Key Points
-
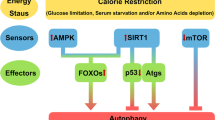
Caloric restriction is known to retard ageing and delay functional decline as well as the onset of disease in most organisms.
-
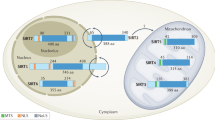
Mammalian sirtuins (SIRT1–SIRT7) have roles in nutrient sensing, energy metabolism and genome stability, and may mediate some of the effects of caloric restriction.
-
Sirtuin 1 (SIRT1), the most studied of the sirtuins, is an unusual target for drug development because it exerts many different effects that are relevant to health and could have a role in lifespan modulation via caloric restriction.
-
Sirtuin-activating compounds are the subject of a growing field in medicinal chemistry, and several SIRT1 activators have been described, including resveratrol and SRT1720.
-
Resveratrol and SRT1720 have been shown to increase healthspan, improve insulin sensitivity and alleviate other harmful effects of obesity in mice, but their mechanisms of action remain controversial.
-
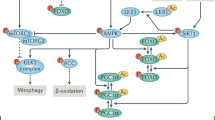
Many of resveratrol's effects on metabolism may be mediated by SIRT1-dependent deacetylation of PPARγ co-activator 1α (PGC1α), a key regulator of mitochondrial biogenesis, and involve AMP-activated protein kinase (AMPK), another key regulator of cellular energy homeostasis.
-
Resveratrol promotes cardiovascular health, and evidence suggests that SIRT1 mediates the inhibitory effect of resveratrol on nuclear factor-κB (NF-κB) activity while also activating nuclear factor erythroid 2-related factor 2 (NRF2) and upregulating NRF2-driven antioxidant systems in endothelial cells.
-
Resveratrol and sirtuins may have neuroprotective effects through several mechanisms, including reduction of inflammation, inhibition of plaque formation and activation of CREB-regulated transcription co-activator 1 (TORC1) signalling.
-
Some recent studies suggest that the beneficial effects of resveratrol and the synthetic SIRT1 activators may also be realized in humans.
-
This Review covers the current status and controversies surrounding the potential of sirtuins as novel pharmacological targets, with a focus on SIRT1.
Abstract
Although the increased lifespan of our populations illustrates the success of modern medicine, the risk of developing many diseases increases exponentially with old age. Caloric restriction is known to retard ageing and delay functional decline as well as the onset of disease in most organisms. Studies have implicated the sirtuins (SIRT1–SIRT7) as mediators of key effects of caloric restriction during ageing. Two unrelated molecules that have been shown to increase SIRT1 activity in some settings, resveratrol and SRT1720, are excellent protectors against metabolic stress in mammals, making SIRT1 a potentially appealing target for therapeutic interventions. This Review covers the current status and controversies surrounding the potential of sirtuins as novel pharmacological targets, with a focus on SIRT1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Le Couteur, D. G., McLachlan, A. J., Quinn, R. J., Simpson, S. J. & de Cabo, R. Aging biology and novel targets for drug discovery. J. Gerontol. A Biol. Sci. Med. Sci. 67, 168–174 (2012).
McCay, C. M. & Crowell, M. F. Prolonging the life span. Sci. Mon. 39, 405–414 (1934).
McCay, C. M., Crowell, M. F. & Maynard, L. A. The effect of retarded growth upon the length of life span and upon the ultimate body size. Nutrition 10, 63–79 (1935).
McCay, C. M., Maynard, L. A., Sperling, G. & Barnes, L. L. Retarded growth, life span, ultimate body size and age changes in the albino rat after feeding diets restricted in calories. Nutr. Rev. 33, 241–253 (1975).
Weindruch, R. & Walford, R. L. The Retardation of Aging and Disease by Dietary Restriction (Charles C. Thomas, 1988).
Ingram, D. K. et al. Calorie restriction mimetics: an emerging research field. Aging Cell 5, 97–108 (2006).
Le Bourg, E. & Rattan, S. I. Can dietary restriction increase longevity in all species, particularly in human beings? Introduction to a debate among experts. Biogerontology 7, 123–125 (2006).
de Grey, A. D. The unfortunate influence of the weather on the rate of ageing: why human caloric restriction or its emulation may only extend life expectancy by 2–3 years. Gerontology 51, 73–82 (2005).
Colman, R. J. et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325, 201–204 (2009). This study provides the first evidence that caloric restriction extends lifespan in non-human primates.
Kuningas, M. et al. Genes encoding longevity: from model organisms to humans. Aging Cell 7, 270–280 (2008).
Miller, R. A. et al. An aging interventions testing program: study design and interim report. Aging Cell 6, 565–575 (2007).
Rae, M. It's never too late: calorie restriction is effective in older mammals. Rejuvenation Res. 7, 3–8 (2004).
Baur, J. A. Resveratrol, sirtuins, and the promise of a DR mimetic. Mech. Ageing Dev. 131, 261–269 (2010).
Spindler, S. R. Caloric restriction: from soup to nuts. Ageing Res. Rev. 9, 324–353 (2010).
Chen, D., Steele, A. D., Lindquist, S. & Guarente, L. Increase in activity during calorie restriction requires Sirt1. Science 310, 1641 (2005). This study demonstrates that SIRT1 is involved in at least some aspects of the response to caloric restriction.
Li, Y., Xu, W., McBurney, M. W. & Longo, V. D. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 8, 38–48 (2008). This study shows that caloric restriction fails to extend lifespan in mice lacking SIRT1.
Oberdoerffer, P. et al. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell 135, 907–918 (2008).
Yu, J. & Auwerx, J. Protein deacetylation by SIRT1: an emerging key post-translational modification in metabolic regulation. Pharmacol. Res. 62, 35–41 (2010).
Lin, S. J., Defossez, P. A. & Guarente, L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289, 2126–2128 (2000).
Rogina, B. & Helfand, S. L. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl Acad. Sci. USA 101, 15998–16003 (2004).
Wang, Y. & Tissenbaum, H. A. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mech. Ageing Dev. 127, 48–56 (2006).
