Key Points
-
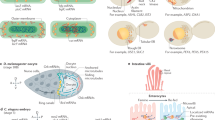
The asymmetrical distribution of mRNAs in cells is used by various organisms to spatially control gene expression.
-
RNA localization has a role in diverse biological processes, such as development, cell motility, neuron connectivity and mating type switching in yeast.
-
Recent technical advents and the development of new methods for mRNA detection in live and fixed cells allow the tracking and quantification of single mRNAs in a variety of cell types.
-
Single-molecule imaging of mRNA in fixed and live cells revealed a complex cooperativity between RNA-binding proteins (RBPs) and motor proteins to regulate active transport of mRNAs.
-
The composition of mRNA–protein (mRNP) complexes is intricate, and future research will reveal how they assemble into RNA granules with unique localization and functions.
-
Neurons and unicellular organisms, such as yeast and bacteria, use both convergent and disparate mechanisms of targeting mRNAs to different regions.
Abstract
The spatial regulation of protein translation is an efficient way to create functional and structural asymmetries in cells. Recent research has furthered our understanding of how individual cells spatially organize protein synthesis, by applying innovative technology to characterize the relationship between mRNAs and their regulatory proteins, single-mRNA trafficking dynamics, physiological effects of abrogating mRNA localization in vivo and for endogenous mRNA labelling. The implementation of new imaging technologies has yielded valuable information on mRNA localization, for example, by observing single molecules in tissues. The emerging movements and localization patterns of mRNAs in morphologically distinct unicellular organisms and in neurons have illuminated shared and specialized mechanisms of mRNA localization, and this information is complemented by transgenic and biochemical techniques that reveal the biological consequences of mRNA mislocalization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
08 July 2015
In the original article, the two reference citations in the following sentence were incorrect: "... conversely, only 600–800 mammalian RBPs have been recognized so far (Refs 88,89; see the RBP database) ...". The correct references have now been added to the online version of this articleas as references 177 and 178.
References
Weatheritt, R. J., Gibson, T. J. & Babu, M. M. Asymmetric mRNA localization contributes to fidelity and sensitivity of spatially localized systems. Nature Struct. Mol. Biol. 21, 833–839 (2014).
Jeffery, W. R., Tomlinson, C. R. & Brodeur, R. D. Localization of actin messenger RNA during early ascidian development. Dev. Biol. 99, 408–417 (1983).
Lawrence, J. B. & Singer, R. H. Intracellular localization of messenger RNAs for cytoskeletal proteins. Cell 45, 407–415 (1986).
Melton, D. A. Translocation of a localized maternal mRNA to the vegetal pole of Xenopus oocytes. Nature 328, 80–82 (1987).
Berleth, T. et al. The role of localization of bicoid RNA in organizing the anterior pattern of the Drosophila embryo. EMBO J. 7, 1749–1756 (1988).
Long, R. M. et al. Mating type switching in yeast controlled by asymmetric localization of ASH1 mRNA. Science 277, 383–387 (1997). This is the first demonstration of mRNA localization in yeast.
Garner, C. C., Tucker, R. P. & Matus, A. Selective localization of messenger RNA for cytoskeletal protein MAP2 in dendrites. Nature 336, 674–677 (1988).
Lecuyer, E. et al. Global analysis of mRNA localization reveals a prominent role in organizing cellular architecture and function. Cell 131, 174–187 (2007).
Cajigas, I. J. et al. The local transcriptome in the synaptic neuropil revealed by deep sequencing and high-resolution imaging. Neuron 74, 453–466 (2012).
Jeffery, W. R. The spatial distribution of maternal mRNA is determined by a cortical cytoskeletal domain in Chaetopterus eggs. Dev. Biol. 110, 217–229 (1985).
Pondel, M. D. & King, M. L. Localized maternal mRNA related to transforming growth factor β mRNA is concentrated in a cytokeratin-enriched fraction from Xenopus oocytes. Proc. Natl Acad. Sci. USA 85, 7612–7616 (1988).
Yisraeli, J. K., Sokol, S. & Melton, D. A. A two-step model for the localization of maternal mRNA in Xenopus oocytes: involvement of microtubules and microfilaments in the translocation and anchoring of Vg1 mRNA. Development 108, 289–298 (1990).
Sundell, C. L. & Singer, R. H. Requirement of microfilaments in sorting of actin messenger RNA. Science 253, 1275–1277 (1991).
Litman, P., Barg, J. & Ginzburg, I. Microtubules are involved in the localization of tau mRNA in primary neuronal cell cultures. Neuron 13, 1463–1474 (1994).
Macdonald, P. M. & Struhl, G. Cis-acting sequences responsible for anterior localization of bicoid mRNA in Drosophila embryos. Nature 336, 595–598 (1988).
Yisraeli, J. K. & Melton, D. A. The material mRNA Vg1 is correctly localized following injection into Xenopus oocytes. Nature 336, 592–595 (1988).
MacDonald, P. M. bicoid mRNA localization signal: phylogenetic conservation of function and RNA secondary structure. Development 110, 161–171 (1990).
Mowry, K. L. & Melton, D. A. Vegetal messenger RNA localization directed by a 340-nt RNA sequence element in Xenopus oocytes. Science 255, 991–994 (1992).
