Key Points
-
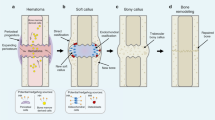
Benign cartilage lesions (enchondromas and osteochondromas) can result from the deregulation of the hedgehog signalling pathway, which is involved in normal bone development.
-
Malignant chondrosarcomas can derive from benign cartilage lesions, with enchondromas developing into central chondrosarcomas and osteochondromas developing into peripheral chondrosarcomas.
-
Peripheral chondrosarcomas have different molecular abnormalities from central chondrosarcomas.
-
The progression from benign to malignant cartilage tumours is not driven by hedgehog signalling, but blocking this pathway may be a potential therapeutic target by inducing tumour cartilage cell differentiation.
-
Genetically modified mice show that p53 and insulin-like growth factor are deregulated when enchondromas progress to central chondrosarcomas.
-
Cytogenetic studies show that cyclin-dependent kinase 4, hypoxia-inducible factor, matrix metalloproteinases, SRC and AKT are activated in central chondrosarcomas, suggesting potential new therapeutic approaches.
Abstract
As a group, cartilage tumours are the most common primary bone lesions. They range from benign lesions, such as enchondromas and osteochondromas, to malignant chondrosarcoma. The benign lesions result from the deregulation of the hedgehog signalling pathway, which is involved in normal bone development. These lesions can be the precursors of malignant chondrosarcomas, which are notoriously resistant to conventional chemotherapy and radiotherapy. Cytogenetic studies and mouse models are beginning to identify genes and signalling pathways that have roles in tumour progression, such as hedgehog, p53, insulin-like growth factor, cyclin-dependent kinase 4, hypoxia-inducible factor, matrix metalloproteinases, SRC and AKT, suggesting potential new therapeutic approaches.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Fletcher, C. D. M., Unni, K. K. & Mertens, F. (eds). in World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. 234–257 (IARC Press, Lyon. France, 2002).
Kronenberg, H. M. Developmental regulation of the growth plate. Nature 423, 332–336 (2003).
Tiet, T. D. & Alman, B. A. Developmental pathways in musculoskeletal neoplasia: involvement of the Indian Hedgehog-parathyroid hormone-related protein pathway. Pediatr. Res. 53, 539–543 (2003).
Pathi, S., Rutenberg, J. B., Johnson, R. L. & Vortkamp, A. Interaction of Ihh and BMP/Noggin signaling during cartilage differentiation. Dev. Biol. 209, 239–253 (1999).
Lyons, K. M., Pelton, R. W. & Hogan, B. L. Patterns of expression of murine Vgr-1 and BMP-2a RNA suggest that transforming growth factor-beta-like genes coordinately regulate aspects of embryonic development. Genes Dev. 3, 1657–1668 (1989).
Bitgood, M. J. & McMahon, A. P. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev. Biol. 172, 126–138 (1995).
St-Jacques, B., Hammerschmidt, M. & McMahon, A. P. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 13, 2072–2086 (1999).
Minina, E. et al. BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development 128, 4523–4534 (2001).
Schwartz, H. S. et al. The malignant potential of enchondromatosis. J. Bone Joint Surg. Am. 69, 269–274 (1987).
Tiet, T. D. et al. Constitutive hedgehog signaling in chondrosarcoma up-regulates tumor cell proliferation. Am. J. Pathol. 168, 321–330 (2006).
Schrage, Y. M. et al. Aberrant heparan sulfate proteoglycan localization, despite normal exostosin, in central chondrosarcoma. Am. J. Pathol. 174, 979–988 (2009).
Hopyan, S. et al. A mutant PTH/PTHrP type I receptor in enchondromatosis. Nature Genet. 30, 306–310 (2002). This study shows that enchondromas are caused by mutations activating hedgehog signalling.
Couvineau, A. et al. PTHR1 mutations associated with Ollier disease result in receptor loss of function. Hum. Mol. Genet. 17, 2766–2775 (2008).
van Beerendonk, H. M. et al. Molecular analysis of the INK4A/INK4A-ARF gene locus in conventional (central) chondrosarcomas and enchondromas: indication of an important gene for tumour progression. J. Pathol. 202, 359–366 (2004).