Greer, E. L. & Brunet, A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell 8, 113–127 (2009).
Kaeberlein, M., Kirkland, K. T., Fields, S. & Kennedy, B. K. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2, E296 (2004).
Kaeberlein, M., McVey, M. & Guarente, L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 13, 2570–2580 (1999).
Tissenbaum, H. A. & Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410, 227–230 (2001).
Bauer, J. H. et al. dSir2 and Dmp53 interact to mediate aspects of CR-dependent lifespan extension in D. melanogaster. Aging 1, 38–48 (2009).
Burnett, C. et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature 477, 482–485 (2011). This study provides the strongest evidence against a role for Sir2 in lifespan extension in worms and flies, in contrast to other reports.
Viswanathan, M. & Guarente, L. Regulation of Caenorhabditis elegans lifespan by sir-2.1 transgenes. Nature 477, E1–E2 (2011).
Bordone, L. et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 6, 759–767 (2007). This study demonstrates that SIRT1 overexpression is sufficient to confer metabolic benefits in mice.
Asher, G. et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134, 317–328 (2008).
Nakahata, Y. et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134, 329–340 (2008).
Firestein, R. et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS ONE 3, e2020 (2008).
Yoshizaki, T. et al. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol. Cell Biol. 29, 1363–1374 (2009).
Jeong, H. et al. Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nature Med. 18, 159–165 (2011).
Kim, D. et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 26, 3169–3179 (2007).
Libert, S. et al. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell 147, 1459–1472 (2011).
Guarente, L. Sirtuins in aging and disease. Cold Spring Harb. Symp. Quant. Biol. 72, 483–488 (2007).
Howitz, K. T. et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425, 191–196 (2003). This study proposes resveratrol as a CRM that acts on Sir2 and mediates lifespan extension in yeast.
Baur, J. A. et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444, 337–342 (2006). This study shows that a SIRT1 activator improves insulin sensitivity and reduces mortality in obese mice.
Baur, J. A. & Sinclair, D. A. Therapeutic potential of resveratrol: the in vivo evidence. Nature Rev. Drug Discov. 5, 493–506 (2006).
Jang, M. et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275, 218–220 (1997).
Richard, T. et al. Neuroprotective properties of resveratrol and derivatives. Ann. NY Acad. Sci. 1215, 103–108 (2011).
Barger, J. L. et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS ONE 3, e2264 (2008). This study shows that a low dose of resveratrol mimics caloric restriction at the transcriptional level, and may delay some aspects of ageing.
Pearson, K. J. et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 8, 157–168 (2008). This paper provides a detailed characterization of the long-term effects of resveratrol treatment initiated late in life in male mice. The study reports an abundance of health benefits for all groups, but lifespan extension is beneficial only in mice fed a high-fat diet or mice fed every other day.
Borra, M. T., Smith, B. C. & Denu, J. M. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 280, 17187–17195 (2005).
Kaeberlein, M. et al. Substrate-specific activation of sirtuins by resveratrol. J. Biol. Chem. 280, 17038–17045 (2005). References 45 and 46 provide the first evidence that SIRT1 activation by resveratrol might not be direct.
Milne, J. C. et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450, 712–716 (2007).
Feige, J. N. et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 8, 347–358 (2008). This study shows that a second SIRT1 activator, SRT1720, exerts many of the same metabolic benefits as resveratrol.
Minor, R. K. et al. SRT1720 improves survival and healthspan of obese mice. Sci. Rep. 1, 70 (2011).
Dai, H. et al. SIRT1 activation by small molecules: kinetic and biophysical evidence for direct interaction of enzyme and activator. J. Biol. Chem. 285, 32695–32703 (2010).
Huber, J. L., McBurney, M. W., Distefano, P. S. & McDonagh, T. SIRT1-independent mechanisms of the putative sirtuin enzyme activators SRT1720 and SRT2183. Future Med. Chem. 2, 1751–1759 (2010).
Pacholec, M. et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J. Biol. Chem. 285, 8340–8351 (2010). This paper challenges the idea that resveratrol or more recently developed compounds are direct SIRT1 activators.
Boily, G. et al. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS ONE 3, e1759 (2008).
McBurney, M. W. et al. The mammalian SIR2α protein has a role in embryogenesis and gametogenesis. Mol. Cell Biol. 23, 38–54 (2003).
Michan, S. et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J. Neurosci. 30, 9695–9707 (2010).
Guarente, L. & Franklin, H. Epstein lecture: sirtuins, aging, and medicine. N. Engl. J. Med. 364, 2235–2244 (2011).
Haigis, M. C. & Sinclair, D. A. Mammalian sirtuins: biological insights and disease relevance. Annu. Rev. Pathol. 5, 253–295 (2010).
Rine, J., Strathern, J. N., Hicks, J. B. & Herskowitz, I. A suppressor of mating-type locus mutations in Saccharomyces cerevisiae: evidence for and identification of cryptic mating-type loci. Genetics 93, 877–901 (1979).
Michishita, E., Park, J. Y., Burneskis, J. M., Barrett, J. C. & Horikawa, I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol. Biol. Cell 16, 4623–4635 (2005).
Frye, R. A. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem. Biophys. Res. Commun. 260, 273–279 (1999).
North, B. J., Marshall, B. L., Borra, M. T., Denu, J. M. & Verdin, E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol. Cell 11, 437–444 (2003).
Shi, T., Wang, F., Stieren, E. & Tong, Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J. Biol. Chem. 280, 13560–13567 (2005).
Vaziri, H. et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107, 149–159 (2001).
Haigis, M. C. et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell 126, 941–954 (2006).
Liszt, G., Ford, E., Kurtev, M. & Guarente, L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J. Biol. Chem. 280, 21313–21320 (2005).
Du, J. et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 334, 806–809 (2011). This study demonstrates that at least one sirtuin, SIRT5, has activities beyond deacetylation and ADP ribosylation.
Vakhrusheva, O. et al. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ. Res. 102, 703–710 (2008).
Imai, S., Armstrong, C. M., Kaeberlein, M. & Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800 (2000).
