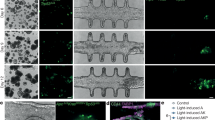
Abstract
This protocol describes how to form a 3D cell culture with explicit, endothelialized microvessels. The approach leads to fully enclosed, perfusable vessels in a bioremodelable hydrogel (type I collagen). The protocol uses microfabrication to enable user-defined geometries of the vascular network and microfluidic perfusion to control mass transfer and hemodynamic forces. These microvascular networks (μVNs) allow for multiweek cultures of endothelial cells or cocultures with parenchymal or tissue cells in the extra-lumen space. The platform enables real-time fluorescence imaging of living engineered tissues, in situ confocal fluorescence of fixed cultures and transmission electron microscopy (TEM) imaging of histological sections. This protocol enables studies of basic vascular and blood biology, provides a model for diseases such as tumor angiogenesis or thrombosis and serves as a starting point for constructing prevascularized tissues for regenerative medicine. After one-time microfabrication steps, the system can be assembled in less than 1 d and experiments can run for weeks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Choi, N.W. et al. Microfluidic scaffolds for tissue engineering. Nat. Mater. 6, 908–915 (2007).
Zheng, Y. et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc. Natl. Acad. Sci. USA 109, 9342–9347 (2012).
Verbridge, S.S. et al. Physicochemical regulation of endothelial sprouting in a 3-D microfluidic angiogenesis model. J. Biomed. Mater. Res. A doi:10.1002/jbm.a.34587 (5 April 2013).
Stroock, A.D. & Fischbach, C. Microfluidic culture models of tumor angiogenesis. Tissue Eng. Part A 16, 2143–2146 (2010).
Verbridge, S.S. et al. Oxygen-controlled 3-D cultures to analyze tumor angiogenesis. Tissue Eng Part A 16, 2157–2159 (2010).
Song, J.W. & Munn, L.L. Fluid forces control endothelial sprouting. Proc. Natl. Acad. Sci. USA 108, 15342–15347 (2011).
Song, J.W., Bazou, D. & Munn, L.L. Anastomosis of endothelial sprouts forms new vessels in a tissue analogue of angiogenesis. Integr. Biol. 4, 857–862 (2012).
Shin, Y. et al. In vitro 3D collective sprouting angiogenesis under orchestrated ANG-1 and VEGF gradients. Lab Chip 11, 2175–2181 (2011).
Shin, Y. et al. Microfluidic assay for simultaneous culture of multiple cell types on surfaces or within hydrogels. Nat. Protoc. 7, 1247–1259 (2012).
Davis, G.E., Bayless, K.J. & Mavila, A. Molecular basis of endothelial cell morphogenesis in three-dimensional extracellular matrices. Anat. Rec. 268, 252–275 (2002).
Yannas, I.V. & Burke, J.F. Design of an artificial skin I. basic design principles. J. Biomed. Mater. Res. 14, 65–81 (1980).
Langer, R. & Vacanti, J.P. Tissue engineering. Science 260, 920–926 (1993).
Jain, R.K., Au, P., Tam, J., Duda, D.G. & Fukumura, D. Engineering vascularized tissue. Nat. Biotechnol. 23, 821–823 (2005).
Stroock, A.D. & Cabodi, M. Microfluidic biomaterials. MRS Bulletin 31, 114–119 (2006).
Mikos, A.G. et al. Prevascularization of porous biodegradable polymers. Biotechnol. Bioeng. 42, 716–723 (1993).
Levenberg, S. et al. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 23, 879–884 (2005).
Du, Y. et al. Sequential assembly of cell-laden hydrogel constructs to engineer vascular-like microchannels. Biotechnol. Bioeng. 108, 1693–1703 (2011).
Neumann, T., Nicholson, B.S. & Sanders, J.E. Tissue engineering of perfused microvessels. Microvasc. Res. 66, 59–67 (2003).
Cabodi, M. et al. A microfluidic biomaterial. J. Am. Chem. Soc. 127, 13788–13789 (2005).
Chrobak, K.M., Potter, D.R. & Tien, J. Formation of perfused, functional microvascular tubes in vitro. Microvasc. Res. 71, 185–196 (2006).
Miller, J.S. et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 11, 768–774 (2012).
Whitesides, G.M., Ostuni, E., Takayama, S., Jiang, X. & Ingber, D.E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 3, 335–373 (2001).
Qin, D., Xia, Y. & Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 5, 491–502 (2010).
Tang, M.D., Golden, A.P. & Tien, J. Molding of three-dimensional microstructures of gels. J. Am. Chem. Soc. 125, 12988–12989 (2003).
Nelson, C.M., Vanduijn, M.M., Inman, J.L., Fletcher, D.A. & Bissell, M.J. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science 314, 298–300 (2006).
Bellan, L.M. et al. Fabrication of an artificial 3-dimensional vascular network using sacrificial sugar structures. Soft Matter 5, 1354–1357 (2009).
