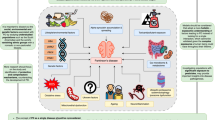
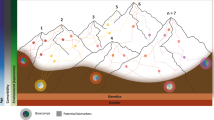
Abstract
Parkinson's disease is caused by the premature death of neurons in the midbrain. By contrast, cancer spawns from cells that refuse to die. We would therefore expect their pathogenic mechanisms to be very different. However, recent genetic studies and emerging functional work show that strikingly similar and overlapping pathways are involved in both diseases. We consider these areas of convergence and discuss how insights from one disease can inform us about, and possibly help us to treat, the other.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jankovic, J. Parkinson's disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatr. 79, 368–376 (2008).
Doshay, L. J. Problem situations in the treatment of paralysis agitans. J. Am. Med. Assoc. 156, 680–684 (1954).
Polymeropoulos, M. H. et al. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047 (1997).
Spillantini, M. G. et al. α-synuclein in Lewy bodies. Nature 388, 839–840 (1997).
Braak, H. & Braak, E. Pathoanatomy of Parkinson's disease. J. Neurol. 247 (Suppl. 2), II3–10 (2000).
Satake, W. et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nature Genet. 41, 1303–1307 (2009).
Simon-Sanchez, J. et al. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nature Genet. 41, 1308–1312 (2009).
Hardy, J. Genetic analysis of pathways to Parkinson disease. Neuron 68, 201–206 (2010).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Greenman, C. et al. Patterns of somatic mutation in human cancer genomes. Nature 446, 153–158 (2007).
Rubinsztein, D. C. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 443, 780–786 (2006).
Schulz, J. B. & Falkenburger, B. H. Neuronal pathology in Parkinson's disease. Cell Tissue Res. 318, 135–147 (2004).
McNaught, K. S., Belizaire, R., Isacson, O., Jenner, P. & Olanow, C. W. Altered proteasomal function in sporadic Parkinson's disease. Exp. Neurol. 179, 38–46 (2003).
Scott, M. D. & Frydman, J. Aberrant protein folding as the molecular basis of cancer. Methods Mol. Biol. 232, 67–76 (2003).
Soussi, T. & Wiman, K. G. Shaping genetic alterations in human cancer: the p53 mutation paradigm. Cancer Cell 12, 303–312 (2007).
Brooks, C. L. & Gu, W. p53 regulation by ubiquitin. FEBS Lett. 585, 2803–2809 (2011).
Rotter, V. p53, a transformation-related cellular-encoded protein, can be used as a biochemical marker for the detection of primary mouse tumor cells. Proc. Natl Acad. Sci. USA 80, 2613–2617 (1983).
Moll, U. M., Riou, G. & Levine, A. J. Two distinct mechanisms alter p53 in breast cancer: mutation and nuclear exclusion. Proc. Natl Acad. Sci. USA 89, 7262–7266 (1992).
Gannon, J. V., Greaves, R., Iggo, R. & Lane, D. P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 9, 1595–1602 (1990).
Xu, J. et al. Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nature Chem. Biol. 7, 285–295 (2011).
Kim, C. H. et al. Role of reactive oxygen species-dependent protein aggregation in metabolic stress-induced necrosis. Int. J. Oncol. 37, 97–102 (2010).
Kitada, T. et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608 (1998).
Shimura, H. et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nature Genet. 25, 302–305 (2000).
Tanaka, K., Suzuki, T., Hattori, N. & Mizuno, Y. Ubiquitin, proteasome and parkin. Biochim. Biophys. Acta 1695, 235–247 (2004).
Chung, K. K. et al. S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science 304, 1328–1331 (2004).
LaVoie, M. J., Ostaszewski, B. L., Weihofen, A., Schlossmacher, M. G. & Selkoe, D. J. Dopamine covalently modifies and functionally inactivates parkin. Nature Med. 11, 1214–1221 (2005).
Winklhofer, K. F., Henn, I. H., Kay-Jackson, P. C., Heller, U. & Tatzelt, J. Inactivation of parkin by oxidative stress and C-terminal truncations: a protective role of molecular chaperones. J. Biol. Chem. 278, 47199–47208 (2003).