Smith, J. S. et al. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc. Natl Acad. Sci. USA 97, 6658–6663 (2000). References 68 and 69 identify the enzymatic activity of SIRT1.
Cen, Y., Youn, D. Y. & Sauve, A. A. Advances in characterization of human sirtuin isoforms: chemistries, targets and therapeutic applications. Curr. Med. Chem. 18, 1919–1935 (2011).
Verdin, E., Hirschey, M. D., Finley, L. W. & Haigis, M. C. Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem. Sci. 35, 669–675 (2010).
Lombard, D. B. et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol. Cell Biol. 27, 8807–8814 (2007).
Nakagawa, T., Lomb, D. J., Haigis, M. C. & Guarente, L. SIRT5 deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 137, 560–570 (2009).
Someya, S. et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143, 802–812 (2010). This study establishes a role for SIRT3 in the protective effects of caloric restriction.
Hirschey, M. D. et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464, 121–125 (2010).
Zhao, S. et al. Regulation of cellular metabolism by protein lysine acetylation. Science 327, 1000–1004 (2010).
Alcendor, R. R., Kirshenbaum, L. A., Imai, S., Vatner, S. F. & Sadoshima, J. Silent information regulator 2α, a longevity factor and class III histone deacetylase, is an essential endogenous apoptosis inhibitor in cardiac myocytes. Circ. Res. 95, 971–980 (2004). This study shows that SIRT1 can have biphasic effects, based on the level of its overexpression.
Satoh, A., Stein, L. & Imai, S. The role of mammalian sirtuins in the regulation of metabolism, aging, and longevity. Handb. Exp. Pharmacol. 206, 125–162 (2011).
Vaquero, A. et al. SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation. Nature 450, 440–444 (2007).
Wang, R. H. et al. Interplay among BRCA1, SIRT1, and Survivin during BRCA1-associated tumorigenesis. Mol. Cell 32, 11–20 (2008).
Alcendor, R. R. et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ. Res. 100, 1512–1521 (2007).
Banks, A. S. et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 8, 333–341 (2008). Reference 82 describes transgenic mice that express an extra copy of SIRT1 that is under the control of its native promoter, which results in protection from diabetes during obesity.
Herranz, D. et al. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nature Commun. 1, 3 (2010). This study shows that despite providing protection from DNA damage and cancer, SIRT1-transgenic mice do not live longer.
Hsu, C. P. et al. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation 122, 2170–2182 (2010).
Pfluger, P. T., Herranz, D., Velasco-Miguel, S., Serrano, M. & Tschop, M. H. Sirt1 protects against high-fat diet-induced metabolic damage. Proc. Natl Acad. Sci. USA 105, 9793–9798 (2008). Reference 85 describes transgenic mice that express an extra copy of SIRT1 that is under the control of its native promoter, which results in protection from diabetes during obesity.
Zhang, Q. J. et al. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc. Res. 80, 191–199 (2008).
Luo, J. et al. Negative control of p53 by Sir2α promotes cell survival under stress. Cell 107, 137–148 (2001).
Yeung, F. et al. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 23, 2369–2380 (2004).
Brunet, A. et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303, 2011–2015 (2004).
van der Horst, A. et al. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2(SIRT1). J. Biol. Chem. 279, 28873–28879 (2004).
Picard, F. et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature 429, 771–776 (2004).
Nemoto, S., Fergusson, M. M. & Finkel, T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J. Biol. Chem. 280, 16456–16460 (2005).
Rodgers, J. T. et al. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434, 113–118 (2005). This paper demonstrates the role of SIRT1 in energy homeostasis via deacetylation of PGC1α.
Moynihan, K. A. et al. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2, 105–117 (2005).
Baur, J. A. Biochemical effects of SIRT1 activators. Biochim. Biophys. Acta 1804, 1626–1634 (2010).
Dong, Y. et al. SIRT1 is associated with a decrease in acute insulin secretion and a sex specific increase in risk for type 2 diabetes in Pima Indians. Mol. Genet. Metab. 104, 661–665 (2011).
Kim, S. et al. Telomere maintenance genes SIRT1 and XRCC6 impact age-related decline in telomere length but only SIRT1 is associated with human longevity. Biogerontology 13, 119–131 (2011).
Takaoka, M. J. Of the phenolic substances of white hellebore (Veratrum grandiflorum Loes. fil.). J. Faculty Sci. Hokkaido Imperial University 3, 1–16 (1940).
Wood, J. G. et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430, 686–689 (2004).
Bass, T. M., Weinkove, D., Houthoofd, K., Gems, D. & Partridge, L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech. Ageing Dev. 128, 546–552 (2007).
Bauer, J. H., Goupil, S., Garber, G. B. & Helfand, S. L. An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 101, 12980–12985 (2004).
Jarolim, S. et al. A novel assay for replicative lifespan in Saccharomyces cerevisiae. FEMS Yeast Res. 5, 169–177 (2004).
Agarwal, B. & Baur, J. A. Resveratrol and life extension. Ann. NY Acad. Sci. 1215, 138–143 (2011).
Valenzano, D. R. et al. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr. Biol. 16, 296–300 (2006).
Weindruch, R. & Sohal, R. S. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N. Engl. J. Med. 337, 986–994 (1997).
Gledhill, J. R., Montgomery, M. G., Leslie, A. G. & Walker, J. E. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc. Natl Acad. Sci. USA 104, 13632–13637 (2007).
Park, S. et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 148, 421–433 (2012). This study proposes PDE4 as a central target of resveratrol.
Lan, F., Cacicedo, J. M., Ruderman, N. & Ido, Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J. Biol. Chem. 283, 27628–27635 (2008).
Dasgupta, B. & Milbrandt, J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl Acad. Sci. USA 104, 7217–7222 (2007).
Beher, D. et al. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem. Biol. Drug Des. 74, 619–624 (2009).
Canto, C. & Auwerx, J. Targeting sirtuin 1 to improve metabolism: all you need is NAD+? Pharmacol. Rev. 64, 166–187 (2011).
Canto, C. et al. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 11, 213–219 (2010). This paper highlights the complex relationship between SIRT1 and AMPK.