Gavis, E. R. & Lehmann, R. Localization of nanos RNA controls embryonic polarity. Cell 71, 301–313 (1992).
Litman, P., Behar, L., Elisha, Z., Yisraeli, J. K. & Ginzburg, I. Exogenous tau RNA is localized in oocytes: possible evidence for evolutionary conservation of localization mechanisms. Dev. Biol. 176, 86–94 (1996).
Kislauskis, E. H., Li, Z., Singer, R. H. & Taneja, K. L. Isoform-specific 3′-untranslated sequences sort α-cardiac and β-cytoplasmic actin messenger RNAs to different cytoplasmic compartments. J. Cell Biol. 123, 165–172 (1993).
Schwartz, S. P., Aisenthal, L., Elisha, Z., Oberman, F. & Yisraeli, J. K. A. 69-kDa RNA-binding protein from Xenopus oocytes recognizes a common motif in two vegetally localized maternal mRNAs. Proc. Natl Acad. Sci. USA 89, 11895–11899 (1992).
Ferrandon, D., Elphick, L., Nusslein-Volhard, C. & St Johnston, D. Staufen protein associates with the 3′UTR of bicoid mRNA to form particles that move in a microtubule-dependent manner. Cell 79, 1221–1232 (1994).
Ross, A. F., Oleynikov, Y., Kislauskis, E. H., Taneja, K. L. & Singer, R. H. Characterization of a β-actin mRNA zipcode-binding protein. Mol. Cell. Biol. 17, 2158–2165 (1997).
Robb, D. L., Heasman, J., Raats, J. & Wylie, C. A kinesin-like protein is required for germ plasm aggregation in Xenopus. Cell 87, 823–831 (1996).
Eliscovich, C., Buxbaum, A. R., Katz, Z. B. & Singer, R. H. mRNA on the move: the road to its biological destiny. J. Biol. Chem. 288, 20361–20368 (2013).
Singer, R. H. & Ward, D. C. Actin gene expression visualized in chicken muscle tissue culture by using in situ hybridization with a biotinated nucleotide analog. Proc. Natl Acad. Sci. USA 79, 7331–7335 (1982).
Lawrence, J. B. & Singer, R. H. Quantitative analysis of in situ hybridization methods for the detection of actin gene expression. Nucleic Acids Res. 13, 1777–1799 (1985). This study reports the first optimization of FISH. The authors quantified β-actin mRNA in fibroblast by counting and normalizing autoradiography granules.
Femino, A. M., Fay, F. S., Fogarty, K. & Singer, R. H. Visualization of single RNA transcripts in situ. Science 280, 585–590 (1998). This is the first demonstration of single-molecule FISH in cells.
Raj, A., van den Bogaard, P., Rifkin, S. A., van Oudenaarden, A. & Tyagi, S. Imaging individual mRNA molecules using multiple singly labeled probes. Nature Methods 5, 877–879 (2008).
Shaffer, S. M., Wu, M. T., Levesque, M. J. & Raj, A. Turbo FISH: a method for rapid single molecule RNA FISH. PLoS ONE 8, e75120 (2013).
Levsky, J. M., Shenoy, S. M., Pezo, R. C. & Singer, R. H. Single-cell gene expression profiling. Science 297, 836–840 (2002).
Jakt, L. M., Moriwaki, S. & Nishikawa, S. A continuum of transcriptional identities visualized by combinatorial fluorescent in situ hybridization. Development 140, 216–225 (2013).
Battich, N., Stoeger, T. & Pelkmans, L. Image-based transcriptomics in thousands of single human cells at single-molecule resolution. Nature Methods 10, 1127–1133 (2013).
Wang, S. X. & Hazelrigg, T. Implications for Bcd messenger-RNA localization from spatial-distribution of Exu protein in Drosophila oogenesis. Nature 369, 400–403 (1994).
Kohrmann, M. et al. Microtubule-dependent recruitment of Staufen–green fluorescent protein into large RNA-containing granules and subsequent dendritic transport in living hippocampal neurons. Mol. Biol. Cell 10, 2945–2953 (1999).
Alami, N. H. et al. Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron 81, 536–543 (2014). This is a demonstration of altered RBP-dependent granule transport in neurons in the absence of the neuronal RBP TDP-43.
Bertrand, E. et al. Localization of ASH1 mRNA particles in living yeast. Mol. Cell 2, 437–445 (1998). This is the first use of an MS2 system to follow mRNA localization in live cells.
Beach, D. L., Salmon, E. D. & Bloom, K. Localization and anchoring of mRNA in budding yeast. Curr. Biol. 9, 569–578 (1999).
Brodsky, A. S. & Silver, P. A. Identifying proteins that affect mRNA localization in living cells. Methods 26, 151–155 (2002).
Chao, J. A., Patskovsky, Y., Almo, S. C. & Singer, R. H. Structural basis for the coevolution of a viral RNA–protein complex. Nature Struct. Mol. Biol. 15, 103–105 (2008).
Lange, S. et al. Simultaneous transport of different localized mRNA species revealed by live-cell imaging. Traffic 9, 1256–1267 (2008).
