Abstract
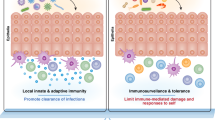
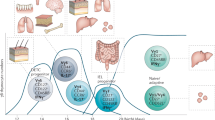
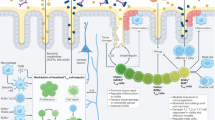
In this Opinion article, we discuss the function of tissues as a crucial checkpoint for the regulation of effector T cell responses, and the notion that interleukin-15 (IL-15) functions as a danger molecule that communicates to the immune system that the tissue is under attack and poises it to mediate tissue destruction. More specifically, we propose that expression of IL-15 in tissues promotes T helper 1 cell-mediated immunity and provides co-stimulatory signals to effector cytotoxic T cells to exert their effector functions and drive tissue destruction. Therefore, we think that IL-15 contributes to tissue protection by promoting the elimination of infected cells but that when its expression is chronically dysregulated, it can promote the development of complex T cell-mediated disorders associated with tissue destruction, such as coeliac disease and type 1 diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cousens, L. P. et al. Two roads diverged: interferon α/β- and interleukin 12-mediated pathways in promoting T cell interferon γ responses during viral infection. J. Exp. Med. 189, 1315–1328 (1999).
Taylor, G. A., Feng, C. G. & Sher, A. Control of IFN-γ-mediated host resistance to intracellular pathogens by immunity-related GTPases (p47 GTPases). Microbes Infect. 9, 1644–1651 (2007).
Valentine, L., Potts, R. & Premenko-Lanier, M. CD8+ T cell-derived IFN-γ prevents infection by a second heterologous virus. J. Immunol. 189, 5841–5848 (2012).
Antonelli, A., Ferrari, S. M., Corrado, A., Di Domenicantonio, A. & Fallahi, P. Autoimmune thyroid disorders. Autoimmun. Rev. 14, 174–180 (2015).
Jabri, B. & Sollid, L. M. Tissue-mediated control of immunopathology in coeliac disease. Nat. Rev. Immunol. 9, 858–870 (2009).
Walker, L. S. & von Herrath, M. CD4 T cell differentiation in type 1 diabetes. Clin. Exp. Immunol. http://dx.doi.org/10.1111/cei.12672 (2015).
Rochman, Y., Spolski, R. & Leonard, W. J. New insights into the regulation of T cells by γc family cytokines. Nat. Rev. Immunol. 9, 480–490 (2009).
Waldmann, T. A. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 6, 595–601 (2006).
Bergamaschi, C. et al. Circulating IL-15 exists as heterodimeric complex with soluble IL-15Rα in human and mouse serum. Blood 120, e1–e8 (2012).
Mortier, E., Woo, T., Advincula, R., Gozalo, S. & Ma, A. IL-15Rα chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J. Exp. Med. 205, 1213–1225 (2008).
Ota, N., Takase, M., Uchiyama, H., Olsen, S. K. & Kanagawa, O. No requirement of trans presentations of IL-15 for human CD8 T cell proliferation. J. Immunol. 185, 6041–6048 (2010).
Liao, W., Lin, J. X. & Leonard, W. J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 38, 13–25 (2013).
Wuest, S. C. et al. A role for interleukin-2 trans-presentation in dendritic cell-mediated T cell activation in humans, as revealed by daclizumab therapy. Nat. Med. 17, 604–609 (2011).
Mishra, A., Sullivan, L. & Caligiuri, M. A. Molecular pathways: interleukin-15 signaling in health and in cancer. Clin. Cancer Res. 20, 2044–2050 (2014).
Colpitts, S. L. et al. Cutting edge: the role of IFN-α receptor and MyD88 signaling in induction of IL-15 expression in vivo. J. Immunol. 188, 2483–2487 (2012).
Zhou, R., Wei, H., Sun, R., Zhang, J. & Tian, Z. NKG2D recognition mediates Toll-like receptor 3 signaling-induced breakdown of epithelial homeostasis in the small intestines of mice. Proc. Natl Acad. Sci. USA 104, 7512–7515 (2007).
Abadie, V. & Jabri, B. IL-15: a central regulator of celiac disease immunopathology. Immunol. Rev. 260, 221–234 (2014).