Fulco, M. et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev. Cell 14, 661–673 (2008).
Lagouge, M. et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127, 1109–1122 (2006).
Vang, O. et al. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS ONE 6, e19881 (2011).
Wenz, T., Rossi, S. G., Rotundo, R. L., Spiegelman, B. M. & Moraes, C. T. Increased muscle PGC-1α expression protects from sarcopenia and metabolic disease during aging. Proc. Natl Acad. Sci. USA 106, 20405–20410 (2009). This study shows that overexpression of PGC1α, a SIRT1 target, in skeletal muscle is sufficient to improve health and extend lifespan in mice.
Um, J. H. et al. AMPK-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 59, 554–563 (2009). This paper establishes the requirement for AMPK in many of the metabolic benefits of resveratrol.
Timmers, S. et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 14, 612–622 (2011).
Jager, S., Handschin, C., St-Pierre, J. & Spiegelman, B. M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl Acad. Sci. USA 104, 12017–12022 (2007).
Cheng, H. L. et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc. Natl Acad. Sci. USA 100, 10794–10799 (2003).
Price, N. L. et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 15, 675–690 (2012). This study shows that SIRT1 is required for the beneficial effects of resveratrol on mitochondrial function in skeletal muscle.
Gerhart-Hines, Z. et al. The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD+. Mol. Cell 44, 851–863 (2011).
Smoliga, J. M., Baur, J. A. & Hausenblas, H. A. Resveratrol and health — a comprehensive review of human clinical trials. Mol. Nutr. Food Res. 55, 1129–1141 (2011).
Brasnyo, P. et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 106, 383–389 (2011). This study establishes that resveratrol improves insulin sensitivity in humans in the same way as it does in mice.
Crandall, J. P. et al. Pilot study of resveratrol in older adults with impaired glucose tolerance. J. Gerontol. A Biol. Sci. Med. Sci. 4 Jan 2012 (doi:10.1093/gerona/glr235).
Le Couteur, D. G. & Lakatta, E. G. A vascular theory of aging. J. Gerontol. A Biol. Sci. Med. Sci. 65, 1025–1027 (2010).
Fukao, H. et al. Effect of trans-resveratrol on the thrombogenicity and atherogenicity in apolipoprotein E-deficient and low-density lipoprotein receptor-deficient mice. Blood Coagul. Fibrinolysis 15, 441–446 (2004).
Wang, Z. et al. Dealcoholized red wine containing known amounts of resveratrol suppresses atherosclerosis in hypercholesterolemic rabbits without affecting plasma lipid levels. Int. J. Mol. Med. 16, 533–540 (2005).
Zou, J. et al. Effect of resveratrol on intimal hyperplasia after endothelial denudation in an experimental rabbit model. Life Sci. 68, 153–163 (2000).
Takemura, A. et al. Sirtuin 1 retards hyperphosphatemia-induced calcification of vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 31, 2054–2062 (2011).
Csiszar, A. et al. Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension 54, 668–675 (2009).
Schreiner, C. E. et al. Resveratrol blocks Akt activation in angiotensin II- or EGF-stimulated vascular smooth muscle cells in a redox-independent manner. Cardiovasc. Res. 90, 140–147 (2011).
Csiszar, A. et al. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J. Gerontol. A Biol. Sci. Med. Sci. 4 Jan 2012 (doi:glr228 [pii] 10.1093/gerona/glr228).
Wallerath, T. et al. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 106, 1652–1658 (2002).
Wang, Z. et al. Effects of red wine and wine polyphenol resveratrol on platelet aggregation in vivo and in vitro. Int. J. Mol. Med. 9, 77–79 (2002).
Stef, G., Csiszar, A., Lerea, K., Ungvari, Z. & Veress, G. Resveratrol inhibits aggregation of platelets from high-risk cardiac patients with aspirin resistance. J. Cardiovasc. Pharmacol. 48, 1–5 (2006).
Shigematsu, S. et al. Resveratrol, a red wine constituent polyphenol, prevents superoxide-dependent inflammatory responses induced by ischemia/reperfusion, platelet-activating factor, or oxidants. Free Radic. Biol. Med. 34, 810–817 (2003).
Csiszar, A., Labinskyy, N., Orosz, Z. & Ungvari, Z. Altered mitochondrial energy metabolism may play a role in vascular aging. Med. Hypotheses 67, 904–908 (2006).
Ungvari, Z. et al. Resveratrol increases vascular oxidative stress resistance. Am. J. Physiol. Heart Circ. Physiol. 292, H2417–H2424 (2007).
Xia, N. et al. Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. J. Pharmacol. Exp. Ther. 335, 149–154 (2010).
Kaneko, H. et al. Resveratrol prevents the development of abdominal aortic aneurysm through attenuation of inflammation, oxidative stress, and neovascularization. Atherosclerosis 217, 350–357 (2011).
Kim, J. W. et al. Inhibition of neointimal formation by trans-resveratrol: role of phosphatidyl inositol 3-kinase-dependent Nrf2 activation in heme oxygenase-1 induction. Mol. Nutr. Food Res. 54, 1497–1505 (2010).
Zhang, H., Zhang, J., Ungvari, Z. & Zhang, C. Resveratrol improves endothelial function: role of TNFα and vascular oxidative stress. Arterioscler. Thromb. Vasc. Biol. 29, 1164–1171 (2009).
Chow, S. E., Hshu, Y. C., Wang, J. S. & Chen, J. K. Resveratrol attenuates oxLDL-stimulated NADPH oxidase activity and protects endothelial cells from oxidative functional damages. J. Appl. Physiol. 102, 1520–1527 (2007).
Ungvari, Z. I. et al. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am. J. Physiol. Heart Circ. Physiol. 294, H2121–H2128 (2008).
Ungvari, Z. et al. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 297, H1876–H1881 (2009).
Taubert, D. & Berkels, R. Upregulation and activation of eNOS by resveratrol. Circulation 107, e78–e79 (2003).