Hocine, S., Raymond, P., Zenklusen, D., Chao, J. A. & Singer, R. H. Single-molecule analysis of gene expression using two-color RNA labeling in live yeast. Nature Methods 10, 119–121 (2013).
Wu, B., Chao, J. A. & Singer, R. H. Fluorescence fluctuation spectroscopy enables quantitative imaging of single mRNAs in living cells. Biophys. J. 102, 2936–2944 (2012).
Wu, B., Chen, J. & Singer, R. H. Background free imaging of single mRNAs in live cells using split fluorescent proteins. Sci. Rep. 4, 3615 (2014).
Carrocci, T. J. & Hoskins, A. A. Imaging of RNAs in live cells with spectrally diverse small molecule fluorophores. Analyst 139, 44–47 (2014).
Dolgosheina, E. V. et al. RNA mango aptamer-fluorophore: a bright, high-affinity complex for RNA labeling and tracking. ACS Chem. Biol. 9, 2412–2420 (2014).
Paige, J. S., Wu, K. Y. & Jaffrey, S. R. RNA mimics of green fluorescent protein. Science 333, 642–646 (2011).
Katz, Z. B. et al. β-actin mRNA compartmentalization enhances focal adhesion stability and directs cell migration. Genes Dev. 26, 1885–1890 (2012). Forced mRNA localization at focal adhesions demonstrates how altered mRNA localization affects cell motility.
Long, R. M., Gu, W., Lorimer, E., Singer, R. H. & Chartrand, P. She2p is a novel RNA-binding protein that recruits the Myo4p–She3p complex to ASH1 mRNA. EMBO J. 19, 6592–6601 (2000).
Haim-Vilmovsky, L., Gadir, N., Herbst, R. H. & Gerst, J. E. A genomic integration method for the simultaneous visualization of endogenous mRNAs and their translation products in living yeast. RNA 17, 2249–2255 (2011).
Larson, D. R., Zenklusen, D., Wu, B., Chao, J. A. & Singer, R. H. Real-time observation of transcription initiation and elongation on an endogenous yeast gene. Science 332, 475–478 (2011).
Lionnet, T. et al. A transgenic mouse for in vivo detection of endogenous labeled mRNA. Nature Methods 8, 165–170 (2011).
Zimyanin, V. L. et al. In vivo Imaging of oskar mRNA transport reveals the mechanism of posterior localization. Cell 134, 843–853 (2008). Single-molecule imaging of an endogenous mRNA reveals a slight bias in active transport that leads to localization to the oocyte posterior.
Jaramillo, A. M., Weil, T. T., Goodhouse, J., Gavis, E. R. & Schupbach, T. The dynamics of fluorescently labeled endogenous gurken mRNA in Drosophila. J. Cell Sci. 121, 887–894 (2008).
Weil, T. T., Parton, R., Davis, I. & Gavis, E. R. Changes in bicoid mRNA anchoring highlight conserved mechanisms during the oocyte-to-embryo transition. Curr. Biol. 18, 1055–1061 (2008).
Forrest, K. M. & Gavis, E. R. Live imaging of endogenous RNA reveals a diffusion and entrapment mechanism for nanos mRNA localization in Drosophila. Curr. Biol. 13, 1159–1168 (2003).
Park, H. Y. et al. Visualization of dynamics of single endogenous mRNA labeled in live mouse. Science 343, 422–424 (2014).
Mali, P., Esvelt, K. M. & Church, G. M. Cas9 as a versatile tool for engineering biology. Nature Methods 10, 957–963 (2013).
Fusco, D. et al. Single mRNA molecules demonstrate probabilistic movement in living mammalian cells. Curr. Biol. 13, 161–167 (2003).
Amrute-Nayak, M. & Bullock, S. L. Single-molecule assays reveal that RNA localization signals regulate dynein–dynactin copy number on individual transcript cargoes. Nature Cell Biol. 14, 416–423 (2012). Single-molecule measurements in vitro of mRNAs with altered sequences and binding proteins demonstrate the roles they have in active mRNA transport.
Buxbaum, A. R., Wu, B. & Singer, R. H. Single β-actin mRNA detection in neurons reveals a mechanism for regulating its translatability. Science 343, 419–422 (2014).
Batish, M., van den Bogaard, P., Kramer, F. R. & Tyagi, S. Neuronal mRNAs travel singly into dendrites. Proc. Natl Acad. Sci. USA 109, 4645–4650 (2012).
Mikl, M., Vendra, G. & Kiebler, M. A. Independent localization of MAP2, CaMKIIα and β-actin RNAs in low copy numbers. EMBO Rep. 12, 1077–1084 (2011).
Jambhekar, A. & Derisi, J. L. Cis-acting determinants of asymmetric, cytoplasmic RNA transport. RNA 13, 625–642 (2007).
Shahbabian, K. & Chartrand, P. Control of cytoplasmic mRNA localization. Cell. Mol. Life Sci. 69, 535–552 (2012).
Zid, B. M. & O'Shea, E. K. Promoter sequences direct cytoplasmic localization and translation of mRNAs during starvation in yeast. Nature 514, 117–121 (2014).
Besse, F. & Ephrussi, A. Translational control of localized mRNAs: restricting protein synthesis in space and time. Nature Rev. Mol. Cell Biol. 9, 971–980 (2008).