Waldmann, T. A. The biology of IL-15: implications for cancer therapy and the treatment of autoimmune disorders. J. Investig. Dermatol. Symp. Proc. 16, S28–S30 (2013).
Harada, S. et al. Production of interleukin-7 and interleukin-15 by fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum. 42, 1508–1516 (1999).
McInnes, I. B. et al. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat. Med. 2, 175–182 (1996).
Vaknin-Dembinsky, A., Brass, S. D., Gandhi, R. & Weiner, H. L. Membrane bound IL-15 is increased on CD14 monocytes in early stages of MS. J. Neuroimmunol. 195, 135–139 (2008).
Bouchaud, G. et al. Epidermal IL-15Rα acts as an endogenous antagonist of psoriasiform inflammation in mouse and man. J. Exp. Med. 210, 2105–2117 (2013).
Villadsen, L. S. et al. Resolution of psoriasis upon blockade of IL-15 biological activity in a xenograft mouse model. J. Clin. Invest. 112, 1571–1580 (2003).
Aringer, M. et al. Serum interleukin-15 is elevated in systemic lupus erythematosus. Rheumatology (Oxford) 40, 876–881 (2001).
Robak, E. et al. Proinflammatory interferon-gamma-inducing monokines (interleukin-12, interleukin-18, interleukin-15)—serum profile in patients with systemic lupus erythematosus. Eur. Cytokine Netw. 13, 364–368 (2002).
Chen, J. et al. Insulin-dependent diabetes induced by pancreatic β cell expression of IL-15 and IL-15Rα. Proc. Natl Acad. Sci. USA 110, 13534–13539 (2013).
Sadlack, B. et al. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 75, 253–261 (1993).
Willerford, D. M. et al. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity 3, 521–530 (1995).
Mlecnik, B. et al. Functional network pipeline reveals genetic determinants associated with in situ lymphocyte proliferation and survival of cancer patients. Sci. Transl Med. 6, 228ra37 (2014).
Sheridan, B. S. & Lefrancois, L. Intraepithelial lymphocytes: to serve and protect. Curr. Gastroenterol. Rep. 12, 513–521 (2010).
Kalinski, P., Hilkens, C. M., Wierenga, E. A. & Kapsenberg, M. L. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol. Today 20, 561–567 (1999).
Forrester, J. V., Xu, H., Lambe, T. & Cornall, R. Immune privilege or privileged immunity? Mucosal Immunol. 1, 372–381 (2008).
Simpson, E. A historical perspective on immunological privilege. Immunol. Rev. 213, 12–22 (2006).
McKenna, K. C. & Kapp, J. A. Ocular immune privilege and CTL tolerance. Immunol. Res. 29, 103–112 (2004).
Weaver, C. T. & Hatton, R. D. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat. Rev. Immunol. 9, 883–889 (2009).
Iwata, M. et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538 (2004).
Johansson-Lindbom, B. et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 202, 1063–1073 (2005).
Mora, J. R. et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature 424, 88–93 (2003).
Coombes, J. L. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007).
Mucida, D. et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317, 256–260 (2007).
Sun, C. M. et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204, 1775–1785 (2007).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
DePaolo, R. W. et al. A specific role for TLR1 in protective TH17 immunity during mucosal infection. J. Exp. Med. 209, 1437–1444 (2012).
Schenkel, J. M. & Masopust, D. Tissue-resident memory T cells. Immunity 41, 886–897 (2014).
Thome, J. J. & Farber, D. L. Emerging concepts in tissue-resident T cells: lessons from humans. Trends Immunol. 36, 428–435 (2015).
Deshpande, P. et al. IL-7- and IL-15-mediated TCR sensitization enables T cell responses to self-antigens. J. Immunol. 190, 1416–1423 (2013).
Liu, R. B. et al. IL-15 in tumor microenvironment causes rejection of large established tumors by T cells in a noncognate T cell receptor-dependent manner. Proc. Natl Acad. Sci. USA 110, 8158–8163 (2013).
Roberts, A. I. et al. NKG2D receptors induced by IL-15 costimulate CD28-negative effector CTL in the tissue microenvironment. J. Immunol. 167, 5527–5530 (2001).