Robich, M. P. et al. Resveratrol modifies risk factors for coronary artery disease in swine with metabolic syndrome and myocardial ischemia. Eur. J. Pharmacol. 664, 45–53 (2011).
Wong, R. H. et al. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr. Metab. Cardiovasc. Dis. 21, 851–856 (2011).
Magyar, K. et al. Cardioprotection by resveratrol: a human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 50, 179–187 (2012).
Adabbo, F. et al. The Krebs cycle and mitochondrial mass are early victims of endothelial dysfunction: proteomic approach. Am. J. Pathol. 174, 34–43 (2009).
Csiszar, A. et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 297, H13–H20 (2009).
Wang, Z. et al. Regulation of proliferation and gene expression in cultured human aortic smooth muscle cells by resveratrol and standardized grape extracts. Biochem. Biophys. Res. Commun. 346, 367–376 (2006).
Kleinedler, J. J. et al. Synergistic effect of resveratrol and quercetin released from drug-eluting polymer coatings for endovascular devices. J. Biomed. Mater. Res. B Appl. Biomater. 99, 266–275 (2011).
Gehm, B. D., McAndrews, J. M., Chien, P. Y. & Jameson, J. L. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc. Natl Acad. Sci. USA 94, 14138–14143 (1997).
Klinge, C. M., Wickramasinghe, N. S., Ivanova, M. M. & Dougherty, S. M. Resveratrol stimulates nitric oxide production by increasing estrogen receptor α-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 22, 2185–2197 (2008).
Khandelwal, A. R., Hebert, V. Y. & Dugas, T. R. Essential role of ER-α-dependent NO production in resveratrol-mediated inhibition of restenosis. Am. J. Physiol. Heart Circ. Physiol. 299, H1451–H1458 (2010).
Bowers, J. L., Tyulmenkov, V. V., Jernigan, S. C. & Klinge, C. M. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors α and β. Endocrinology 141, 3657–3667 (2000).
Dubey, R. K. et al. Resveratrol, a red wine constituent, blocks the antimitogenic effects of estradiol on human female coronary artery smooth muscle cells. J. Clin. Endocrinol. Metab. 95, E9–E17 (2010).
Hsu, C. P., Odewale, I., Alcendor, R. R. & Sadoshima, J. Sirt1 protects the heart from aging and stress. Biol. Chem. 389, 221–231 (2008).
Mattagajasingh, I. et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl Acad. Sci. USA 104, 14855–14860 (2007).
Tanno, M. et al. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J. Biol. Chem. 285, 8375–8382 (2010).
Sulaiman, M. et al. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 298, H833–H843 (2010).
Breitenstein, A. et al. Sirt1 inhibition promotes in vivo arterial thrombosis and tissue factor expression in stimulated cells. Cardiovasc. Res. 89, 464–472 (2011).
Miyazaki, R. et al. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 28, 1263–1269 (2008).
Biala, A. et al. Resveratrol induces mitochondrial biogenesis and ameliorates Ang II-induced cardiac remodeling in transgenic rats harboring human renin and angiotensinogen genes. Blood Press. 19, 196–205 (2010).
Renaud, S. & de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 339, 1523–1526 (1992).
Danz, E. D., Skramsted, J., Henry, N., Bennett, J. A. & Keller, R. S. Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radic. Biol. Med. 46, 1589–1597 (2009).
Zhang, C. et al. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through SIRT1-mediated deacetylation of p53. Cardiovasc. Res. 90, 538–545 (2011).
Wang, Z. et al. Effect of resveratrol on platelet aggregation in vivo and in vitro. Chin. Med. J. 115, 378–380 (2002).
Ungvari, Z. et al. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am. J. Physiol. Heart Circ. Physiol. 300, H1133–H1140 (2011).
Albani, D., Polito, L. & Forloni, G. Sirtuins as novel targets for Alzheimer's disease and other neurodegenerative disorders: experimental and genetic evidence. J. Alzheimers Dis. 19, 11–26 (2010).
Julien, C. et al. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J. Neuropathol. Exp. Neurol. 68, 48–58 (2009).
Girbovan, C., Morin, L. & Plamondon, H. Repeated resveratrol administration confers lasting protection against neuronal damage but induces dose-related alterations of behavioral impairments after global ischemia. Behav. Pharmacol. 23, 1–13 (2012).
Shin, J. A. et al. Therapeutic effects of resveratrol during acute periods following experimental ischemic stroke. J. Neuroimmunol. 227, 93–100 (2010).
Della-Morte, D. et al. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience 159, 993–1002 (2009).
Lu, K. T. et al. Neuroprotective effects of resveratrol on cerebral ischemia-induced neuron loss mediated by free radical scavenging and cerebral blood flow elevation. J. Agric. Food Chem. 54, 3126–3131 (2006).
Parker, J. A. et al. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nature Genet. 37, 349–350 (2005).
Inoue, H. et al. Brain protection by resveratrol and fenofibrate against stroke requires peroxisome proliferator-activated receptor α in mice. Neurosci. Lett. 352, 203–206 (2003).
Frisardi, V. et al. Metabolic-cognitive syndrome: a cross-talk between metabolic syndrome and Alzheimer's disease. Ageing Res. Rev. 9, 399–417 (2010).
Capiralla, H. et al. Resveratrol mitigates lipopolysaccharide- and Aβ-mediated microglial inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J. Neurochem. 120, 461–472 (2012).
Bonda, D. J. et al. The sirtuin pathway in ageing and Alzheimer disease: mechanistic and therapeutic considerations. Lancet Neurol. 10, 275–279 (2011).
Araki, T., Sasaki, Y. & Milbrandt, J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 305, 1010–1013 (2004).
Gao, J. et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466, 1105–1109 (2010).
Satoh, A. et al. SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. J. Neurosci. 30, 10220–10232 (2010).
Donmez, G., Wang, D., Cohen, D. E. & Guarente, L. SIRT1 suppresses β-amyloid production by activating the α-secretase gene ADAM10. Cell 142, 320–332 (2010).
Min, S. W. et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 67, 953–966 (2010).