Ziviani, E., Tao, R. N. & Whitworth, A. J. Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc. Natl Acad. Sci. USA 107, 5018–5023 (2010).
Gegg, M. E. et al. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum. Mol. Genet. 19, 4861–4870 (2010).
Rakovic, A. et al. Mutations in PINK1 and Parkin impair ubiquitination of Mitofusins in human fibroblasts. PLoS ONE 6, e16746 (2011).
Saito, S. et al. Definition of a commonly deleted region in ovarian cancers to a 300-kb segment of chromosome 6q27. Cancer Res. 56, 5586–5589 (1996).
Orphanos, V. et al. Allelic imbalance of chromosome 6q in ovarian tumours. Br. J. Cancer 71, 666–669 (1995).
Kong, F. M., Anscher, M. S., Washington, M. K., Killian, J. K. & Jirtle, R. L. M6P/IGF2R is mutated in squamous cell carcinoma of the lung. Oncogene 19, 1572–1578 (2000).
Negrini, M. et al. Suppression of tumorigenicity of breast cancer cells by microcell-mediated chromosome transfer: studies on chromosomes 6 and 11. Cancer Res. 54, 1331–1336 (1994).
Cesari, R. et al. Parkin, a gene implicated in autosomal recessive juvenile parkinsonism, is a candidate tumor suppressor gene on chromosome 6q25-q27. Proc. Natl Acad. Sci. USA 100, 5956–5961 (2003).
Veeriah, S. et al. Somatic mutations of the Parkinson's disease-associated gene PARK2 in glioblastoma and other human malignancies. Nature Genet. 42, 77–82 (2010).
Klein, C., Lohmann-Hedrich, K., Rogaeva, E., Schlossmacher, M. G. & Lang, A. E. Deciphering the role of heterozygous mutations in genes associated with parkinsonism. Lancet Neurol. 6, 652–662 (2007).
Tay, S. P. et al. Parkin enhances the expression of cyclin-dependent kinase 6 and negatively regulates the proliferation of breast cancer cells. J. Biol. Chem. 285, 29231–29238 (2010).
da Costa, C. A. et al. Transcriptional repression of p53 by parkin and impairment by mutations associated with autosomal recessive juvenile Parkinson's disease. Nature Cell Biol. 11, 1370–1375 (2009).
Rodriguez-Gonzalez, A. et al. Role of the aggresome pathway in cancer: targeting histone deacetylase 6-dependent protein degradation. Cancer Res. 68, 2557–2560 (2008).
Olzmann, J. A. & Chin, L. S. Parkin-mediated K63-linked polyubiquitination: a signal for targeting misfolded proteins to the aggresome-autophagy pathway. Autophagy 4, 85–87 (2008).
Kawaguchi, Y. et al. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell 115, 727–738 (2003).
Tang, M. et al. Interactions of Wnt/β-catenin signaling and sonic hedgehog regulate the neurogenesis of ventral midbrain dopamine neurons. J. Neurosci. 30, 9280–9291 (2010).
Cicero, S. & Herrup, K. Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J. Neurosci. 25, 9658–9668 (2005).
Nguyen, M. D., Mushynski, W. E. & Julien, J. P. Cycling at the interface between neurodevelopment and neurodegeneration. Cell Death Differ. 9, 1294–1306 (2002).
Simon, D. K. et al. Somatic mitochondrial DNA mutations in cortex and substantia nigra in aging and Parkinson's disease. Neurobiol. Aging 25, 71–81 (2004).
Bender, A. et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nature Genet. 38, 515–517 (2006).
Yang, J. L., Weissman, L., Bohr, V. A. & Mattson, M. P. Mitochondrial DNA damage and repair in neurodegenerative disorders. DNA Repair 7, 1110–1120 (2008).
Ikeda, Y., Matsunaga, Y., Takiguchi, M. & Ikeda, M. A. Expression of cyclin E in postmitotic neurons during development and in the adult mouse brain. Gene Expr. Patterns 11, 64–71 (2011).
Staropoli, J. F. et al. Parkin is a component of an SCF-like ubiquitin ligase complex and protects postmitotic neurons from kainate excitotoxicity. Neuron 37, 735–749 (2003).