Abaza, I. & Gebauer, F. Trading translation with RNA-binding proteins. RNA 14, 404–409 (2008).
Patel, V. L. et al. Spatial arrangement of an RNA zipcode identifies mRNAs under post-transcriptional control. Genes Dev. 26, 43–53 (2012).
Ascano, M., Hafner, M., Cekan, P., Gerstberger, S. & Tuschl, T. Identification of RNA-protein interaction networks using PAR-CLIP. Wiley Interdiscip. Rev.RNA 3, 159–177 (2012).
Darnell, J. C. et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146, 247–261 (2011).
Heraud-Farlow, J. E. et al. Staufen2 regulates neuronal target RNAs. Cell Rep. 5, 1511–1518 (2013).
Geng, C. & Macdonald, P. M. Imp associates with squid and Hrp48 and contributes to localized expression of gurken in the oocyte. Mol. Cell. Biol. 26, 9508–9516 (2006).
McDermott, S. M. & Davis, I. Drosophila hephaestus/polypyrimidine tract binding protein is required for dorso-ventral patterning and regulation of signalling between the germline and soma. PLoS ONE 8, e69978 (2013).
Uchiumi, T. et al. YB-1 is important for an early stage embryonic development: neural tube formation and cell proliferation. J. Biol. Chem. 281, 40440–40449 (2006).
Klein, M. E., Younts, T. J., Castillo, P. E. & Jordan, B. A. RNA-binding protein Sam68 controls synapse number and local β-actin mRNA metabolism in dendrites. Proc. Natl Acad. Sci. USA 110, 3125–3130 (2013).
Hartman, T. R. et al. RNA helicase A is necessary for translation of selected messenger RNAs. Nature Struct. Mol. Biol. 13, 509–516 (2006).
Fukuda, N. et al. The transacting factor CBF-A/Hnrnpab binds to the A2RE/RTS element of protamine 2 mRNA and contributes to its translational regulation during mouse spermatogenesis. PLoS Genet. 9, e1003858 (2013).
Glinka, M. et al. The heterogeneous nuclear ribonucleoprotein-R is necessary for axonal β-actin mRNA translocation in spinal motor neurons. Hum. Mol. Genet. 19, 1951–1966 (2010).
Todd, A. G. et al. SMN, Gemin2 and Gemin3 associate with β-actin mRNA in the cytoplasm of neuronal cells in vitro. J. Mol. Biol. 401, 681–689 (2010).
Ma, B. et al. Huntingtin mediates dendritic transport of β-actin mRNA in rat neurons. Sci. Rep. 1, 140 (2011).
Kanai, Y., Dohmae, N. & Hirokawa, N. Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron 43, 513–525 (2004).
Elvira, G. et al. Characterization of an RNA granule from developing brain. Mol. Cell Proteom. 5, 635–651 (2006).
Fritzsche, R. et al. Interactome of two diverse RNA granules links mRNA localization to translational repression in neurons. Cell Rep. 5, 1749–1762 (2013). This study demonstrates the overlap of RNA granule components from different RBP- and mRNA-containing granules, suggesting the extent of similarity of different mRNA complexes.
Kato, M. et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149, 753–767 (2012). This paper demonstrates that RBPs contain sequences that facilitate their aggregation into RNA granules.
Li, P. et al. Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336–340 (2012).
Donnelly, C. J. et al. Limited availability of ZBP1 restricts axonal mRNA localization and nerve regeneration capacity. EMBO J. 30, 4665–4677 (2011).
Bullock, S. L., Nicol, A., Gross, S. P. & Zicha, D. Guidance of bidirectional motor complexes by mRNA cargoes through control of dynein number and activity. Curr. Biol. 16, 1447–1452 (2006).
Gagnon, J. A. & Mowry, K. L. Molecular motors: directing traffic during RNA localization. Crit. Rev. Biochem. Mol. Biol. 46, 229–239 (2011).
Bullock, S. L. Translocation of mRNAs by molecular motors: think complex? Semin. Cell Dev. Biol. 18, 194–201 (2007).
Sladewski, T. E., Bookwalter, C. S., Hong, M. S. & Trybus, K. M. Single-molecule reconstitution of mRNA transport by a class V myosin. Nature Struct. Mol. Biol. 20, 952–957 (2013). This study shows that the multimerization of localization elements on mRNAs linearly correlates with the number of molecular motors to which they bind.
Soundararajan, H. C. & Bullock, S. L. The influence of dynein processivity control, MAPs, and microtubule ends on directional movement of a localising mRNA. Elife 3, e01596 (2014).
Messitt, T. J. et al. Multiple kinesin motors coordinate cytoplasmic RNA transport on a subpopulation of microtubules in Xenopus oocytes. Dev. Cell 15, 426–436 (2008).
Gross, S. P., Vershinin, M. & Shubeita, G. T. Cargo transport: two motors are sometimes better than one. Curr. Biol. 17, R478–R486 (2007).
McKenney, R. J., Huynh, W., Tanenbaum, M. E., Bhabha, G. & Vale, R. D. Activation of cytoplasmic dynein motility by dynactin–cargo adapter complexes. Science 345, 337–341 (2014).