Donmez, G. et al. SIRT1 protects against α-synuclein aggregation by activating molecular chaperones. J. Neurosci. 32, 124–132 (2012).
Mudo, G. et al. Transgenic expression and activation of PGC-1α protect dopaminergic neurons in the MPTP mouse model of Parkinson's disease. Cell. Mol. Life Sci. 69, 1153–1165 (2012).
Outeiro, T. F. et al. Sirtuin 2 inhibitors rescue α-synuclein-mediated toxicity in models of Parkinson's disease. Science 317, 516–519 (2007).
Albani, D. et al. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by α-synuclein or amyloid-β (1–42) peptide. J. Neurochem. 110, 1445–1456 (2009).
Jiang, M. et al. Neuroprotective role of Sirt1 in mammalian models of Huntington's disease through activation of multiple Sirt1 targets. Nature Med. 18, 153–158 (2011).
Harikumar, K. B. & Aggarwal, B. B. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle 7, 1020–1035 (2008).
Gao, X., Xu, Y. X., Janakiraman, N., Chapman, R. A. & Gautam, S. C. Immunomodulatory activity of resveratrol: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production. Biochem. Pharmacol. 62, 1299–1308 (2001).
Kim, G. Y. et al. Resveratrol inhibits phenotypic and functional maturation of murine bone marrow-derived dendritic cells. Int. Immunopharmacol. 4, 245–253 (2004).
Kimura, Y., Okuda, H. & Arichi, S. Effects of stilbenes on arachidonate metabolism in leukocytes. Biochim. Biophys. Acta 834, 275–278 (1985).
Tao, H. Y. et al. The grape component resveratrol interferes with the function of chemoattractant receptors on phagocytic leukocytes. Cell. Mol. Immunol. 1, 50–56 (2004).
Wirleitner, B., Schroecksnadel, K., Winkler, C., Schennach, H. & Fuchs, D. Resveratrol suppresses interferon-γ-induced biochemical pathways in human peripheral blood mononuclear cells in vitro. Immunol. Lett. 100, 159–163 (2005).
Dioum, E. M. et al. Regulation of hypoxia-inducible factor 2α signaling by the stress-responsive deacetylase sirtuin 1. Science 324, 1289–1293 (2009).
Lim, J. H. et al. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1α. Mol. Cell 38, 864–878 (2010).
Cohen, H. Y. et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305, 390–392 (2004).
Motta, M. C. et al. Mammalian SIRT1 represses forkhead transcription factors. Cell 116, 551–563 (2004).
Westerheide, S. D., Anckar, J., Stevens, S. M. Jr, Sistonen, L. & Morimoto, R. I. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science 323, 1063–1066 (2009).
Gracia-Sancho, J., Villarreal, G. Jr, Zhang, Y. & Garcia-Cardena, G. Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc. Res. 85, 514–519 (2010).
Callaway, E. Questions hang over red-wine chemical. Nature 2 Feb 2012 (doi:10.1038/nature.2012.9970).
Hou, X. et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 283, 20015–20026 (2008).
Lin, J. N. et al. Resveratrol modulates tumor cell proliferation and protein translation via SIRT1-dependent AMPK activation. J. Agric. Food Chem. 58, 1584–1592 (2010).
Yoshizaki, T. et al. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 298, E419–E428 (2010).
Kim, D. H. et al. SIRT1 activation by resveratrol ameliorates cisplatin-induced renal injury through deacetylation of p53. Am. J. Physiol. Renal Physiol. 301, F427–F435 (2011).
Vetterli, L., Brun, T., Giovannoni, L., Bosco, D. & Maechler, P. Resveratrol potentiates glucose-stimulated insulin secretion in INS-1E β-cells and human islets through a SIRT1-dependent mechanism. J. Biol. Chem. 286, 6049–6060 (2011).
Shindler, K. S. et al. Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. J. Neuroophthalmol. 30, 328–339 (2010).
Park, J. M. et al. Role of resveratrol in FOXO1-mediated gluconeogenic gene expression in the liver. Biochem. Biophys. Res. Commun. 403, 329–334 (2010).
Yang, J., Wang, N., Li, J., Zhang, J. & Feng, P. Effects of resveratrol on NO secretion stimulated by insulin and its dependence on SIRT1 in high glucose cultured endothelial cells. Endocrine 37, 365–372 (2010).
He, X., Andersson, G., Lindgren, U. & Li, Y. Resveratrol prevents RANKL-induced osteoclast differentiation of murine osteoclast progenitor RAW 264.7 cells through inhibition of ROS production. Biochem. Biophys. Res. Commun. 401, 356–362 (2010).
Li, J., Qu, X., Ricardo, S. D., Bertram, J. F. & Nikolic-Paterson, D. J. Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am. J. Pathol. 177, 1065–1071 (2010).
Kao, C. L. et al. Resveratrol protects human endothelium from H2O2-induced oxidative stress and senescence via SirT1 activation. J. Atheroscler. Thromb. 17, 970–979 (2010).
Fischer-Posovszky, P. et al. Resveratrol regulates human adipocyte number and function in a Sirt1-dependent manner. Am. J. Clin. Nutr. 92, 5–15 (2010).
Ohguchi, K. et al. SIRT1 modulates expression of matrix metalloproteinases in human dermal fibroblasts. Br. J. Dermatol. 163, 689–694 (2010).
Xia, L., Ding, F., Zhu, J. H. & Fu, G. S. Resveratrol attenuates apoptosis of pulmonary microvascular endothelial cells induced by high shear stress and proinflammatory factors. Hum. Cell 24, 127–133 (2011).
Bemis, J. E. et al. Discovery of oxazolo[4,5-b]pyridines and related heterocyclic analogs as novel SIRT1 activators. Bioorg. Med. Chem. Lett. 19, 2350–2353 (2009).
Vu, C. B. et al. Discovery of imidazo[1,2-b]thiazole derivatives as novel SIRT1 activators. J. Med. Chem. 52, 1275–1283 (2009).
Mai, A. et al. Study of 1,4-dihydropyridine structural scaffold: discovery of novel sirtuin activators and inhibitors. J. Med. Chem. 52, 5496–5504 (2009).