McGuigan, A.P. & Sefton, M.V. Vascularized organoid engineered by modular assembly enables blood perfusion. Proc. Natl. Acad. Sci. USA 103, 11461–11466 (2006).
Gauvin, R., Guillemette, M., Dokmeci, M. & Khademhosseini, A. Application of microtechnologies for the vascularization of engineered tissues. Vasc. Cell 3, 24 (2011).
Borenstein, J.T. et al. Microfabrication technology for vascularized tissue engineering. Biomed. Microdevices 4, 167–175 (2002).
Fidkowski, C. et al. Endothelialized microvasculature based on a biodegradable elastomer. Tissue Eng. 11, 302–309 (2005).
Bettinger, C.J. et al. Silk fibroin microfluidic devices. Adv. Mater. 19, 2847–2850 (2007).
Ling, Y. et al. A cell-laden microfluidic hydrogel. Lab Chip 7, 756–762 (2007).
Nelson, C.M. & Tien, J. Microstructured extracellular matrices in tissue engineering and development. Curr. Opin. Biotechnol. 17, 518–523 (2006).
Golden, A.P. & Tien, J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip 7, 720–725 (2007).
Iruela-Arispe, M.L. & Davis, G.E. Cellular and molecular mechanisms of vascular lumen formation. Dev. Cell 16, 222–231 (2009).
Asahara, T., Kawamoto, A. & Masuda, H. Concise review: circulating endothelial progenitor cells for vascular medicine. Stem Cells 29, 1650–1655 (2011).
Feener, E.P. & King, G.L. Vascular dysfunction in diabetes mellitus. Lancet 350 (suppl. 1), SI9–S13 (1997).
Muschler, G.F., Nakamoto, C. & Griffith, L.G. Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg. Am. 86-A, 1541–1558 (2004).
Sung, J.H., Kam, C. & Shuler, M.L. A microfluidic device for a pharmacokinetic-pharmacodynamic (PK-PD) model on a chip. Lab Chip 10, 446–455 (2010).
Wong, K.H., Chan, J.M., Kamm, R.D. & Tien, J. Microfluidic models of vascular functions. Annu. Rev. Biomed. Eng. 14, 205–230 (2012).
Cross, V.L. et al. Dense collagen matrices with microstructure and cellular remodeling for three-dimensional cell culture. Biomaterials 31, 8596–8607 (2010).
King, K.R., Wang, C.C.J., Kaazempur-Mofrad, M.R., Vacanti, J.P. & Borenstein, J.T. Biodegradable microfluidics. Adv. Mater. 16, 2007–2012 (2004).
Vickerman, V., Blundo, J., Chung, S. & Kamm, R. Design, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging. Lab Chip 8, 1468–1477 (2008).
Bornstein, M.B. Reconstituted rattail collagen used as substrate for tissue cultures on coverslips in Maximow slides and roller tubes. Lab Invest. 7, 134–137 (1958).
Rajan, N., Habermehl, J., Cote, M.F., Doillon, C.J. & Mantovani, D. Preparation of ready-to-use, storable and reconstituted type I collagen from rat tail tendon for tissue engineering applications. Nat. Protoc. 1, 2753–2758 (2006).
Haessler, U., Pisano, M., Wu, M. & Swartz, M.A. Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and CCL19. Proc. Natl. Acad. Sci. USA 108, 5614–5619 (2011).
Franco, C. & Gerhardt, H. Tissue engineering: blood vessels on a chip. Nature 488, 465–466 (2012).
Acknowledgements
We acknowledge the technical assistance of G. Swan. We thank C. Murry and S. Schwartz for helpful discussions. We acknowledge the Life Sciences Core Laboratories Center at Cornell University and the Lynn and Mike Garvey Imaging Laboratory in the Institute of Stem Cell and Regenerative Medicine at University of Washington. We acknowledge the financial support from an American Heart Association Scientist Development Grant (Y.Z.); the US National Institutes of Health (NIH) (grant no. R01HL091153 to J.A.L.; and NIH grant no. RC1 CA146065); the Cornell Center on the Microenvironment and Metastasis (no. NCI-U54 CA143876); the Human Frontiers in Science Program; the Cornell Nanobiotechnology Center (no. NSF-STC; ECS-9876771); the Cornell Center for Nanoscale Science and Technology (no. NSF-NNIN ECS 03-35765); an Empire State Development Division of Science, Technology and Innovation (NYSTAR) Center for Advanced Technology (CAT) award; a New York State J.D. Watson Award (A.D.S.); and an Arnold and Mabel Beckman Foundation Young Investigator Award (A.D.S.) P.F.D. acknowledges a National Science Foundation Graduate Fellowship.