Lang, K. S. et al. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat. Med. 11, 138–145 (2005).
de Kauwe, A. L. et al. Resistance to celiac disease in humanized HLA-DR3-DQ2-transgenic mice expressing specific anti-gliadin CD4+ T cells. J. Immunol. 182, 7440–7450 (2009).
DePaolo, R. W. et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature 471, 220–224 (2011).
Kim, S. K., Schluns, K. S. & Lefrancois, L. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J. Immunol. 163, 4125–4132 (1999).
Marietta, E. et al. A new model for dermatitis herpetiformis that uses HLA-DQ8 transgenic NOD mice. J. Clin. Invest. 114, 1090–1097 (2004).
Husby, S. et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 54, 136–160 (2012).
Setty, M. et al. Distinct and synergistic contributions of epithelial stress and adaptive immunity to functions of intraepithelial killer cells and active celiac disease. Gastroenterology 149, 681–691 (2015).
Long, S. A., Buckner, J. H. & Greenbaum, C. J. IL-2 therapy in type 1 diabetes: “Trials” and tribulations. Clin. Immunol. 149, 324–331 (2013).
Ring, A. M. et al. Mechanistic and structural insight into the functional dichotomy between IL-2 and IL-15. Nat. Immunol. 13, 1187–1195 (2012).
Kennedy, M. K. et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191, 771–780 (2000).
Imamichi, H., Sereti, I. & Lane, H. C. IL-15 acts as a potent inducer of CD4+CD25hi cells expressing FOXP3. Eur. J. Immunol. 38, 1621–1630 (2008).
Lin, S. J. et al. Expansion of regulatory T cells from umbilical cord blood and adult peripheral blood CD4+CD25+ T cells. Immunol. Res. 60, 105–111 (2014).
Litjens, N. H. et al. Allogeneic mature human dendritic cells generate superior alloreactive regulatory T cells in the presence of IL-15. J. Immunol. 194, 5282–5293 (2015).
Marshall, D., Sinclair, C., Tung, S. & Seddon, B. Differential requirement for IL-2 and IL-15 during bifurcated development of thymic regulatory T cells. J. Immunol. 193, 5525–5533 (2014).
Raynor, J. et al. IL-15 fosters age-driven regulatory T cell accrual in the face of declining IL-2 levels. Front. Immunol. 4, 161 (2013).
D'Cruz, L. M. & Klein, L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat. Immunol. 6, 1152–1159 (2005).
Fontenot, J. D., Rasmussen, J. P., Gavin, M. A. & Rudensky, A. Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 6, 1142–1151 (2005).
Maloy, K. J. & Powrie, F. Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nat. Immunol. 6, 1071–1072 (2005).
Lenardo, M. et al. Mature T lymphocyte apoptosis—immune regulation in a dynamic and unpredictable antigenic environment. Annu. Rev. Immunol. 17, 221–253 (1999).
Ohta, N. et al. IL-15-dependent activation-induced cell death-resistant Th1 type CD8 αβ+NK1.1+ T cells for the development of small intestinal inflammation. J. Immunol. 169, 460–468 (2002).
Waldmann, T. A., Dubois, S. & Tagaya, Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity 14, 105–110 (2001).
Ku, C. C., Murakami, M., Sakamoto, A., Kappler, J. & Marrack, P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science 288, 675–678 (2000).
Burkett, P. R. et al. IL-15Rα expression on CD8+ T cells is dispensable for T cell memory. Proc. Natl Acad. Sci. USA 100, 4724–4729 (2003).
Dubois, S., Mariner, J., Waldmann, T. A. & Tagaya, Y. IL-15Rα recycles and presents IL-15 in trans to neighboring cells. Immunity 17, 537–547 (2002).
Ben Ahmed, M. et al. IL-15 renders conventional lymphocytes resistant to suppressive functions of regulatory T cells through activation of the phosphatidylinositol 3-kinase pathway. J. Immunol. 182, 6763–6770 (2009).
Ruprecht, C. R. et al. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J. Exp. Med. 201, 1793–1803 (2005).
Jabri, B. et al. Selective expansion of intraepithelial lymphocytes expressing the HLA-E-specific natural killer receptor CD94 in celiac disease. Gastroenterology 118, 867–879 (2000).
Mention, J. J. et al. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology 125, 730–745 (2003).