Lopes, J. P. & Agostinho, P. Cdk5: multitasking between physiological and pathological conditions. Prog. Neurobiol. 94, 49–63 (2011).
Hoglinger, G. U. et al. The pRb/E2F cell-cycle pathway mediates cell death in Parkinson's disease. Proc. Natl Acad. Sci. USA 104, 3585–3590 (2007).
Ko, H. S. et al. Accumulation of the authentic parkin substrate aminoacyl-tRNA synthetase cofactor, p38/JTV-1, leads to catecholaminergic cell death. J. Neurosci. 25, 7968–7978 (2005).
Di Fonzo, A. et al. FBXO7 mutations cause autosomal recessive, early-onset parkinsonian-pyramidal syndrome. Neurology 72, 240–245 (2009).
Jackson, P. K. & Eldridge, A. G. The SCF ubiquitin ligase: an extended look. Mol. Cell 9, 923–925 (2002).
Laman, H. et al. Transforming activity of Fbxo7 is mediated specifically through regulation of cyclin D/cdk6. EMBO J. 24, 3104–3116 (2005).
Chang, Y. F., Cheng, C. M., Chang, L. K., Jong, Y. J. & Yuo, C. Y. The F-box protein Fbxo7 interacts with human inhibitor of apoptosis protein cIAP1 and promotes cIAP1 ubiquitination. Biochem. Biophys. Res. Commun. 342, 1022–1026 (2006).
Bonifati, V. et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299, 256–259 (2003).
Bretaud, S., Allen, C., Ingham, P. W. & Bandmann, O. p53-dependent neuronal cell death in a DJ-1-deficient zebrafish model of Parkinson's disease. J. Neurochem. 100, 1626–1635 (2007).
Fan, J. et al. DJ-1 decreases Bax expression through repressing p53 transcriptional activity. J. Biol. Chem. 283, 4022–4030 (2008).
Giaime, E. et al. Loss of function of DJ-1 triggered by Parkinson's disease-associated mutation is due to proteolytic resistance to caspase-6. Cell Death Differ. 17, 158–169 (2010).
Healy, D. G. et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. Lancet Neurol. 7, 583–590 (2008).
Ambros, V. The functions of animal microRNAs. Nature 431, 350–355 (2004).
Gehrke, S., Imai, Y., Sokol, N. & Lu, B. Pathogenic LRRK2 negatively regulates microRNA-mediated translational repression. Nature 466, 637–641 (2010).
Garzon, R., Marcucci, G. & Croce, C. M. Targeting microRNAs in cancer: rationale, strategies and challenges. Nature Rev. Drug Discov. 9, 775–789 (2010).
Langston, J. W., Ballard, P., Tetrud, J. W. & Irwin, I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219, 979–980 (1983).
Schapira, A. H. et al. Mitochondrial complex I deficiency in Parkinson's disease. Lancet 1, 1269 (1989).
Betarbet, R. et al. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nature Neurosci. 3, 1301–1306 (2000).
Warburg, O. On the origin of cancer cells. Science 123, 309–314 (1956).
Gargini, R., Garcia-Escudero, V. & Izquierdo, M. Therapy mediated by mitophagy abrogates tumor progression. Autophagy 7, 466–476 (2011).
Kim, J. H. et al. Involvement of mitophagy in oncogenic K-Ras-induced transformation: overcoming a cellular energy deficit from glucose deficiency. Autophagy 7 (2011).
Valente, E. M. et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science 304, 1158–1160 (2004).
Pridgeon, J. W., Olzmann, J. A., Chin, L. S. & Li, L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 5, e172 (2007).
Park, J. et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441, 1157–1161 (2006).
Clark, I. E. et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 441, 1162–1166 (2006).
Exner, N. et al. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J. Neurosci. 27, 12413–12418 (2007).
Narendra, D., Tanaka, A., Suen, D. F. & Youle, R. J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803 (2008).
Martin, S. A., Hewish, M., Sims, D., Lord, C. J. & Ashworth, A. Parallel high-throughput RNA interference screens identify PINK1 as a potential therapeutic target for the treatment of DNA mismatch repair-deficient cancers. Cancer Res. 71, 1836–1848 (2011).
