sci. USA 108, 3047–3052 (2011).
Neufeld, K. M., Kang, N., Bienenstock, J. & Foster, J. A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 23, 255–264 (2010). References 48–50 are important studies linking the gut microbiota to neurodevelopmental processes and behaviour. They independently show that germ-free mice have alterations in concentrations of neurotransmitters and neurotrophic factors in the brain, and have reduced anxiety-like behaviour.
Gareau, M. G. et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 60, 307–317 (2011). One of the first studies to assess cognitive function in germ-free mice, therefore showing that the gut microbiota may be a therapeutic target for cognitive enhancement.
Cryan, J. F. & Sweeney, F. F. The age of anxiety: role of animal models of anxiolytic action in drug discovery. Br. J. Pharmacol. 164, 1129–1161 (2011).
Bergami, M. et al. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc. Natl Acad. Sci. USA 105, 15570–15575 (2008).
Akimova, E., Lanzenberger, R. & Kasper, S. The serotonin-1A receptor in anxiety disorders. Biol. Psychiatry 66, 627–635 (2009).
Barkus, C. et al. Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion. Eur. J. Pharmacol. 626, 49–56 (2010).
Jacobson, L. H. & Cryan, J. F. Feeling strained? Influence of genetic background on depression-related behavior in mice: a review. Behav. Genet. 37, 171–213 (2007).
Benson, A. K. et al. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc. Natl Acad. Sci. USA 107, 18933–18938 (2010).
Esworthy, R. S., Smith, D. D. & Chu, F. F. A. Strong impact of genetic background on gut microflora in mice. Int. J. Inflam. 2010, 986046 (2010).
Kovacs, A. et al. Genotype is a stronger determinant than sex of the mouse gut microbiota. Microb. Ecol. 61, 423–428 (2011).
Bercik, P. et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141, 599–609.e3 (2011). A key study showing the utility of microbiota transplantation in mice to examine the microbiota–gut–brain axis.
Bercik, P. et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 139, 2102–2112.e1 (2010).
Lyte, M., Li, W., Opitz, N., Gaykema, R. & Goehler, L. E. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol. Behav. 89, 350–357 (2006).
Kennedy, P. J. et al. Gut memories: towards a cognitive neurobiology of irritable bowel syndrome. Neurosci. Biobehav. Rev. 36, 310–340 (2012).
O'Malley, D., Quigley, E. M., Dinan, T. G. & Cryan, J. F. Do interactions between stress and immune responses lead to symptom exacerbations in irritable bowel syndrome? Brain Behav. Immun. 25, 1333–1341 (2011).
Gaykema, R. P., Goehler, L. E. & Lyte, M. Brain response to cecal infection with Campylobacter jejuni: analysis with Fos immunohistochemistry. Brain Behav. Immun. 18, 238–245 (2004).
Goehler, L. E., Park, S. M., Opitz, N., Lyte, M. & Gaykema, R. P. Campylobacter jejuni infection increases anxiety-like behavior in the holeboard: possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain Behav. Immun. 22, 354–366 (2008).
Wang, X. et al. Evidences for vagus nerve in maintenance of immune balance and transmission of immune information from gut to brain in STM-infected rats. World J. Gastroenterol. 8, 540–545 (2002).
Gareau, M. G., Sherman, P. M. & Walker, W. A. Probiotics and the gut microbiota in intestinal health and disease. Nature Rev. Gastroenterol. Hepatol. 7, 503–514 (2010).
Quigley, E. M. Probiotics in functional gastrointestinal disorders: what are the facts? Curr. Opin. Pharmacol. 8, 704–708 (2008).
Clarke, G., Cryan, J. F., Dinan, T. G. & Quigley, E. M. Review article: probiotics for the treatment of irritable bowel syndrome — focus on lactic acid bacteria. Aliment. Pharmacol. Ther. 35, 403–413 (2012).
Logan, A. C. & Katzman, M. Major depressive disorder: probiotics may be an adjuvant therapy. Med. Hypotheses 64, 533–538 (2005).
Rao, S., Srinivasjois, R. & Patole, S. Prebiotic supplementation in full-term neonates: a systematic review of randomized controlled trials. Arch. Pediatr. Adolesc. Med. 163, 755–764 (2009).
Messaoudi, M. et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 105, 755–764 (2011). One of the first human studies assessing the psychotropic-like effects of probiotics.