Fuchs, A. et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity 38, 769–781 (2013).
Cui, G. et al. Characterization of the IL-15 niche in primary and secondary lymphoid organs in vivo. Proc. Natl Acad. Sci. USA 111, 1915–1920 (2014).
Doherty, T. M., Seder, R. A. & Sher, A. Induction and regulation of IL-15 expression in murine macrophages. J. Immunol. 156, 735–741 (1996).
Dubois, S. P., Waldmann, T. A. & Muller, J. R. Survival adjustment of mature dendritic cells by IL-15. Proc. Natl Acad. Sci. USA 102, 8662–8667 (2005).
Mattei, F., Schiavoni, G., Belardelli, F. & Tough, D. F. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J. Immunol. 167, 1179–1187 (2001).
Orinska, Z. et al. IL-15 constrains mast cell-dependent antibacterial defenses by suppressing chymase activities. Nat. Med. 13, 927–934 (2007).
Miranda-Carus, M. E. et al. Peripheral blood T lymphocytes from patients with early rheumatoid arthritis express RANKL and interleukin-15 on the cell surface and promote osteoclastogenesis in autologous monocytes. Arthritis Rheum. 54, 1151–1164 (2006).
Schneider, R. et al. B cell-derived IL-15 enhances CD8 T cell cytotoxicity and is increased in multiple sclerosis patients. J. Immunol. 187, 4119–4128 (2011).
Ogasawara, K. et al. Requirement for IRF-1 in the microenvironment supporting development of natural killer cells. Nature 391, 700–703 (1998).
Rappl, G. et al. Dermal fibroblasts sustain proliferation of activated T cells via membrane-bound interleukin-15 upon long-term stimulation with tumor necrosis factor-α. J. Invest. Dermatol. 116, 102–109 (2001).
Ma, L. J., Acero, L. F., Zal, T. & Schluns, K. S. Trans-presentation of IL-15 by intestinal epithelial cells drives development of CD8αα IELs. J. Immunol. 183, 1044–1054 (2009).
Reinecker, H. C., MacDermott, R. P., Mirau, S., Dignass, A. & Podolsky, D. K. Intestinal epithelial cells both express and respond to interleukin 15. Gastroenterology 111, 1706–1713 (1996).
Zdrenghea, M. T. et al. RSV infection modulates IL-15 production and MICA levels in respiratory epithelial cells. Eur. Respir. J. 39, 712–720 (2012).
Xing, L. et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat. Med. 20, 1043–1049 (2014).
Ruckert, R. et al. Inhibition of keratinocyte apoptosis by IL-15: a new parameter in the pathogenesis of psoriasis? J. Immunol. 165, 2240–2250 (2000).
Bo, H. et al. Elevated expression of transmembrane IL-15 in immune cells correlates with the development of murine lupus: a potential target for immunotherapy against SLE. Scand. J. Immunol. 69, 119–129 (2009).
Maiuri, L. et al. Interleukin 15 mediates epithelial changes in celiac disease. Gastroenterology 119, 996–1006 (2000).
Kirman, I. & Nielsen, O. H. Increased numbers of interleukin-15-expressing cells in active ulcerative colitis. Am. J. Gastroenterol. 91, 1789–1794 (1996).
Liu, Z. et al. IL-15 is highly expressed in inflammatory bowel disease and regulates local T cell-dependent cytokine production. J. Immunol. 164, 3608–3615 (2000).
Vainer, B., Nielsen, O. H., Hendel, J., Horn, T. & Kirman, I. Colonic expression and synthesis of interleukin 13 and interleukin 15 in inflammatory bowel disease. Cytokine 12, 1531–1536 (2000).
Agostini, C. et al. Role of IL-15, IL-2, and their receptors in the development of T cell alveolitis in pulmonary sarcoidosis. J. Immunol. 157, 910–918 (1996).
Waldmann, T. A. & Tagaya, Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu. Rev. Immunol. 17, 19–49 (1999).
Ohteki, T., Suzue, K., Maki, C., Ota, T. & Koyasu, S. Critical role of IL-15–IL-15R for antigen-presenting cell functions in the innate immune response. Nat. Immunol. 2, 1138–1143 (2001).