Kim, R. H. et al. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyrindine (MPTP) and oxidative stress. Proc. Natl Acad. Sci. USA 102, 5215–5220 (2005).
Shendelman, S., Jonason, A., Martinat, C., Leete, T. & Abeliovich, A. DJ-1 is a redox-dependent molecular chaperone that inhibits α-synuclein aggregate formation. PLoS Biol. 2, e362 (2004).
Clements, C. M., McNally, R. S., Conti, B. J., Mak, T. W. & Ting, J. P. DJ-1, a cancer- and Parkinson's disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc. Natl Acad. Sci. USA 103, 15091–15096 (2006).
van der Brug, M. P. et al. RNA binding activity of the recessive parkinsonism protein DJ-1 supports involvement in multiple cellular pathways. Proc. Natl Acad. Sci. USA 105, 10244–10249 (2008).
Irrcher, I. et al. Loss of the Parkinson's disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum. Mol. Genet. 19, 3734–3746 (2010).
Kamp, F. et al. Inhibition of mitochondrial fusion by α-synuclein is rescued by PINK1, Parkin and DJ-1. EMBO J. 29, 3571–3589 (2010).
Xiong, H. et al. Parkin, PINK1, and DJ-1 form a ubiquitin E3 ligase complex promoting unfolded protein degradation. J. Clin. Invest. 119, 650–660 (2009).
Yang, Y. et al. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc. Natl Acad. Sci. USA 103, 10793–10798 (2006).
Nagakubo, D. et al. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem. Biophys. Res. Commun. 231, 509–513 (1997).
Hay, N. & Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 18, 1926–1945 (2004).
Liu, P., Cheng, H., Roberts, T. M. & Zhao, J. J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nature Rev. Drug Discov. 8, 627–644 (2009).
Paisan-Ruiz, C. LRRK2 gene variation and its contribution to Parkinson disease. Hum. Mutat. 30, 1153–1160 (2009).
Cookson, M. R. The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson's disease. Nature Rev. Neurosci. 11, 791–797 (2010).
Imai, Y. et al. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J. 27, 2432–2443 (2008).
Syntichaki, P., Troulinaki, K. & Tavernarakis, N. eIF4E function in somatic cells modulates ageing in Caenorhabditis elegans. Nature 445, 922–926 (2007).
Kumar, A. et al. The Parkinson's disease associated LRRK2 exhibits weaker in vitro phosphorylation of 4E-BP compared to autophosphorylation. PLoS ONE 5, e8730 (2010).
Ohta, E., Kawakami, F., Kubo, M. & Obata, F. LRRK2 directly phosphorylates Akt1 as a possible physiological substrate: impairment of the kinase activity by Parkinson's disease-associated mutations. FEBS Lett. 585, 2165–2170 (2011).
Looyenga, B. D. et al. Chromosomal amplification of leucine-rich repeat kinase-2 (LRRK2) is required for oncogenic MET signaling in papillary renal and thyroid carcinomas. Proc. Natl Acad. Sci. USA 108, 1439–1444 (2011).
Gera, J. F. et al. AKT activity determines sensitivity to mammalian target of rapamycin (mTOR) inhibitors by regulating cyclin D1 and c-myc expression. J. Biol. Chem. 279, 2737–2746 (2004).
Tain, L. S. et al. Rapamycin activation of 4E-BP prevents parkinsonian dopaminergic neuron loss. Nature Neurosci. 12, 1129–1135 (2009).
Murata, H. et al. A new cytosolic pathway from a Parkinson disease-associated kinase, BRPK/PINK1: activation of AKT via mTORC2. J. Biol. Chem. 286, 7182–7189 (2011).
Unoki, M. & Nakamura, Y. Growth-suppressive effects of BPOZ and EGR2, two genes involved in the PTEN signaling pathway. Oncogene 20, 4457–4465 (2001).
Liaw, D. et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nature Genet. 16, 64–67 (1997).
MacKeigan, J. P. et al. Proteomic profiling drug-induced apoptosis in non-small cell lung carcinoma: identification of RS/DJ-1 and RhoGDIα. Cancer Res. 63, 6928–6934 (2003).
Kim, R. H. et al. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell 7, 263–273 (2005).