Arseneault-Breard, J. et al. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br. J. Nutr. 107, 1793–1799 (2012).
Bravo, J. A. et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl Acad. Sci. USA 108, 16050–16055 (2011). An important study demonstrating the ability of a potential probiotic to modify the stress response, behaviours relevant to anxiety, depression and cognition and alter central levels of GABA receptors. Moreover, it demonstrates that these effects are dependent on the vagus nerve.
Bercik, P. et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut–brain communication. Neurogastroenterol. Motil. 23, 1132–1139 (2011).
Ma, X. et al. Lactobacillus reuteri ingestion prevents hyperexcitability of colonic DRG neurons induced by noxious stimuli. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G868–G875 (2009).
Kunze, W. A. et al. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J. Cell. Mol. Med. 13, 2261–2270 (2009).
Tanida, M. et al. Effects of intraduodenal injection of Lactobacillus johnsonii La1 on renal sympathetic nerve activity and blood pressure in urethane-anesthetized rats. Neurosci. Lett. 389, 109–114 (2005).
Maes, M. et al. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 10, 66 (2012).
Desbonnet, L. et al. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 170, 1179–1188 (2010).
Desbonnet, L., Garrett, L., Clarke, G., Bienenstock, J. & Dinan, T. G. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 43, 164–174 (2008).
Wall, R. et al. Contrasting effects of Bifidobacterium breve NCIMB 702258 and Bifidobacterium breve DPC 6330 on the composition of murine brain fatty acids and gut microbiota. Am. J. Clin. Nutr. 95, 1278–1287 (2012).
Innis, S. M. Dietary (n-3) fatty acids and brain development. J. Nutr. 137, 855–859 (2007).
Rapoport, S. I. Brain arachidonic and docosahexaenoic acid cascades are selectively altered by drugs, diet and disease. Prostaglandins Leukot. Essent. Fatty Acids 79, 153–156 (2008).
Luchtman, D. W. & Song, C. Cognitive enhancement by omega-3 fatty acids from child-hood to old age: findings from animal and clinical studies. Neuropharmacology 27 Jul 2012 (doi:10.1016/j.neuropharm.2012.07.019).
Tillisch, K. et al. Modulation of the brain–gut axis after 4-week intervention with a probiotic fermented dairy product. Gastroenterology 142, S-115 (2012).
Craig, A. D. How do you feel — now? The anterior insula and human awareness. Nature Rev. Neurosci. 10, 59–70 (2009).
Paulus, M. P. & Stein, M. B. An insular view of anxiety. Biol. Psychiatry 60, 383–387 (2006).
Verdu, E. F. et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut 55, 182–190 (2006).
Larauche, M., Mulak, A. & Tache, Y. Stress and visceral pain: from animal models to clinical therapies. Exp. Neurol. 233, 49–67 (2012).
Mayer, E. A. et al. Functional GI disorders: from animal models to drug development. Gut 57, 384–404 (2008).
Mertz, H. et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology 118, 842–848 (2000).
Gibney, S. M., Gosselin, R. D., Dinan, T. G. & Cryan, J. F. Colorectal distension-induced prefrontal cortex activation in the Wistar–Kyoto rat: implications for irritable bowel syndrome. Neuroscience 165, 675–683 (2010).
O'Mahony, C. M., Sweeney, F. F., Daly, E., Dinan, T. G. & Cryan, J. F. Restraint stress-induced brain activation patterns in two strains of mice differing in their anxiety behaviour. Behav. Brain Res. 213, 148–154 (2010).
Wang, Z. et al. Regional brain activation in conscious, nonrestrained rats in response to noxious visceral stimulation. Pain 138, 233–243 (2008).
Gareau, M. G., Jury, J., MacQueen, G., Sherman, P. M. & Perdue, M. H. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut 56, 1522–1528 (2007).
McKernan, D. P., Fitzgerald, P., Dinan, T. G. & Cryan, J. F. The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroenterol. Motil. 22, 1029–1035 (2010).
Rousseaux, C. et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nature Med. 13, 35–37 (2007).
Ait-Belgnaoui, A. et al. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: a possible action through interaction with epithelial cell cytoskeleton contraction. Gut 55, 1090–1094 (2006).
Johnson, A. C., Greenwood- Van Meerveld, B. & McRorie, J. Effects of Bifidobacterium infantis 35624 on post-inflammatory visceral hypersensitivity in the rat. Dig. Dis. Sci. 56, 3179–3186 (2011).