Author information
Authors and Affiliations
Contributions
J.P.M., P.F.D., Y.Z., S.S.V., J.C, N.W.C., A.D.-S., J.A.L., T.N.C., C.F., and A.D.S. designed the research; J.P.M., P.F.D., Y.Z., S.S.V., J.C., M.C., P.K., and B.H. performed research; J.P.M., P.F.D., Y.Z., J.C., M.C., J.A.L., and A.D.S. analyzed data; and J.P.M., P.F.D., Y.Z. and A.D.S. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
Technical drawings of the aluminum jig for casting the PDMS stamp. Technical drawings, including critical dimensions, for the three aluminum jig components for molding the PDMS stamp. A machine shop should be able to fabricate all of the necessary pieces based on these schematics. (PDF 138 kb)
Supplementary Figure 2
Technical drawings for the microfluidic polycarbonate culture device. Technical drawings, including critical dimensions, for the top and bottom pieces of the microfluidic culture device. A machine shop should be able to fabricate all of the necessary pieces based on these schematics. (PDF 66 kb)
Supplementary Figure 3
Photographs for detail steps not presented in Figure 2, including the process for device assembly (steps 24-48), seeding (steps 49-53) and gravity-driven perfusion culture (steps 54A and 54B) processes. (i) Place the top piece of the microfluidic culture device onto the PDMS stamp, aligning the Plexiglas reservoir ports with the PDMS channel inlets. (ii) Insert the 16 gauge stainless steel dowel pins into the reservoir ports (to prevent collagen from entering the reservoir ports during injection) on the top piece of the microfluidic device and inject collagen gel into the cavity via the injection port with a 1 ml taper tip syringe. (iii) Use a micropipette to dispense ∼ 170 μL collagen onto the glass microscope coverslip housed in the bottom piece of the microfluidic culture device. (iv) Gently place the flat square of PDMS on top of the collagen-coated coverslip. (v) Homogeneously distribute the collagen across the coverslip by gently depressing and scraping across the back of the PDMS square with a tweezers to spread the collagen. Allow both layers of collagen to gel to cure for 1 hour. (vi) Remove the PDMS stamp by injecting 250 μL of PBS around the interface of stamp with Plexiglas and carefully lift off the stamp. (vii) Dispense PBS around the perimeter of the flat PDMS square and gently remove the 10square from the bottom Plexiglas piece. (viii) Carefully assemble the top and bottom pieces of the microfluidic device, keeping both collagen surfaces wetted with PBS. (ix) Gently tighten all four screws in a rotating manner to ensure a level and uniform join. (x) Turn the device over, aspirate excess PBS from the reservoirs, and add a small volume of culture medium. (xi) After equilibration for one hour in an incubator at 37 °C and 5% CO2, aspirate the medium and add a 10 μL cell suspension into the reservoir inlet port using a gel loading pipette. (xii) Allow cells to adhere for 30 minutes in an incubator. (xiii) Add cell culture media to the inlet reservoir and inspect the device fabrication for channel integrity and absence of air bubbles. (PDF 333 kb)
Supplementary Figure 4
Successful seeding of cells into channels of the microfluidic device. Supplementary image showing successful seeding of cells into channels of microfluidic culture device, at an appropriate density (Steps 49-53). Scale bar 200 μm. (PDF 10392 kb)
Supplementary Video 1
Supplementary video showing detail preparation and assembly of microfluidic culture device (Steps 21-53). (MP4 47324 kb)
Supplementary Video 2
Supplementary video showing successful seeding of cells into channels of microfluidic culture device, at an appropriate density (Steps 49-53). (MP4 2350 kb)
Supplementary Video 3
Supplementary video showing unsuccessful seeding of cells into channels of microfluidic culture device (Steps 49-53), due to an inadequate density. (MP4 499 kb)
Supplementary Video 4
Supplementary video showing unsuccessful seeding of cells into channels of microfluidic culture device (Steps 49-53), due to an excessive flow rate. (MP4 273 kb)
Supplementary Video 5
Supplementary video for time course shown in Figure 3. GFP-expressing endothelial cells exhibit dynamic migration within the endothelium in live fluorescence imaging experiments under physiological shear flow (∼11 mL/min, 17 dyne/cm2). Time course = 48-72 hours after onset of perfusion; scale bar = 50 μm. (MP4 1482 kb)
Supplementary Data
Electronic CAD file (AutoCAD, *.dwg format) for creating the lithographic mask to microfabricate a silicon master via photolithography (Step 2). (ZIP 63 kb)
Rights and permissions
About this article
Cite this article
Morgan, J., Delnero, P., Zheng, Y. et al. Formation of microvascular networks in vitro. Nat Protoc 8, 1820–1836 (2013). https://doi.org/10.1038/nprot.2013.110
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2013.110
This article is cited by
-
Image-based modeling of vascular organization to evaluate anti-angiogenic therapy
Biology Direct (2023)
-
Modeling angiogenesis in the human brain in a tissue-engineered post-capillary venule
Angiogenesis (2023)
-
A modular microfluidic system based on a multilayered configuration to generate large-scale perfusable microvascular networks
Microsystems & Nanoengineering (2021)
-
Biomechanical study on implantable and interventional medical devices
Acta Mechanica Sinica (2021)
-
Microfabricated blood vessels for modeling the vascular transport barrier
Nature Protocols (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.