Bovée, J. V. M. G. & Hogendoorn, P. C. W. Multiple osteochondromas. in World Health Organization Classification of Tumours. Pathology and Genetics Of Tumours of Soft Tissue and Bone (eds Fletcher, C. D. M., Unni, K. K. & Mertens, F.) 360–362 (IARC Press, Lyon, France, 2002).
Bovée, J. V. M. G. Multiple osteochondromas. Orphanet. J. Rare Dis. 3, 3 (2008).
Jennes, I. et al. Multiple osteochondromas: mutation update and description of the multiple osteochondromas mutation database (MOdb). Hum. Mutat. 30, 1620–1627 (2009).
Bovée, J. V. M. G. et al. EXT-mutation analysis and loss of heterozygosity in sporadic and hereditary osteochondromas and secondary chondrosarcomas. Am. J. Hum. Genet. 65, 689–698 (1999).
Hameetman, L. et al. The Role of EXT1 in non hereditary osteochondroma: identification of homozygous deletions. J. Natl Cancer Inst. 99, 396–406 (2007).
Jones, K. B. et al. A mouse model of osteochondromagenesis from clonal inactivation of Ext1 in chondrocytes. Proc. Natl Acad. Sci. USA 107, 2054–2059 (2010).
Bovee, J. V. M. G. EXTra hit for mouse osteochondroma. Proc. Natl Acad. Sci. USA 107, 1813–1814 (2010).
McCormick, C. et al. The putative tumour suppressor EXT1 alters the expression of cell-surface heparan sulfate. Nature Genet. 19, 158–161 (1998). This study shows the function of EXT , which is mutated in osteochondromas.
Koziel, L., Kunath, M., Kelly, O. G. & Vortkamp, A. Ext1-dependent heparan sulfate regulates the range of Ihh signaling during endochondral ossification. Dev. Cell 6, 801–813 (2004).
Hameetman, L. et al. Decreased EXT expression and intracellular accumulation of HSPG in osteochondromas and peripheral chondrosarcomas. J. Pathol. 211, 399–409 (2007).
Benoist-Lasselin, C. et al. Defective chondrocyte proliferation and differentiation in osteochondromas of MHE patients. Bone 39, 17–26 (2006).
Clement, A. et al. Regulation of zebrafish skeletogenesis by ext2/dackel and papst1/pinscher. PLoS. Genet. 4, e1000136 (2008).
Fiorenza, F. et al. Risk factors for survival and local control in chondrosarcoma of bone. J. Bone Joint Surg. Br. 84, 93–99 (2002).
Ayala, G., Liu, C., Nicosia, R., Horowitz, S. & Lackman, R. Microvasculature and VEGF expression in cartilaginous tumors. Hum. Pathol. 31, 341–346 (2000).
Geirnaerdt, M. J., Hogendoorn, P. C. W., Bloem, J. L., Taminiau, A. H. M. & Van der Woude, H. J. Cartilaginous tumors: fast contrast-enhanced MR imaging. Radiology 214, 539–546 (2000).
Eefting, D. et al. Assessment of interobserver variability and histologic parameters to improve reliability in classification and grading of central cartilaginous tumors. Am. J. Surg. Pathol. 33, 50–57 (2009).
Skeletal Lesions Interobserver Correlation among Expert Diagnosticians (SLICED) Study Group. Reliability of histopathologic and radiologic grading of cartilaginous neoplasms in long bones. J. Bone Joint Surg. Am. 89, 2113–2123 (2007).
Gelderblom, H. et al. The clinical approach towards chondrosarcoma. Oncologist 13, 320–329 (2008).
Riedel, R. F. et al. The clinical management of chondrosarcoma. Curr. Treat Options Oncol. 10, 94–106 (2009).
Lee, F. Y. et al. Chondrosarcoma of bone: an assessment of outcome. J. Bone Joint Surg. Am. 81, 326–338 (1999).
Hameetman, L. et al. Peripheral chondrosarcoma progression is accompanied by decreased Indian Hedgehog (IHH) signalling. J. Pathol. 209, 501–511 (2006).