Wang, B. et al. Lactobacillus reuteri ingestion and IKCa channel blockade have similar effects on rat colon motility and myenteric neurones. Neurogastroenterol. Motil. 22, 98–107 (2010).
de Theije, C.G. et al. Pathways underlying the gut-to-brain connection in autism spectrum disorders as future targets for disease management. Eur. J. Pharmacol. 668, S70–S80 (2011).
Williams, B. L., Hornig, M., Parekh, T. & Lipkin, W. I. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. MBio 3, e00261–e00211 (2012).
Finegold, S. M. et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 16, 444–453 (2010).
Finegold, S. M. et al. Gastrointestinal microflora studies in late-onset autism. Clin. Infect. Dis. 35, S6–S16 (2002).
Parracho, H. M., Bingham, M. O., Gibson, G. R. & McCartney, A. L. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 54, 987–991 (2005).
Adams, J. B., Johansen, L. J., Powell, L. D., Quig, D. & Rubin, R. A. Gastrointestinal flora and gastrointestinal status in children with autism — comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 11, 22 (2011).
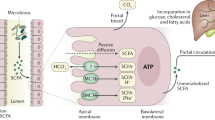
Wang, L. et al. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig. Dis. Sci. 57, 2096–2102 (2012).
Thomas, R. H. et al. The enteric bacterial metabolite propionic acid alters brain and plasma phospholipid molecular species: further development of a rodent model of autism spectrum disorders. J. Neuroinflamm. 9, 153 (2012).
MacFabe, D. F., Cain, N. E., Boon, F., Ossenkopp, K. P. & Cain, D. P. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: relevance to autism spectrum disorder. Behav. Brain Res. 217, 47–54 (2011).
Sandler, R. H. et al. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J. Child Neurol. 15, 429–435 (2000).
Turnbaugh, P. J. & Gordon, J. I. The core gut microbiome, energy balance and obesity. J. Physiol. 587, 4153–4158 (2009).
Backhed, F., Manchester, J. K., Semenkovich, C. F. & Gordon, J. I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl Acad. Sci. USA 104, 979–984 (2007).
Morton, G. J., Cummings, D. E., Baskin, D. G., Barsh, G. S. & Schwartz, M. W. Central nervous system control of food intake and body weight. Nature 443, 289–295 (2006).
Schellekens, H., Finger, B. C., Dinan, T. G. & Cryan, J. F. Ghrelin signalling and obesity: at the interface of stress, mood and food reward. Pharmacol. Ther. 135, 316–326 (2012).
Manco, M. Gut microbiota and developmental programming of the brain: from evidence in behavioral endophenotypes to novel perspective in obesity. Front. Cell. Infect. Microbiol. 2, 109 (2012).
Davey, K. J. et al. Gender-dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology 221, 155–169 (2012).
Berer, K. et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541 (2011).
Lee, Y. K., Menezes, J. S., Umesaki, Y. & Mazmanian, S. K. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 108, 4615–4622 (2011).
O'Toole, P. W. & Cooney, J. C. Probiotic bacteria influence the composition and function of the intestinal microbiota. Interdiscip. Perspect. Infect. Dis. 2008, 175285 (2008).
Forsythe, P. & Bienenstock, J. Immunomodulation by commensal and probiotic bacteria. Immunol. Invest. 39, 429–448 (2010).
Duerkop, B. A., Vaishnava, S. & Hooper, L. V. Immune responses to the microbiota at the intestinal mucosal surface. Immunity 31, 368–376 (2009).
Sternberg, E. M. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nature Rev. Immunol. 6, 318–328 (2006).
Dantzer, R., O'Connor, J. C., Freund, G. G., Johnson, R. W. & Kelley, K. W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Rev. Neurosci. 9, 46–56 (2008).
Wang, H. et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421, 384–388 (2003).
Thayer, J. F. & Sternberg, E. M. Neural concomitants of immunity-focus on the vagus nerve. Neuroimage 47, 908–910 (2009).
de Lartigue, G., de La Serre, C. B. & Raybould, H. E. Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol. Behav. 105, 100–105 (2011).
Ruddick, J. P. et al. Tryptophan metabolism in the central nervous system: medical implications. Expert Rev. Mol. Med. 8, 1–27 (2006).