Yang, Y. et al. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc. Natl Acad. Sci. USA 102, 13670–13675 (2005).
Aleyasin, H. et al. DJ-1 protects the nigrostriatal axis from the neurotoxin MPTP by modulation of the AKT pathway. Proc. Natl Acad. Sci. USA 107, 3186–3191 (2010).
Qian, B. Z. & Pollard, J. W. Macrophage diversity enhances tumor progression and metastasis. Cell 141, 39–51 (2010).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Beutler, E. Gaucher disease: new molecular approaches to diagnosis and treatment. Science 256, 794–799 (1992).
Shiran, A., Brenner, B., Laor, A. & Tatarsky, I. Increased risk of cancer in patients with Gaucher disease. Cancer 72, 219–224 (1993).
de Fost, M. et al. Increased incidence of cancer in adult Gaucher disease in Western Europe. Blood Cells Mol. Dis. 36, 53–58 (2006).
Shoenfeld, Y. et al. Gaucher's disease: a disease with chronic stimulation of the immune system. Arch. Pathol. Lab. Med. 106, 388–391 (1982).
Allen, M. J., Myer, B. J., Khokher, A. M., Rushton, N. & Cox, T. M. Pro-inflammatory cytokines and the pathogenesis of Gaucher's disease: increased release of interleukin-6 and interleukin-10. QJM 90, 19–25 (1997).
Velayati, A., Yu, W. H. & Sidransky, E. The role of glucocerebrosidase mutations in Parkinson disease and Lewy body disorders. Curr. Neurol. Neurosci. Rep. 10, 190–198 (2010).
Neumann, J. et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain 132, 1783–1794 (2009).
Ron, I. & Horowitz, M. ER retention and degradation as the molecular basis underlying Gaucher disease heterogeneity. Hum. Mol. Genet. 14, 2387–2398 (2005).
Cuervo, A. M., Stefanis, L., Fredenburg, R., Lansbury, P. T. & Sulzer, D. Impaired degradation of mutant α-synuclein by chaperone-mediated autophagy. Science 305, 1292–1295 (2004).
Mazzulli, J. R. et al. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 146, 37–52 (2011).
Manning-Bog, A. B., Schule, B. & Langston, J. W. α-synuclein-glucocerebrosidase interactions in pharmacological Gaucher models: a biological link between Gaucher disease and parkinsonism. Neurotoxicology 30, 1127–1132 (2009).
McGeer, P. L., Itagaki, S., Boyes, B. E. & McGeer, E. G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology 38, 1285–1291 (1988).
Depino, A. M. et al. Microglial activation with atypical proinflammatory cytokine expression in a rat model of Parkinson's disease. Eur. J. Neurosci. 18, 2731–2742 (2003).
Burguillos, M. A. et al. Caspase signalling controls microglia activation and neurotoxicity. Nature 472, 319–324 (2011).
Swanton, C. et al. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell 11, 498–512 (2007).
Falkenburger, B. H. & Schulz, J. B. Limitations of cellular models in Parkinson's disease research. J. Neural Transm. Suppl. 70, 261–268 (2006).
Han, S. S., Williams, L. A. & Eggan, K. C. Constructing and deconstructing stem cell models of neurological disease. Neuron 70, 626–644 (2011).
Beck, J. A. et al. Somatic and germline mosaicism in sporadic early-onset Alzheimer's disease. Hum. Mol. Genet. 13, 1219–1224 (2004).
Rowe, I. F., Ridler, M. A. & Gibberd, F. B. Presenile dementia associated with mosaic trisomy 21 in a patient with a Down syndrome child. Lancet 2, 229 (1989).
Post, B., Merkus, M. P., de Haan, R. J. & Speelman, J. D. Prognostic factors for the progression of Parkinson's disease: a systematic review. Mov. Disord. 22, 1839–1851; quiz 1988 (2007).
Newell, G. R., Spitz, M. R. & Sider, J. G. Cancer and age. Semin. Oncol. 16, 3–9 (1989).
Gilbert, W. Origins of Life: the RNA world. Nature 319, 618 (1986).
Gapstur, S. M. & Thun, M. J. Progress in the war on cancer. JAMA 303, 1084–1085 (2010).