Rudin, C. M. et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N. Engl. J. Med. 361, 1173–1178 (2009).
Von Hoff, D. D. et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N. Engl. J. Med. 361, 1164–1172 (2009).
Alman, B. A. & Wunder, J. S. Parathyroid hormone-related protein regulates glioma-associated oncogene transcriptional activation: lessons learned from bone development and cartilage neoplasia. Ann. NY Acad. Sci. 1144, 36–41 (2008).
Ho, L. et al. Gli2 and p53 cooperate to regulate IGFBP-3- mediated chondrocyte apoptosis in the progression from benign to malignant cartilage tumors. Cancer Cell 16, 126–136 (2009). This study shows that progression from enchondroma to chondrosracoma is associated with the loss of p53 and IGF signalling activation.
Olmos, D. et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing's sarcoma: a phase 1 expansion cohort study. Lancet Oncol. 2, 129–135 (2010).
Bovée, J. V. M. G. et al. Loss of heterozygosity and DNA ploidy point to a diverging genetic mechanism in the origin of peripheral and central chondrosarcoma. Genes Chromosom. Cancer 26, 237–246 (1999).
Tallini, G. et al. Correlation between clinicopathological features and karyotype in 100 cartilaginous and chordoid tumours. A report from the Chromosomes and Morphology (CHAMP) Collaborative Study Group. J. Pathol. 196, 194–203 (2002).
Rozeman, L. B. et al. Array-CGH of central chondrosarcoma: identification of RPS6 and CDK4 as candidate target genes for genomic aberrations. Cancer 107, 380–388 (2006).
Schrage, Y.M. et al. Central chondrosarcoma progression is associated with pRb pathway alterations; CDK4 downregulation and p16 overexpression inhibit cell growth in vitro. J. Cell. Mol. Med. 13, 2843–2852 (2009).
Larramendy, M. L. et al. Gains, losses, and amplifications of DNA sequences evaluated by comparative genomic hybridization in chondrosarcomas. Am. J. Pathol. 150, 685–691 (1997).
Bovée, J. V. M. G. et al. Chromosome 9 alterations and trisomy 22 in central chondrosarcoma: a cytogenetic and DNA flow cytometric analysis of chondrosarcoma subtypes. Diagn. Mol. Pathol. 10, 228–236 (2001).
Asp, J. et al. Changes of the p16 gene but not the p53 gene in human chondrosarcoma tissues. Int. J. Cancer 85, 782–786 (2000).
Asp, J., Inerot, S., Block, J. A. & Lindahl, A. Alterations in the regulatory pathway involving p16, pRb and cdk4 in human chondrosarcoma. J. Orthop. Res. 19, 149–154 (2001).
Rozeman, L. B. et al. Absence of IHH and retention of PTHrP signalling in enchondromas and central chondrosarcomas. J. Pathol. 205, 476–482 (2005).
Schrage, Y. M. et al. Kinome profiling of chondrosarcoma reveals Src-pathway activity and dasatinib as option for treatment. Cancer Res. 69, 6216–6222 (2009). This study, which uses kinome profiling in human tumours, suggests several potential therapeutic options.
Jang, J. H. & Chung, C. P. Tenascin-C promotes cell survival by activation of Akt in human chondrosarcoma cell. Cancer Lett. 229, 101–105 (2005).
Engelman, J. A. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nature Rev. Cancer 9, 550–562 (2009).
Bussink, J., van der Kogel, A. J. & Kaanders, J. H. Activation of the PI3-K/AKT pathway and implications for radioresistance mechanisms in head and neck cancer. Lancet Oncol. 9, 288–296 (2008).
Lin, C., Meitner, P. A. & Terek, R. M. PTEN mutation is rare in chondrosarcoma. Diagn. Mol. Pathol. 11, 22–26 (2002).
Lin, C., McGough, R., Aswad, B., Block, J. A. & Terek, R. Hypoxia induces HIF-1α and VEGF expression in chondrosarcoma cells and chondrocytes. J. Orthop. Res. 22, 1175–1181 (2004).