Clarke, G. et al. Tryptophan degradation in irritable bowel syndrome: evidence of indoleamine 2,3-dioxygenase activation in a male cohort. BMC Gastroenterol. 9, 6 (2009).
Nicholson, J. K. et al. Host–gut microbiota metabolic interactions. Science 336, 1262–1267 (2012).
Gundersen, B. B. & Blendy, J. A. Effects of the histone deacetylase inhibitor sodium butyrate in models of depression and anxiety. Neuropharmacology 57, 67–74 (2009).
Lyte, M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays 33, 574–581 (2011).
Matur, E. & Eraslan, E. in New Advances in the Basic and Clinical Gastroenterology (ed. Brzozowski, T. ) (InTech, 2012).
Barrett, E., Ross, R. P., O'Toole, P. W., Fitzgerald, G. F. & Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 113, 411–417 (2012).
Forsythe, P. & Kunze, W. A. Voices from within: gut microbes and the CNS. Cell. Mol. Life Sci. 26 May 2012 (doI:10.1007/s00018-012-1028-z).
Fanning, S. et al. Bifidobacterial surface-exopolysaccharide facilitates commensal–host interaction through immune modulation and pathogen protection. Proc. Natl Acad. Sci. USA 109, 2108–2113 (2012).
Acknowledgements
The authors thank M. Julio-Pieper at Imágenes Ciencia for assistance with figures, and G. Clarke and L. Desbonnet for helpful comments on the paper. The Alimentary Pharmabiotic Centre is a research centre funded by Science Foundation Ireland (SFI), through the Irish Government's National Development Plan. The authors and their work were supported by SFI (grant numbers 02/CE/B124 and 07/CE/B1368).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Microbiota
-
The collection of microorganisms in a particular habitat, such as the microbiota of the skin or gut.
- Stress response
-
The name given to the hormonal and metabolic changes that follow exposure to a threat. It involves the activation of the hypothalamus–pituitary–adrenal axis.
- Microbiome
-
The collective genomes of all of the microorganisms in a microbiota.
- Hypothalamus–pituitary–adrenal (HPA) axis
-
The HPA axis is the endocrine core of the stress system. Its activation results in the release of corticotropin-releasing factor from the hypothalamus, adrenocorticotropic hormone from the pituitary and cortisol (corticosterone in rats and mice) from the adrenal glands.
- Maternal separation
-
A model of stress in early life. Isolation of pups from their mother in early life alters maternal behaviour upon being reunited and results in permanent changes in brain and behaviour in the offspring.
- Probiotic
-
A living microorganism that, when ingested by humans or animals, can beneficially influence health.
- Inflamm-ageing
-
A neologism to reflect the concept that ageing is accompanied by a global reduction in the capacity to cope with various stressors and a concomitant progressive increase in pro-inflammatory status.
- Mono-association
-
The inoculation of germ-free animals with a specific bacterium.
- Bacteriocins
-
Proteinaceous toxins produced by bacteria to inhibit the growth of similar or closely related bacterial strain(s).
- Colonic AH neurons
-
The major intrinsic sensory neurons in the colon. They are termed AH owing to their common electrophysiological properties whereby action potentials are followed by prolonged and substantial after-hyperpolarizing (AH) potentials.
- Dysbiosis
-
A microbial imbalance on or within the body, often localized to the gut.
- Colorectal distension
-
A method for assessing visceral hypersensitivity. It is a noxious visceral stimulus that can be used in studies performed in animals and humans.
Rights and permissions
About this article
Cite this article
Cryan, J., Dinan, T. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13, 701–712 (2012). https://doi.org/10.1038/nrn3346
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3346
This article is cited by
-
Pathophysiology of acute lung injury in patients with acute brain injury: the triple-hit hypothesis
Critical Care (2024)
-
The role of gut microbiota in human metabolism and inflammatory diseases: a focus on elderly individuals
Annals of Microbiology (2024)
-
Comparative effect of selected caloric and non-caloric sweeteners on some neuroinflammatory indices in brain cortex and hippocampus of scopolamine-induced rat
Nutrire (2024)
-
Gut microbiota variations in wild yellow baboons (Papio cynocephalus) are associated with sex and habitat disturbance
Scientific Reports (2024)
-
Transplantation of gut microbiota derived from patients with schizophrenia induces schizophrenia-like behaviors and dysregulated brain transcript response in mice
Schizophrenia (2024)