Smith, J. J. et al. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst. Biol. 3, 31 (2009).
Yamazaki, Y. et al. Treatment with SRT1720, a SIRT1 activator, ameliorates fatty liver with reduced expression of lipogenic enzymes in MSG mice. Am. J. Physiol. Endocrinol. Metab. 297, E1179–E1186 (2009).
Walker, A. K. et al. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 24, 1403–1417 (2010). This paper identifies a role for SIRT1 in the regulation of lipid and cholesterol synthesis through SREBPs.
Ray, K. K. et al. Statins and all-cause mortality in high-risk primary prevention: a meta-analysis of 11 randomized controlled trials involving 65,229 participants. Arch. Intern. Med. 170, 1024–1031 (2010).
McNaughton, S. A., Bates, C. J. & Mishra, G. D. Diet quality is associated with all-cause mortality in adults aged 65 years and older. J. Nutr. 142, 320–325 (2012).
Singer, E. More trouble for Sirtris. Technology Review (MIT) website [online], www.technologyreview.com/blog/editors/25150 (2010).
Smoliga, J. M., Vang, O. & Baur, J. A. Challenges of translating basic research into therapeutics: resveratrol as an example. J. Gerontol. A Biol. Sci. Med. Sci. 67, 158–167 (2011).
Welch, H. G., Albertsen, P. C., Nease, R. F., Bubolz, T. A. & Wasson, J. H. Estimating treatment benefits for the elderly: the effect of competing risks. Ann. Intern. Med. 124, 577–584 (1996).
Olshansky, S. J., Perry, D., Miller, R. A. & Butler, R. N. In pursuit of the longevity dividend: what should we be doing to prepare for the unprecedented aging of humanity? The Scientist 20, 28–36 (2006).
Olshansky, S. J., Perry, D., Miller, R. A. & Butler, R. N. Pursuing the longevity dividend: scientific goals for an aging world. Ann. NY Acad. Sci. 1114, 11–13 (2007).
Olshansky, S. J., Carnes, B. A. & Cassel, C. In search of Methuselah: estimating the upper limits to human longevity. Science 250, 634–640 (1990).
Milne, J. C. & Denu, J. M. The sirtuin family: therapeutic targets to treat diseases of aging. Curr. Opin. Chem. Biol. 12, 11–17 (2008).
Fries, J. F. Aging, natural death, and the compression of morbidity. N. Engl. J. Med. 303, 130–135 (1980).
Le Couteur, D. G. et al. in Calorie Restriction, Aging and Longevity (eds Everitt, A. V., Rattan, S. I. S., Le Couteur, D. A. & de Cabo, R.) 191–216 (Springer Press, 2010).
Hilmer, S. N. et al. Age-related changes in the hepatic sinusoidal endothelium impede lipoprotein transfer in the rat. Hepatology 42, 1349–1354 (2005).
Braidy, N. et al. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS ONE 6, e19194 (2011).
Fried, L. P. et al. Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 56, M146–M156 (2001).
Bandinelli, S., Corsi, A. M., Milaneschi, Y. & Vazzana, R. Frailty and the homeostatic network. Acta. Biomed. 81 (Suppl. 1), 15–18 (2010).
Moore, A. Z. et al. Polymorphisms in the mitochondrial DNA control region and frailty in older adults. PLoS ONE 5, e11069 (2010).
Strong, R. et al. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell 7, 641–650 (2008).
Wanke, V. et al. Caffeine extends yeast lifespan by targeting TORC1. Mol. Microbiol. 69, 277–285 (2008).
Warner, H. R., Ingram, D., Miller, R. A., Nadon, N. L. & Richardson, A. G. Program for testing biological interventions to promote healthy aging. Mech. Ageing Dev. 115, 199–207 (2000).
Doll, R., Peto, R., Boreham, J. & Sutherland, I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ 328, 1519 (2004).
Taylor, D. H. Jr, Hasselblad, V., Henley, S. J., Thun, M. J. & Sloan, F. A. Benefits of smoking cessation for longevity. Am. J. Public Health 92, 990–996 (2002).
Whitlock, G. et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 373, 1083–1096 (2009).
Franco, O. H. et al. Effects of physical activity on life expectancy with cardiovascular disease. Arch. Intern. Med. 165, 2355–2360 (2005).
Jonker, J. T. et al. Physical activity and life expectancy with and without diabetes: life table analysis of the Framingham Heart Study. Diabetes Care 29, 38–43 (2006).
Nusselder, W. J., Franco, O. H., Peeters, A. & Mackenbach, J. P. Living healthier for longer: comparative effects of three heart-healthy behaviors on life expectancy with and without cardiovascular disease. BMC Public Health 9, 487 (2009).
Teramoto, M. & Bungum, T. J. Mortality and longevity of elite athletes. J. Sci. Med. Sport 13, 410–416 (2010).
Bjelakovic, G., Nikolova, D., Gluud, L. L., Simonetti, R. G. & Gluud, C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. CD007176 (2012).
Streppel, M. T., Ocke, M. C., Boshuizen, H. C., Kok, F. J. & Kromhout, D. Long-term wine consumption is related to cardiovascular mortality and life expectancy independently of moderate alcohol intake: the Zutphen Study. J. Epidemiol. Community Health 63, 534–540 (2009).
Weindruch, R., Walford, R. L., Fligiel, S. & Guthrie, D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J. Nutr. 116, 641–654 (1986).
Holloszy, J. O., Smith, E. K., Vining, M. & Adams, S. Effect of voluntary exercise on longevity of rats. J. Appl. Physiol. 59, 826–831 (1985).
Aguiar-Oliveira, M. H. et al. Longevity in untreated congenital growth hormone deficiency due to a homozygous mutation in the GHRH receptor gene. J. Clin. Endocrinol. Metab. 95, 714–721 (2010).
Wilson, R., Gazzala, J. & House, J. Aspirin in primary and secondary prevention in elderly adults revisited. South. Med. J. 105, 82–86 (2012).
Baigent, C. et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 373, 1849–1860 (2009).