Schlager, M. A., Hoang, H. T., Urnavicius, L., Bullock, S. L. & Carter, A. P. In vitro reconstitution of a highly processive recombinant human dynein complex. EMBO J. 33, 1855–1868 (2014).
Thirumurugan, K., Sakamoto, T., Hammer, J. A., Sellers, J. R. & Knight, P. J. The cargo-binding domain regulates structure and activity of myosin 5. Nature 442, 212–215 (2006).
Heym, R. G. et al. In vitro reconstitution of an mRNA-transport complex reveals mechanisms of assembly and motor activation. J. Cell Biol. 203, 971–984 (2013).
Dynes, J. L. & Steward, O. Dynamics of bidirectional transport of Arc mRNA in neuronal dendrites. J. Comp. Neurol. 500, 433–447 (2007). This paper reports Arc mRNA transporting in dendrites and quantifies multiple aspects of transport.
Dynes, J. L. & Steward, O. Arc mRNA docks precisely at the base of individual dendritic spines indicating the existence of a specialized microdomain for synapse-specific mRNA translation. J. Comp. Neurol. 520, 3105–3119 (2012).
Xu, X., Brechbiel, J. L. & Gavis, E. R. Dynein-dependent transport of nanos RNA in Drosophila sensory neurons requires Rumpelstiltskin and the germ plasm organizer Oskar. J. Neurosci. 33, 14791–14800 (2013).
Liu, G. et al. Interactions of elongation factor 1α with F-actin and β-actin mRNA: implications for anchoring mRNA in cell protrusions. Mol. Biol. Cell 13, 579–592 (2002).
de Heredia, M. L. & Jansen, R. P. mRNA localization and the cytoskeleton. Curr. Opin. Cell Biol. 16, 80–85 (2004).
Delanoue, R. & Davis, I. Dynein anchors its mRNA cargo after apical transport in the Drosophila blastoderm embryo. Cell 122, 97–106 (2005).
Gonzalez, I., Buonomo, S. B., Nasmyth, K. & von Ahsen, U. ASH1 mRNA localization in yeast involves multiple secondary structural elements and Ash1 protein translation. Curr. Biol. 9, 337–340 (1999).
Bi, E. & Park, H. O. Cell polarization and cytokinesis in budding yeast. Genetics 191, 347–387 (2012).
Govindarajan, S., Nevo-Dinur, K. & Amster-Choder, O. Compartmentalization and spatio-temporal organization of macromolecules in bacteria. FEMS Microbiol. Rev. 36, 1005–1022 (2012).
Bobola, N., Jansen, R. P., Shin, T. H. & Nasmyth, K. Asymmetric accumulation of Ash1p in postanaphase nuclei depends on a myosin and restricts yeast mating-type switching to mother cells. Cell 84, 699–709 (1996).
Sil, A. & Herskowitz, I. Identification of asymmetrically localized determinant, Ash1p, required for lineage-specific transcription of the yeast HO gene. Cell 84, 711–722 (1996).
Takizawa, P. A., Sil, A., Swedlow, J. R., Herskowitz, I. & Vale, R. D. Actin-dependent localization of an RNA encoding a cell-fate determinant in yeast. Nature 389, 90–93 (1997).
Gonsalvez, G. B., Urbinati, C. R. & Long, R. M. RNA localization in yeast: moving towards a mechanism. Biol. Cell 97, 75–86 (2005).
Heym, R. G. & Niessing, D. Principles of mRNA transport in yeast. Cell. Mol. Life Sci. 69, 1843–1853 (2012).
Zarnack, K. & Feldbrugge, M. mRNA trafficking in fungi. Mol. Genet. Genom. 278, 347–359 (2007).
Powrie, E. A., Zenklusen, D. & Singer, R. H. A nucleoporin, Nup60p, affects the nuclear and cytoplasmic localization of ASH1 mRNA in S. cerevisiae. RNA 17, 134–144 (2011).
Paquin, N. et al. Local activation of yeast ASH1 mRNA translation through phosphorylation of Khd1p by the casein kinase Yck1p. Mol. Cell 26, 795–809 (2007).
Gu, W., Deng, Y., Zenklusen, D. & Singer, R. H. A new yeast PUF family protein, Puf6p, represses ASH1 mRNA translation and is required for its localization. Genes Dev. 18, 1452–1465 (2004).
Shahbabian, K., Jeronimo, C., Forget, A., Robert, F. & Chartrand, P. Co-transcriptional recruitment of Puf6 by She2 couples translational repression to mRNA localization. Nucleic Acids Res. 42, 8692–8704 (2014).
Deng, Y., Singer, R. H. & Gu, W. Translation of ASH1 mRNA is repressed by Puf6p-Fun12p/eIF5B interaction and released by CK2 phosphorylation. Genes Dev. 22, 1037–1050 (2008).
Zipor, G. et al. Localization of mRNAs coding for peroxisomal proteins in the yeast, Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 106, 19848–19853 (2009).
Gadir, N., Haim-Vilmovsky, L., Kraut-Cohen, J. & Gerst, J. E. Localization of mRNAs coding for mitochondrial proteins in the yeast Saccharomyces cerevisiae. RNA 17, 1551–1565 (2011).
Margeot, A. et al. In Saccharomyces cerevisiae, ATP2 mRNA sorting to the vicinity of mitochondria is essential for respiratory function. EMBO J. 21, 6893–6904 (2002).