Haber, D. A., Gray, N. S. & Baselga, J. The evolving war on cancer. Cell 145, 19–24 (2011).
Jansson, B. & Jankovic, J. Low cancer rates among patients with Parkinson's disease. Ann. Neurol. 17, 505–509 (1985).
Moller, H., Mellemkjaer, L., McLaughlin, J. K. & Olsen, J. H. Occurrence of different cancers in patients with Parkinson's disease. BMJ 310, 1500–1501 (1995).
Minami, Y., Yamamoto, R., Nishikouri, M., Fukao, A. & Hisamichi, S. Mortality and cancer incidence in patients with Parkinson's disease. J. Neurol. 247, 429–434 (2000).
Hernan, M. A., Takkouche, B., Caamano-Isorna, F. & Gestal-Otero, J. J. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson's disease. Ann. Neurol. 52, 276–284 (2002).
Olsen, J. H. et al. Atypical cancer pattern in patients with Parkinson's disease. Br. J. Cancer 92, 201–205 (2005).
Bajaj, A., Driver, J. A. & Schernhammer, E. S. Parkinson's disease and cancer risk: a systematic review and meta-analysis. Cancer Causes Control 21, 697–707 (2010).
Liu, R., Gao, X., Lu, Y. & Chen, H. Meta-analysis of the relationship between Parkinson disease and melanoma. Neurology 76, 2002–2009 (2011).
Malagelada, C., Jin, Z. H., Jackson-Lewis, V., Przedborski, S. & Greene, L. A. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson's disease. J. Neurosci. 30, 1166–1175 (2010).
Zhang, J., Yang, P. L. & Gray, N. S. Targeting cancer with small molecule kinase inhibitors. Nature Rev. Cancer 9, 28–39 (2009).
Lee, B. D. et al. Inhibitors of leucine-rich repeat kinase-2 protect against models of Parkinson's disease. Nature Med. 16, 998–1000 (2010).
Nair, B. C., Vallabhaneni, S., Tekmal, R. R. & Vadlamudi, R. K. Roscovitine confers tumor suppressive effect on therapy-resistant breast tumor cells. Breast Cancer Res. 13, R80 (2011).
Smith, P. D. et al. Cyclin-dependent kinase 5 is a mediator of dopaminergic neuron loss in a mouse model of Parkinson's disease. Proc. Natl Acad. Sci. USA 100, 13650–13655 (2003).
Chico, L. K., Van Eldik, L. J. & Watterson, D. M. Targeting protein kinases in central nervous system disorders. Nature Rev. Drug Discov. 8, 892–909 (2009).
Sittler, A. et al. Geldanamycin activates a heat shock response and inhibits huntingtin aggregation in a cell culture model of Huntington's disease. Hum. Mol. Genet. 10, 1307–1315 (2001).
McLean, P. J., Klucken, J., Shin, Y. & Hyman, B. T. Geldanamycin induces Hsp70 and prevents α-synuclein aggregation and toxicity in vitro. Biochem. Biophys. Res. Commun. 321, 665–669 (2004).
Riedel, M., Goldbaum, O., Schwarz, L., Schmitt, S. & Richter-Landsberg, C. 17-AAG induces cytoplasmic α-synuclein aggregate clearance by induction of autophagy. PLoS ONE 5, e8753 (2010).
Geisler, S. et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nature Cell Biol. 12, 119–131 (2010).
Pandey, N., Strider, J., Nolan, W. C., Yan, S. X. & Galvin, J. E. Curcumin inhibits aggregation of α-synuclein. Acta Neuropathol. 115, 479–489 (2008).
Kawashima, M., Suzuki, S. O., Doh-ura, K. & Iwaki, T. α-Synuclein is expressed in a variety of brain tumors showing neuronal differentiation. Acta Neuropathol. 99, 154–160 (2000).
Matsuo, Y. & Kamitani, T. Parkinson's disease-related protein, α-synuclein, in malignant melanoma. PLoS ONE 5, e10481 (2010).
Bruening, W. et al. Synucleins are expressed in the majority of breast and ovarian carcinomas and in preneoplastic lesions of the ovary. Cancer 88, 2154–2163 (2000).