Yu, X. et al. Artificial antigen-presenting cells plus IL-15 and IL-21 efficiently induce melanoma-specific cytotoxic CD8+ CD28+ T lymphocyte responses. Asian Pac. J. Trop. Med. 6, 467–472 (2013).
Anguille, S. et al. Interleukin-15 dendritic cells as vaccine candidates for cancer immunotherapy. Hum. Vaccin. Immunother. 9, 1956–1961 (2013).
Steel, J. C., Waldmann, T. A. & Morris, J. C. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol. Sci. 33, 35–41 (2012).
Pandiyan, P. et al. The role of IL-15 in activating STAT5 and fine-tuning IL-17A production in CD4 T lymphocytes. J. Immunol. 189, 4237–4246 (2012).
Castillo, E. F. & Schluns, K. S. Regulating the immune system via IL-15 transpresentation. Cytokine 59, 479–490 (2012).
Ebert, E. C. Interleukin 15 is a potent stimulant of intraepithelial lymphocytes. Gastroenterology 115, 1439–1445 (1998).
Jabri, B. & Ebert, E. Human CD8+ intraepithelial lymphocytes: a unique model to study the regulation of effector cytotoxic T lymphocytes in tissue. Immunol. Rev. 215, 202–214 (2007).
Meresse, B. et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity 21, 357–366 (2004).
Riha, P. & Rudd, C. E. CD28 co-signaling in the adaptive immune response. Self Nonself 1, 231–240 (2010).
Tang, F. et al. Cytosolic PLA2 is required for CTL-mediated immunopathology of celiac disease via NKG2D and IL-15. J. Exp. Med. 206, 707–719 (2009).
Sutherland, C. L. et al. UL16-binding proteins, novel MHC class I-related proteins, bind to NKG2D and activate multiple signaling pathways in primary NK cells. J. Immunol. 168, 671–679 (2002).
Jabri, B. et al. TCR specificity dictates CD94/NKG2A expression by human CTL. Immunity 17, 487–499 (2002).
Hue, S. et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 21, 367–377 (2004).
Meresse, B. et al. Reprogramming of CTLs into natural killer-like cells in celiac disease. J. Exp. Med. 203, 1343–1355 (2006).
Bauer, S. et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285, 727–729 (1999).
Wu, J. et al. An activating immunoreceptor complex formed by NKG2D and DAP10. Science 285, 730–732 (1999).
Groh, V. et al. Costimulation of CD8αβ T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat. Immunol. 2, 255–260 (2001).
Thome, J. J. et al. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell 159, 814–828 (2014).
Zhang, C., Zhang, J., Niu, J. & Tian, Z. Interleukin-15 improves cytotoxicity of natural killer cells via up-regulating NKG2D and cytotoxic effector molecule expression as well as STAT1 and ERK1/2 phosphorylation. Cytokine 42, 128–136 (2008).
Upshaw, J. L. & Leibson, P. J. NKG2D-mediated activation of cytotoxic lymphocytes: unique signaling pathways and distinct functional outcomes. Semin. Immunol. 18, 167–175 (2006).
Wildin, R. S. et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 27, 18–20 (2001).
Buckner, J. H. Mechanisms of impaired regulation by CD4+CD25+FOXP3+ regulatory T cells in human autoimmune diseases. Nat. Rev. Immunol. 10, 849–859 (2010).
Cao, D. et al. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur. J. Immunol. 33, 215–223 (2003).
Mottonen, M. et al. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin. Exp. Immunol. 140, 360–367 (2005).
Maul, J. et al. Peripheral and intestinal regulatory CD4+ CD25high T cells in inflammatory bowel disease. Gastroenterology 128, 1868–1878 (2005).
Sitohy, B., Hammarstrom, S., Danielsson, A. & Hammarstrom, M. L. Basal lymphoid aggregates in ulcerative colitis colon: a site for regulatory T cell action. Clin. Exp. Immunol. 151, 326–333 (2008).
Marks-Konczalik, J. et al. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc. Natl Acad. Sci. USA 97, 11445–11450 (2000).
Lilley, B. N. & Ploegh, H. L. Viral modulation of antigen presentation: manipulation of cellular targets in the ER and beyond. Immunol. Rev. 207, 126–144 (2005).