Kraut-Cohen, J. et al. Translation- and SRP-independent mRNA targeting to the endoplasmic reticulum in the yeast Saccharomyces cerevisiae. Mol. Biol. Cell 24, 3069–3084 (2013).
Kilchert, C. & Spang, A. Cotranslational transport of ABP140 mRNA to the distal pole of S. cerevisiae. EMBO J. 30, 3567–3580 (2011).
Simpson, C. E., Lui, J., Kershaw, C. J., Sims, P. F. & Ashe, M. P. mRNA localization to Pbodies in yeast is biphasic with many mRNAs captured in a late Bfr1pdependent wave. J. Cell Sci. 127, 1254–1262 (2014).
Andoh, T., Oshiro, Y., Hayashi, S., Takeo, H. & Tani, T. Visual screening for localized RNAs in yeast revealed novel RNAs at the bud-tip. Biochem. Biophys. Res. Commun. 351, 999–1004 (2006).
Aronov, S. et al. mRNAs encoding polarity and exocytosis factors are cotransported with the cortical endoplasmic reticulum to the incipient bud in Saccharomyces cerevisiae. Mol. Cell. Biol. 27, 3441–3455 (2007).
Shepard, K. A. et al. Widespread cytoplasmic mRNA transport in yeast: identification of 22 bud-localized transcripts using DNA microarray analysis. Proc. Natl Acad. Sci. USA 100, 11429–11434 (2003).
Gelin-Licht, R., Paliwal, S., Conlon, P., Levchenko, A. & Gerst, J. E. Scp160-dependent mRNA trafficking mediates pheromone gradient sensing and chemotropism in yeast. Cell Rep. 1, 483–494 (2012).
Miller, O. L. Jr, Hamkalo, B. A. & Thomas, C. A. Jr. Visualization of bacterial genes in action. Science 169, 392–395 (1970).
Nevo-Dinur, K., Nussbaum-Shochat, A., Ben-Yehuda, S. & Amster-Choder, O. Translation-independent localization of mRNA in E. coli. Science 331, 1081–1084 (2011). This paper demonstrated for the first time that mRNAs in bacteria can localize to specific subcellular sites.
Prilusky, J. & Bibi, E. Studying membrane proteins through the eyes of the genetic code revealed a strong uracil bias in their coding mRNAs. Proc. Natl Acad. Sci. USA 106, 6662–6666 (2009).
Martin, K. C., Barad, M. & Kandel, E. R. Local protein synthesis and its role in synapse-specific plasticity. Curr. Opin. Neurobiol. 10, 587–592 (2000).
Miyashiro, K., Dichter, M. & Eberwine, J. On the nature and differential distribution of mRNAs in hippocampal neurites: implications for neuronal functioning. Proc. Natl Acad. Sci. USA 91, 10800–10804 (1994).
Poon, M. M., Choi, S. H., Jamieson, C. A., Geschwind, D. H. & Martin, K. C. Identification of process-localized mRNAs from cultured rodent hippocampal neurons. J. Neurosci. 26, 13390–13399 (2006).
Puthanveettil, S. V. et al. A strategy to capture and characterize the synaptic transcriptome. Proc. Natl Acad. Sci. USA 110, 7464–7469 (2013).
Knowles, R. B. et al. Translocation of RNA granules in living neurons. J. Neurosci. 16, 7812–7820 (1996). Live imaging and tracking of RNA material in neurons reveals oscillatory behaviour and altered movement in response to stimulation.
Davis, L., Banker, G. A. & Steward, O. Selective dendritic transport of RNA in hippocampal neurons in culture. Nature 330, 477–479 (1987).
Tubing, F. et al. Dendritically localized transcripts are sorted into distinct ribonucleoprotein particles that display fast directional motility along dendrites of hippocampal neurons. J. Neurosci. 30, 4160–4170 (2010).
Ling, S. C., Fahrner, P. S., Greenough, W. T. & Gelfand, V. I. Transport of Drosophila fragile X mental retardation protein-containing ribonucleoprotein granules by kinesin-1 and cytoplasmic dynein. Proc. Natl Acad. Sci. USA 101, 17428–17433 (2004).
Estes, P. S., O'Shea, M., Clasen, S. & Zarnescu, D. C. Fragile X protein controls the efficacy of mRNA transport in Drosophila neurons. Mol. Cell Neurosci. 39, 170–179 (2008). The quantification of mRNA movements in neurons with altered FMRP expression demonstrates protein-enhanced processivity and transport of mRNA.
Doyle, M. & Kiebler, M. A. Mechanisms of dendritic mRNA transport and its role in synaptic tagging. EMBO J. 30, 3540–3552 (2011).
Krichevsky, A. M. & Kosik, K. S. Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron 32, 683–696 (2001).
Mayford, M., Baranes, D., Podsypanina, K. & Kandel, E. R. The 3′-untranslated region of CaMKII α is a cis-acting signal for the localization and translation of mRNA in dendrites. Proc. Natl Acad. Sci. USA 93, 13250–13255 (1996).
Miller, S. et al. Disruption of dendritic translation of CaMKIIα impairs stabilization of synaptic plasticity and memory consolidation. Neuron 36, 507–519 (2002).