Kalinski, T. et al. Differential expression of VEGF-A and angiopoietins in cartilage tumors and regulation by interleukin-1beta. Cancer 106, 2028–2038 (2006).
McGough, R. L., Lin, C., Meitner, P., Aswad, B. I. & Terek, R. M. Angiogenic cytokines in cartilage tumors. Clin. Orthop. 397, 62–69 (2002).
Kubo, T. et al. Expression of hypoxia-inducible factor-1α and its relationship to tumour angiogenesis and cell proliferation in cartilage tumours. J. Bone Joint Surg. Br. 90, 364–370 (2008).
Bovée, J. V. M. G., Van den Broek, L. J. C. M., Cleton-Jansen, A. M. & Hogendoorn, P. C. W. Up-regulation of PTHrP and Bcl-2 expression characterizes the progression of osteochondroma towards peripheral chondrosarcoma and is a late event in central chondrosarcoma. Lab. Invest. 80, 1925–1933 (2000). This is one of several studies showing different molecular pathways dysregulated in peripheral and central chondrosarcomas.
Moussavi-Harami, F. et al. Intrinsic radiation resistance in human chondrosarcoma cells. Biochem. Biophys. Res. Commun. 346, 379–385 (2006).
Boeuf, S., Bovee, J. V. M. G., Lehner, B., Hogendoorn, P. C. W. & Richter, W. Correlation of hypoxic signalling to histological grade and outcome in cartilage tumours. Histopathology 56, 641–651 (2009).
Rozeman, L. B. et al. cDNA expression profiling of central chondrosarcomas: Ollier disease resembles solitary tumors and alteration in genes coding for energy metabolism with increasing grade. J. Pathol. 207, 61–71 (2005).
Wyman, J. J. et al. Multidrug resistance-1 and p-glycoprotein in human chondrosarcoma cell lines: expression correlates with decreased intracellular doxorubicin and in vitro chemoresistance. J. Orthop. Res. 17, 935–940 (1999).
Terek, R. M. et al. Chemotherapy and P-glycoprotein expression in chondrosarcoma. J. Orthop. Res. 16, 585–590 (1998).
Rosier, R. N. et al. P-glycoprotein expression in cartilaginous tumors. J. Surg. Oncol. 65, 95–105 (1997).
Kim, D. W. et al. siRNA-based targeting of antiapoptotic genes can reverse chemoresistance in P-glycoprotein expressing chondrosarcoma cells. Mol. Cancer 8, 28 (2009).
Jiang, X. et al. siRNA mediated inhibition of MMP-1 reduces invasive potential of a human chondrosarcoma cell line. J. Cell. Physiol. 202, 723–730 (2005).
Bovée, J. V. M. G. et al. Near-haploidy and subsequent polyploidization characterize the progression of peripheral chondrosarcoma. Am. J. Pathol. 157, 587–1595 (2000).
Schrage, Y. M. et al. COX-2 expression in chondrosarcoma: a role for celecoxib treatment? Eur. J. Cancer 46, 616–624 (2010).
Geirnardt, M. J. et al. Usefullness of radiography in differentiating enchondroma from central grade I chondrosarcoma. Am. J. Roentgenol. 169, 1097–1104 (1997).
Mulder, J. D., Schütte, H. E., Kroon, H. M. & Taconis, W. K. Radiologic Atlas of Bone Tumors. 2nd edn (Elsevier, Amsterdam, 1993).
Miyaji, T. et al. Monoclonal antibody to parathyroid hormone-related protein induces differentiation and apoptosis of chondrosarcoma cells. Cancer Lett. 199, 147–155 (2003).
Cleton-Jansen, A. M. et al. Estrogen signaling is active in cartilaginous tumors: implications for antiestrogen therapy as treatment option of metastasized or irresectable chondrosarcoma. Clin. Cancer Res. 11, 8028–8035 (2005).
Fong, Y. C. et al. 2-methoxyestradiol induces apoptosis and cell cycle arrest in human chondrosarcoma cells. J. Orthop. Res. 25, 1106–1114 (2007).