Maeurer, M., Seliger, B., Trinder, P., Gerdes, J. & Seitzer, U. Interleukin-15 in mycobacterial infection of antigen-presenting cells. Scand. J. Immunol. 50, 280–288 (1999).
Liu, T., Nishimura, H., Matsuguchi, T. & Yoshikai, Y. Differences in interleukin-12 and -15 production by dendritic cells at the early stage of Listeria monocytogenes infection between BALB/c and C57 BL/6 mice. Cell. Immunol. 202, 31–40 (2000).
Dann, S. M. et al. Interleukin-15 activates human natural killer cells to clear the intestinal protozoan Cryptosporidium. J. Infect. Dis. 192, 1294–1302 (2005).
Braun, M. et al. NK cell activation in human hantavirus infection explained by virus-induced IL-15/IL15Rα expression. PLoS Pathog. 10, e1004521 (2014).
Fawaz, L. M., Sharif-Askari, E. & Menezes, J. Up-regulation of NK cytotoxic activity via IL-15 induction by different viruses: a comparative study. J. Immunol. 163, 4473–4480 (1999).
Zhou, R., Wei, H., Sun, R. & Tian, Z. Recognition of double-stranded RNA by TLR3 induces severe small intestinal injury in mice. J. Immunol. 178, 4548–4556 (2007).
Rausch, A. et al. Interleukin-15 mediates protection against experimental tuberculosis: a role for NKG2D-dependent effector mechanisms of CD8+ T cells. Eur. J. Immunol. 36, 1156–1167 (2006).
Perera, L. et al. Expression of nonclassical class I molecules by intestinal epithelial cells. Inflamm. Bowel Dis. 13, 298–307 (2007).
Gonzalez, S., Groh, V. & Spies, T. Immunobiology of human NKG2D and its ligands. Curr. Top. Microbiol. Immunol. 298, 121–138 (2006).
Raulet, D. H., Gasser, S., Gowen, B. G., Deng, W. & Jung, H. Regulation of ligands for the NKG2D activating receptor. Annu. Rev. Immunol. 31, 413–441 (2013).
Gosselin, J., TomoIu, A., Gallo, R. C. & Flamand, L. Interleukin-15 as an activator of natural killer cell-mediated antiviral response. Blood 94, 4210–4219 (1999).
Nguyen, K. B. et al. Coordinated and distinct roles for IFN-αβ, IL-12, and IL-15 regulation of NK cell responses to viral infection. J. Immunol. 169, 4279–4287 (2002).
Sharif-Askari, E., Fawaz, L. M., Tran, P., Ahmad, A. & Menezes, J. Interleukin 15-mediated induction of cytotoxic effector cells capable of eliminating Epstein–Barr virus-transformed/immortalized lymphocytes in culture. J. Natl Cancer Inst. 93, 1724–1732 (2001).
Lanier, L. L. Evolutionary struggles between NK cells and viruses. Nat. Rev. Immunol. 8, 259–268 (2008).
Losy, J., Niezgoda, A. & Zaremba, J. IL-15 is elevated in sera of patients with relapsing-remitting multiple sclerosis. Folia Neuropathol. 40, 151–153 (2002).
Allez, M. et al. CD4+NKG2D+ T cells in Crohn's disease mediate inflammatory and cytotoxic responses through MICA interactions. Gastroenterology 132, 2346–2358 (2007).
Groh, V., Bruhl, A., El-Gabalawy, H., Nelson, J. L. & Spies, T. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc. Natl Acad. Sci. USA 100, 9452–9457 (2003).
de Menthon, M. et al. Excessive interleukin-15 transpresentation endows NKG2D+CD4+ T cells with innate-like capacity to lyse vascular endothelium in granulomatosis with polyangiitis (Wegener's). Arthritis Rheum. 63, 2116–2126 (2011).
Kuczynski, S. et al. IL-15 is elevated in serum patients with type 1 diabetes mellitus. Diabetes Res. Clin. Pract. 69, 231–236 (2005).
Malchow, S. et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science 339, 1219–1224 (2013).
Dong, H. et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8, 793–800 (2002).
Iwai, Y. et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl Acad. Sci. USA 99, 12293–12297 (2002).
Fridman, W. H. et al. Immune infiltration in human cancer: prognostic significance and disease control. Curr. Top. Microbiol. Immunol. 344, 1–24 (2011).