Perry, R. B. et al. Subcellular knockout of importin β1 perturbs axonal retrograde signaling. Neuron 75, 294–305 (2012).
Yoon, B. C. et al. Local translation of extranuclear lamin B promotes axon maintenance. Cell 148, 752–764 (2012).
Lee, J. H. et al. Highly multiplexed subcellular RNA sequencing in situ. Science 343, 1360–1363 (2014). This is the first demonstration of in situ RNA sequencing, which allows localization analysis of multiple RNA species in cells at the single-molecule level.
Slobodin, B. & Gerst, J. E. RaPID: an aptamer-based mRNA affinity purification technique for the identification of RNA and protein factors present in ribonucleoprotein complexes. Methods Mol. Biol. 714, 387–406 (2011).
Rodriguez, A. J., Shenoy, S. M., Singer, R. H. & Condeelis, J. Visualization of mRNA translation in living cells. J. Cell Biol. 175, 67–76 (2006).
Dieterich, D. C. et al. In situ visualization and dynamics of newly synthesized proteins in rat hippocampal neurons. Nature Neurosci. 13, 897–905 (2010).
Yu, J., Xiao, J., Ren, X., Lao, K. & Xie, X. S. Probing gene expression in live cells, one protein molecule at a time. Science 311, 1600–1603 (2006).
Los, G. V. et al. HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem. Biol. 3, 373–382 (2008).
Haimovich, G. et al. Gene expression is circular: factors for mRNA degradation also foster mRNA synthesis. Cell 153, 1000–1011 (2013).
Zenklusen, D., Larson, D. R. & Singer, R. H. Single-RNA counting reveals alternative modes of gene expression in yeast. Nature Struct. Mol. Biol. 15, 1263–1271 (2008).
Mueller, F. et al. FISH-quant: automatic counting of transcripts in 3D FISH images. Nature Methods 10, 277–278 (2013).
Park, H. Y., Trcek, T., Wells, A. L., Chao, J. A. & Singer, R. H. An unbiased analysis method to quantify mRNA localization reveals its correlation with cell motility. Cell Rep. 1, 179–184 (2012).
Park, H. Y., Buxbaum, A. R. & Singer, R. H. Single mRNA tracking in live cells. Methods Enzymol. 472, 387–406 (2010).
Jaqaman, K. et al. Robust single-particle tracking in live-cell time-lapse sequences. Nature Methods 5, 695–702 (2008).
Meijering, E., Dzyubachyk, O. & Smal, I. Methods for cell and particle tracking. Methods Enzymol. 504, 183–200 (2012).
Chenouard, N. et al. Objective comparison of particle tracking methods. Nature Meth 11, 281–289 (2014). This paper describes a multigroup collaborative effort to compare particle-tracking methods.
Oleynikov, Y. & Singer, R. H. Real-time visualization of ZBP1 association with β-actin mRNA during transcription and localization. Curr. Biol. 13, 199–207 (2003).
Latham, V. M., Yu, E. H., Tullio, A. N., Adelstein, R. S. & Singer, R. H. A. Rho-dependent signaling pathway operating through myosin localizes β-actin mRNA in fibroblasts. Curr. Biol. 11, 1010–1016 (2001).
Eom, T., Antar, L. N., Singer, R. H. & Bassell, G. J. Localization of a β-actin messenger ribonucleoprotein complex with zipcode-binding protein modulates the density of dendritic filopodia and filopodial synapses. J. Neurosci. 23, 10433–10444 (2003).
Ferrandon, D., Koch, I., Westhof, E. & Nusslein-Volhard, C. RNA–RNA interaction is required for the formation of specific bicoid mRNA 3′ UTR-STAUFEN ribonucleoprotein particles. EMBO J. 16, 1751–1758 (1997).
Macdonald, P. M. & Kerr, K. Redundant RNA recognition events in bicoid mRNA localization. RNA 3, 1413–1420 (1997).
Macdonald, P. M., Kerr, K., Smith, J. L. & Leask, A. RNA regulatory element BLE1 directs the early steps of bicoid mRNA localization. Development 118, 1233–1243 (1993).
Bashirullah, A., Cooperstock, R. L. & Lipshitz, H. D. Spatial and temporal control of RNA stability. Proc. Natl Acad. Sci. USA 98, 7025–7028 (2001).
Zaessinger, S., Busseau, I. & Simonelig, M. Oskar allows nanos mRNA translation in Drosophila embryos by preventing its deadenylation by Smaug/CCR4. Development 133, 4573–4583 (2006).
Jain, R. A. & Gavis, E. R. The Drosophila hnRNP M homolog Rumpelstiltskin regulates nanos mRNA localization. Development 135, 973–982 (2008).
Becalska, A. N. et al. Aubergine is a component of a nanos mRNA localization complex. Dev. Biol. 349, 46–52 (2011).
Chang, P. et al. Localization of RNAs to the mitochondrial cloud in Xenopus oocytes through entrapment and association with endoplasmic reticulum. Mol. Biol. Cell 15, 4669–4681 (2004).