Aguero-Torres, H., Viitanen, M., Fratiglioni, L. & Louhija, J. The effect of low-dose daily aspirin intake on survival in the Finnish centenarians cohort. J. Am. Geriatr. Soc. 49, 1578–1580 (2001).
Ford, I. et al. Long-term follow-up of the West of Scotland Coronary Prevention Study. N. Engl. J. Med. 357, 1477–1486 (2007).
Bellizzi, D. et al. A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics 85, 258–263 (2005).
Rose, G. et al. Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp. Gerontol. 38, 1065–1070 (2003).
Lescai, F. et al. Human longevity and 11p15.5: a study in 1321 centenarians. Eur. J. Hum. Genet. 17, 1515–1519 (2009).
Qiu, X., Brown, K., Hirschey, M. D., Verdin, E. & Chen, D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 12, 662–667 (2010).
Hallows, W. C. et al. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol. Cell 41, 139–149 (2011).
Palacios, O. M. et al. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1α in skeletal muscle. Aging 1, 771–783 (2009).
Shimazu, T. et al. SIRT3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 and regulates ketone body production. Cell Metab. 12, 654–661 (2010).
Mostoslavsky, R. et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124, 315–329 (2006).
Kaidi, A., Weinert, B. T., Choudhary, C. & Jackson, S. P. Human SIRT6 promotes DNA end resection through CtIP deacetylation. Science 329, 1348–1353 (2010).
Mao, Z. et al. SIRT6 promotes DNA repair under stress by activating PARP1. Science 332, 1443–1446 (2011).
Michishita, E. et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature 452, 492–496 (2008).
Michishita, E. et al. Cell cycle-dependent deacetylation of telomeric histone H3 lysine K56 by human SIRT6. Cell Cycle 8, 2664–2666 (2009).
Yang, B., Zwaans, B. M., Eckersdorff, M. & Lombard, D. B. The sirtuin SIRT6 deacetylates H3 K56Ac in vivo to promote genomic stability. Cell Cycle 8, 2662–2663 (2009).
Kawahara, T. L. et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-κB-dependent gene expression and organismal life span. Cell 136, 62–74 (2009).
Zhong, L. et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1α. Cell 140, 280–293 (2010).
Kanfi, Y. et al. The sirtuin SIRT6 regulates lifespan in male mice. Nature 22 Feb 2012 (doi:10.1038/nature10815). This paper shows for the first time that overexpression of SIRT6 can extend lifespan in male mice.
Berryman, D. E., Christiansen, J. S., Johannsson, G., Thorner, M. O. & Kopchick, J. J. Role of the GH/IGF-1 axis in lifespan and healthspan: lessons from animal models. Growth Horm. IGF Res. 18, 455–471 (2008).
Ramsey, K. M., Mills, K. F., Satoh, A. & Imai, S. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell 7, 78–88 (2008).
Rodgers, J. T. & Puigserver, P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc. Natl Acad. Sci. USA 104, 12861–12866 (2007).
Palacios, J. A. et al. SIRT1 contributes to telomere maintenance and augments global homologous recombination. J. Cell Biol. 191, 1299–1313 (2010).
Kabra, N. et al. SirT1 is an inhibitor of proliferation and tumor formation in colon cancer. J. Biol. Chem. 284, 18210–18217 (2009).
Kakefuda, K. et al. Sirtuin 1 overexpression mice show a reference memory deficit, but not neuroprotection. Biochem. Biophys. Res. Commun. 387, 784–788 (2009).
Hasegawa, K. et al. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J. Biol. Chem. 285, 13045–13056 (2010).
Tang, M. M. et al. Intra-arterial targeted islet-specific expression of Sirt1 protects β cells from streptozotocin-induced apoptosis in mice. Mol. Ther. 19, 60–66 (2010).
Li, Y. et al. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J. 25, 1664–1679 (2011).
Li, L. et al. SIRT1 acts as a modulator of neointima formation following vascular injury in mice. Circ. Res. 108, 1180–1189 (2011).
Oomen, C. A. et al. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front. Aging Neurosci. 1, 4 (2009).
Labbé, A. et al. Resveratrol improves insulin resistance hyperglycemia and hepatosteatosis but not hypertriglyceridemia, inflammation, and life span in a mouse model for Werner syndrome. J. Gerontol. A Med. Sci. Biol. Sci. 66, 264–278 (2011).
Wong, Y. T., Gruber, J., Jenner, A. M., Tay, F. E. & Ruan, R. Chronic resveratrol intake reverses pro-inflammatory cytokine profile and oxidative DNA damage in ageing hybrid mice. Age 33, 229–246 (2011).
Miller, R. A. et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 66, 191–201 (2011).
Jackson, J. R., Ryan, M. J. & Alway, S. E. Long-term supplementation with resveratrol alleviates oxidative stress but does not attenuate sarcopenia in aged mice. J. Gerontol. A Biol. Sci. Med. Sci. 66, 751–764 (2011).
Acknowledgements
The preparation of this manuscript was supported by the Intramural Research Program of the US National Institutes of Health (NIH), the US National Institute on Aging, research grants from the NIH (AT006526 to Z.U. and AG031182 to J.A.B.), the Ellison Medical Foundation, the National Health and Medical Research Council of Australia, and the Ageing and Alzheimer's Research Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Baur, J., Ungvari, Z., Minor, R. et al. Are sirtuins viable targets for improving healthspan and lifespan?. Nat Rev Drug Discov 11, 443–461 (2012). https://doi.org/10.1038/nrd3738
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3738
This article is cited by
-
NAD+ metabolism-based immunoregulation and therapeutic potential
Cell & Bioscience (2023)
-
The multifaceted benefits of walking for healthy aging: from Blue Zones to molecular mechanisms
GeroScience (2023)
-
SIRT4 in ageing
Biogerontology (2023)
-
Preventing spontaneous cerebral microhemorrhages in aging mice: a novel approach targeting cellular senescence with ABT263/navitoclax
GeroScience (2023)
-
Multiple myeloma, a quintessential malignant disease of aging: a geroscience perspective on pathogenesis and treatment
GeroScience (2023)