Li, L. et al. The tumor suppressor UCHL1 forms a complex with p53/MDM2/ARF to promote p53 signaling and is frequently silenced in nasopharyngeal carcinoma. Clin. Cancer Res. 16, 2949–2958 (2010).
Okochi-Takada, E. et al. Silencing of the UCHL1 gene in human colorectal and ovarian cancers. Int. J. Cancer 119, 1338–1344 (2006).
Kagara, I. et al. CpG hypermethylation of the UCHL1 gene promoter is associated with pathogenesis and poor prognosis in renal cell carcinoma. J. Urol. 180, 343–351 (2008).
Hod, Y. Differential control of apoptosis by DJ-1 in prostate benign and cancer cells. J. Cell. Biochem. 92, 1221–1233 (2004).
Parkinson, J. An essay on the shaking palsy. 1817. J. Neuropsychiatry Clin. Neurosci. 14, 223–236; discussion 222 (2002).
Lewy, F. H. in Handbuch der Neurologie (ed. Lewandowsky, M.) 920–933 (Springer, Berlin, 1912).
Tretiakoff, C. Contribution a l'etude de l'anatomie pathologique du locus niger de Soemmering avec quelques deductions relatives a la athogenie des troubles du tonus musculaire et de la maladie de Parkinson (Jouve and Co, Paris, 1919).
Carlsson, A., Lindqvist, M. & Magnusson, T. 3, 4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature 180, 1200 (1957).
Ehringer, H. & Hornykiewicz, O. [Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system]. Klin. Wochenschr. 38, 1236–1239 (1960).
Birkmayer, W. & Hornykiewicz, O. [The L-3, 4-dioxyphenylalanine (DOPA)-effect in Parkinson-akinesia]. Wien. Klin. Wochenschr. 73, 787–788 (1961).
Cotzias, G. C., Papavasiliou, P. S. & Gellene, R. Modification of Parkinsonism--chronic treatment with L-dopa. N. Engl. J. Med. 280, 337–345 (1969).
Leroy, E. et al. The ubiquitin pathway in Parkinson's disease. Nature 395, 451–452 (1998).
Zimprich, A. et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44, 601–607 (2004).
Paisan-Ruiz, C. et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron 44, 595–600 (2004).
Lwin, A., Orvisky, E., Goker-Alpan, O., LaMarca, M. E. & Sidransky, E. Glucocerebrosidase mutations in subjects with parkinsonism. Mol. Genet. Metab. 81, 70–73 (2004).
Ramirez, A. et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nature Genet. 38, 1184–1191 (2006).
Nalls, M. A. et al. Imputation of sequence variants for identification of genetic risks for Parkinson's disease: a meta-analysis of genome-wide association studies. Lancet 377, 641–649 (2011).
Acknowledgements
The work was funded by a Wellcome Trust Medical Research Council (MRC) Parkinson's Disease Consortium grant to University College London Institute of Neurology, the University of Sheffield and the MRC Protein Phosphorylation Unit at the University of Dundee (grant number WT089698). H.P-F. is funded by the MRC (award number G0700183).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Devine, M., Plun-Favreau, H. & Wood, N. Parkinson's disease and cancer: two wars, one front. Nat Rev Cancer 11, 813–823 (2011). https://doi.org/10.1038/nrc3150
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3150
This article is cited by
-
Longitudinal study of the inverse relationship between Parkinson’s disease and cancer in Korea
npj Parkinson's Disease (2023)
-
Caffeine improves mitochondrial function in PINK1B9-null mutant Drosophila melanogaster
Journal of Bioenergetics and Biomembranes (2023)
-
A study of gene expression by RNA-seq in patients with prostate cancer and in patients with Parkinson disease: an example of inverse comorbidity
Molecular Biology Reports (2021)
-
Parkinson’s Disease is Associated with Dysregulations of a Dopamine-Modulated Gene Network Relevant to Sleep and Affective Neurobehaviors in the Striatum
Scientific Reports (2019)
-
Looking beyond the brain to improve the pathogenic understanding of Parkinson’s disease: implications of whole transcriptome profiling of Patients’ skin
BMC Neurology (2017)