Gavis, E. R. & Lehmann, R. Translational regulation of nanos by RNA localization. Nature 369, 315–318 (1994).
dos Santos, V. T., Bisson-Filho, A. W. & Gueiros-Filho, F. J. DivIVA-mediated polar localization of ComN, a posttranscriptional regulator of Bacillus subtilis. J. Bacteriol. 194, 3661–3669 (2012).
Bohl, F., Kruse, C., Frank, A., Ferring, D. & Jansen, R. P. She2p, a novel RNA-binding protein tethers ASH1 mRNA to the Myo4p myosin motor via She3p. EMBO J. 19, 5514–5524 (2000).
Parton, R. M. et al. A PAR-1-dependent orientation gradient of dynamic microtubules directs posterior cargo transport in the Drosophila oocyte. J. Cell Biol. 194, 121–135 (2011).
Castello, A et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell 149, 1393–1406 (2012).
Baltz, A. G. et al. The mRNA-bound proteome and its global occupancy profile on protein-coding transcripts. Mol. Cell 46, 674–690 (2012).
Acknowledgements
The authors are grateful to T. Trcek, R. Lehmann and O. Amster-Choder for contributing their images for Figure 1. The authors also thank E. Tutucci, Y. J. Yoon, S. Preibisch and B. Wu for comments on the manuscript. R.H.S. is funded by the US National Institutes of Health (NIH) grants NIH/NIGMS 2R01GM057071, NIH/NIBIB 5R01EB013571 and NIH/NINDS 9R01NS083085. G.H. is funded by the Gruss Lipper postdoctoral fellowship (EGL charitable foundation) (Albert Einstein College of Medicine), Dean of faculty fellowship (Weizmann Institute of Science (WIS)) and Clore postdoctoral fellowship (WIS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (figure)
Fluorescence in situ hybridization (FISH) technique variations. (PDF 113 kb)
Supplementary information S2 (table)
Visualizing single mRNAs in fixed and live cells (PDF 307 kb)
Supplementary information S3 (movie)
Altered β-actin mRNA behaviour in different cell types. (AVI 13171 kb)
Related links
Glossary
- Single-molecule FISH
-
(smFISH). A fluorescence in situ hybridization (FISH) technique that uses multiple unique short probes against a single mRNA, which greatly increases signal-to-noise ratio and enables detection of single mRNA molecules.
- SNAP tag
-
A protein fusion tag derived from the human enzyme O6-methylguanine DNA methyltransferase. The protein can covalently bind to a synthetic chemical ligand that can be labelled with a fluorescent dye.
- Aptamers
-
Short nucleic acid sequences with unique folding properties that can bind to a specific target molecule and be used for fluorescent tagging.
- Myosin
-
A family of actin-based, ATP-dependent motor proteins.
- Kinesin
-
A class of molecular motors that use ATP to move along microtubule filaments and that transport many cellular components. There are 14 subtypes in the kinesin superfamily, most of which transport cargo to the plus ends of microtubules.
- Dynein
-
A motor protein family that uses ATP to transport cargo along microtubules, typically towards their minus ends. Axonemal dynein has roles in cilia and flagella, whereas cytoplasmic dynein transports mRNAs, among other cargos.
- Syncytial blastoderms
-
A specific stage of Drosophila spp. embryogenesis during which the embryo becomes a single multinucleated cell.
- Vegetal cortex
-
The lower pole on the animal vegetal axis of oocytes where the yolk resides.
- Bud tip
-
The point opposite to the bud neck (which connects the bud to the mother cell) in budding yeast.
- Mating type
-
The budding yeast has two mating types, a and α. Mating of a and α haploid cells produces a diploid cell that can later undergo meiosis to form spores. Haploid cells can switch mating types.
- Processing bodies
-
(P-bodies). Cytoplasmic granules that contain mRNA-degrading proteins, full-length mRNAs and mRNA fragments. Their function is unclear but is related to mRNA degradation.
- Synaptic plasticity
-
Changes in the strength of synaptic transmission in response to changes in synaptic activity, possibly during learning and memory formation.
- Long-term potentiation
-
Long-lasting increase in the efficacy of synaptic transmission between two neurons owing to enhanced neuronal signalling or activity.
- HaloTag
-
A protein fusion tag derived from the enzyme DhaA from Rhodococcus rhodochrous. The protein can covalently bind to a synthetic chemical ligand that can be labelled with a fluorescent dye.
Rights and permissions
About this article
Cite this article
Buxbaum, A., Haimovich, G. & Singer, R. In the right place at the right time: visualizing and understanding mRNA localization. Nat Rev Mol Cell Biol 16, 95–109 (2015). https://doi.org/10.1038/nrm3918
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3918
This article is cited by
-
Spatiotemporal control of RNA metabolism and CRISPR–Cas functions using engineered photoswitchable RNA-binding proteins
Nature Protocols (2024)
-
MinD-RNase E interplay controls localization of polar mRNAs in E. coli
The EMBO Journal (2024)
-
Arabidopsis cyclophilins direct intracellular transport of mobile mRNA via organelle hitchhiking
Nature Plants (2024)
-
Analysis of subcellular RNA fractions demonstrates significant genetic regulation of gene expression in human brain post-transcriptionally
Scientific Reports (2023)
-
A translational regulator MHZ9 modulates ethylene signaling in rice
Nature Communications (2023)