Rubinstein, M. P. et al. Converting IL-15 to a superagonist by binding to soluble IL-15Rα. Proc. Natl Acad. Sci. USA 103, 9166–9171 (2006).
Wong, H. C., Jeng, E. K. & Rhode, P. R. The IL-15-based superagonist ALT-803 promotes the antigen-independent conversion of memory CD8 T cells into innate-like effector cells with antitumor activity. Oncoimmunology 2, e26442 (2013).
Maas, R. A., Dullens, H. F. & Den Otter, W. Interleukin-2 in cancer treatment: disappointing or (still) promising? A review. Cancer Immunol. Immunother. 36, 141–148 (1993).
Theofilopoulos, A. N., Baccala, R., Beutler, B. & Kono, D. H. Type I interferons α/β in immunity and autoimmunity. Annu. Rev. Immunol. 23, 307–336 (2005).
Hammad, H. & Lambrecht, B. N. Barrier epithelial cells and the control of type 2 immunity. Immunity 43, 29–40 (2015).
De Nitto, D., Monteleone, I., Franze, E., Pallone, F. & Monteleone, G. Involvement of interleukin-15 and interleukin-21, two γ-chain-related cytokines, in celiac disease. World J. Gastroenterol. 15, 4609–4614 (2009).
Spolski, R. & Leonard, W. J. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu. Rev. Immunol. 26, 57–79 (2008).
Sutherland, A. P. et al. IL-21 promotes CD8+ CTL activity via the transcription factor T-bet. J. Immunol. 190, 3977–3984 (2013).
Umlauf, S. W. et al. Molecular regulation of the IL-2 gene: rheostatic control of the immune system. Immunol. Rev. 133, 177–197 (1993).
Acknowledgements
The authors thank patients with coeliac disease and their family members, as well as the University of Chicago Coeliac Disease Center, for supporting their research. The authors also thank L.B. Barreiro and B. Sally for critical reading of the manuscript. The work was supported by grants from the Digestive Diseases Research Core Center (DK42086) at the University of Chicago, from the US National Institutes of Health (R01DK67180 and R01DK098435) to B.J. and from the SickKids Foundation (NI15-040) to V.A.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Activation-induced cell death
-
(AICD). A phenomenon in T cells, in which activation through the T cell receptor results in apoptosis. CD95 (also known as Fas) and its ligand (CD95L) are the main regulators of AICD, and the engagement of CD95 ultimately leads to DNA cleavage by caspase-activated DNase (CAD).
- Latent autoimmune diabetes in adults
-
(LADA). A disorder characterized by the presence of diabetes-associated autoantibodies and islet-reactive T cells in the absence of β-cell destruction and overt diabetes.
- Lymphokine-activated killer activity
-
(LAK activity). The ability of T cells to lyse target cells in the absence of specific antigenic stimuli and MHC restriction. LAK cells can be generated in vitro in the presence of interleukin-15 (IL-15) or high concentrations of IL-2.
- Potential coeliac disease
-
A form of coeliac disease defined by the presence of transglutaminase- and gluten-specific antibodies and compatible HLA molecules in the absence of villous atrophy.
- Tissue-resident effector memory T cells
-
(TRM cells). A population of non-circulating memory T cells with an effector-like phenotype that have entered tissues during the effector phase of immune responses and can permanently reside in tissues.
Rights and permissions
About this article
Cite this article
Jabri, B., Abadie, V. IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction. Nat Rev Immunol 15, 771–783 (2015). https://doi.org/10.1038/nri3919
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3919
This article is cited by
-
Lung cancer cell-intrinsic IL-15 promotes cell migration and sensitizes murine lung tumors to anti-PD-L1 therapy
Biomarker Research (2024)
-
Excessive IL-15 promotes cytotoxic CD4 + CD28− T cell-mediated renal injury in lupus nephritis
Immunity & Ageing (2022)
-
Maladaptive consequences of inflammatory events shape individual immune identity
Nature Immunology (2022)
-
Significance of bystander T cell activation in microbial infection
Nature Immunology (2022)
-
Upregulation of IL-15 in the placenta alters trophoblasts behavior contributing to gestational diabetes mellitus
Cell & Bioscience (2021)