Klenke, F. M. et al. Tyrosine kinase inhibitor SU6668 represses chondrosarcoma growth via antiangiogenesis in vivo. BMC Cancer 7, 49 (2007).
Sakimura, R. et al. The effects of histone deacetylase inhibitors on the induction of differentiation in chondrosarcoma cells. Clin. Cancer Res. 13, 275–282 (2007).
Yamamoto, S. et al. Suberoylanilide hydroxamic acid (SAHA) induces apoptosis or autophagy-associated cell death in chondrosarcoma cell lines. Anticancer Res. 28, 1585–1591 (2008).
Shen, Z. N. et al. Suppression of chondrosarcoma cells by 15-deoxy-Δ12,14-prostaglandin J2 is associated with altered expression of Bax/Bcl-xL and p21. Biochem. Biophys. Res. Commun. 328, 375–382 (2005).
Lai, T. J. et al. Alendronate inhibits cell invasion and MMP-2 secretion in human chondrosarcoma cell line. Acta Pharmacol. Sin. 28, 1231–1235 (2007).
Kubo, T. et al. Inhibitory effects of a new bisphosphonate, minodronate, on proliferation and invasion of a variety of malignant bone tumor cells. J. Orthop. Res. 24, 1138–1144 (2006).
Parsch, D., Brassat, U., Brummendorf, T. H. & Fellenberg, J. Consequences of telomerase inhibition by BIBR1532 on proliferation and chemosensitivity of chondrosarcoma cell lines. Cancer Invest. 26, 590–596 (2008).
Tomek, S. et al. Trail-induced apoptosis and interaction with cytotoxic agents in soft tissue sarcoma cell lines. Eur. J. Cancer 39, 1318–1329 (2003).
Sun, X., Wei, L., Chen, Q. & Terek, R. M. CXCR4/SDF1 mediate hypoxia induced chondrosarcoma cell invasion through ERK signaling and increased MMP1 expression. Mol. Cancer 9, 17 (2010).
Acknowledgements
The authors would like to thank Y. Schrage and L. Hameetman for their help with the tables. J.V.M.G.B was financially supported by The Netherlands Organization for Scientific Research (917-76-315).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1
Mouse models to study the formation of cartilaginous tumours with respect to EXT and growth plate signalling (PDF 185 kb)
Supplementary information S2
Model organisms to study the formation of cartilaginous tumours with respect to EXT and growth plate signalling (PDF 188 kb)
Related links
Glossary
- Long bone
-
A bone that is longer than it is wide; for example, the femur in the thigh and the humerus in the upper arm.
- Osteoblast
-
A cell responsible for bone formation. It expresses bone sialoprotein and osteocalcin and produces osteoid, which is composed mainly of Type I collagen.
- Growth plate
-
A specialized region of cartilage, located near the ends of bone, which is responsible for the longitudinal growth of long bones.
- Epiphysis
-
The rounded end of a long bone, located closest to the adjacent joint.
- Metaphysis
-
The wider portion of a long bone adjacent to the growth plate, but closer to the middle of the bone.
- Muco-myxoid matrix
-
Increase of mucopolysaccharides in the extracellular matrix, resulting in pale-to-lightly basophilic staining using haematoxylin and eosin.
Rights and permissions
About this article
Cite this article
Bovée, J., Hogendoorn, P., Wunder, J. et al. Cartilage tumours and bone development: molecular pathology and possible therapeutic targets. Nat Rev Cancer 10, 481–488 (2010). https://doi.org/10.1038/nrc2869
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc2869
This article is cited by
-
A single-cell atlas of conventional central chondrosarcoma reveals the role of endoplasmic reticulum stress in malignant transformation
Communications Biology (2024)
-
IDH mutations in G2-3 conventional central bone chondrosarcoma: a mono institutional experience
BMC Cancer (2023)
-
Shikonin derivatives cause apoptosis and cell cycle arrest in human chondrosarcoma cells via death receptors and MAPK regulation
BMC Cancer (2022)
-
Insights into skeletal stem cells
Bone Research (2022)
-
Role of nuclear factor of activated T cells in chondrogenesis osteogenesis and osteochondroma formation
Journal of Endocrinological Investigation (2022)