Abstract
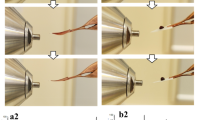
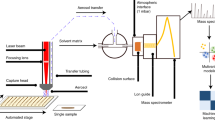
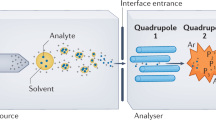
This protocol describes how to perform automated solid-phase microextraction (SPME) and thin-film microextraction (TFME) in a 96-well plate format for high-throughput analysis of drugs, metabolites and any other analytes of interest in biological fluids using liquid chromatography–electrospray tandem mass spectrometry. Sample preparation time required is typically 1 min per sample; hence, the throughput achievable with automated SPME/TFME is comparable with automated 96-well liquid–liquid extraction and solid-phase extraction methods, but greater than most online solid-phase extraction methods. The technique is applicable to complex samples such as whole blood without additional pretreatment. The amount of analyte extracted by SPME/TFME is proportional to the free (unbound) concentration of the analyte; hence, SPME/TFME can be used to determine both total and free concentrations of analytes from a single biofluid sample and to perform automated ligand–receptor binding studies in order to determine binding affinity and/or overall extent of ligand binding to a complex biofluid.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Maurer, H.H. Advances in analytical toxicology: the current role of liquid chromatography–mass spectrometry in drug quantification in blood and oral fluid. Anal. Bioanal. Chem. 381, 110–118 (2005).
Saint-Marcoux, F., Sauvage, F. & Marquet, P. Current role of LC–MS in therapeutic drug monitoring. Anal. Bioanal. Chem. 388, 1327–1349 (2007).
Srinivas, N.R. Changing need for bioanalysis during drug development. Biomed. Chromatogr. 22, 235 (2008).
Niessen, W.A. Liquid Chromatography—Mass Spectrometry (ed. Niessen, W.A.) 1–600 (Taylor & Francis, Boca Raton, Florida, 2006).
Chang, M.S., Ji, Q., Zhang, J. & El-Shourbagy, T.A. Historical review of sample preparation for chromatographic bioanalysis: pros and cons. Drug Dev. Res. 68, 107–133 (2007).
Wells, D.A. Sample preparation for drug discovery bioanalysis. In Integrated Strategies for Drug Discovery using Mass Spectrometry (ed. Lee, M.S.) 477–542 (Wiley-Interscience, Hoboken, New Jersey, 2005).
Niessen, W.M.A., Manini, P. & Andreoli, R. Matrix effects in quantitative pesticide analysis using liquid chromatography–mass spectrometry. Mass Spectrom. Rev. 25, 881–899 (2006).
Tonidandel, L. & Seraglia, R. Matrix effect, signal suppression and enhancement in LC-ESI-MS. In Advances in LC–MS Instrumentation (ed. Cappiello, A.) 193–210 (Elsevier, Amsterdam, The Netherlands, 2007).
Bakhtiar, R. & Majumdar, T.K. Tracking problems and possible solutions in the quantitative determination of small molecule drugs and metabolites in biological fluids using liquid chromatography–mass spectrometry. J. Pharmacol. Toxicol. Methods 55, 227–243 (2007).
Niessen, W.A. Atmospheric pressure ionization. In Liquid Chromatography–Mass Spectrometry (ed. Niessen, W.A.) 141–176 (Taylor & Francis, Boca Raton, Florida, 2006).
Niessen, W.A. Quantitative bioanalysis using LC–MS. In Liquid Chromatography–Mass Spectrometry (ed. Niessen W.A.) 289–330 (Taylor & Francis, Boca Raton, Florida, 2006).
Wells, D.A. High Throughput Bioanalytical Sample Preparation: Methods and Automation Strategies (ed. Wells, D.A.) 1–610 (Elsevier, UK, 2003).
Souverain, S., Rudaz, S. & Veuthey, J.L. Restricted access materials and large particle supports for on-line sample preparation: an attractive approach for biological fluids analysis. J. Chromatogr. B 801, 141–156 (2004).
Arthur, C.L. & Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 62, 2145–2148 (1990).
Solid Phase Microextraction, Theory and Practice (ed. Pawliszyn, J.) (Wiley-WCH, New York, 1997).
Musteata, F.M. & Pawliszyn, J. Bioanalytical applications of solid-phase microextraction. Trends Analyt. Chem. 26, 36–45 (2007).
Kumazawa, T., Lee, X., Sato, K. & Suzuki, O. Solid-phase microextraction and liquid chromatography/mass spectrometry in drug analysis. Anal. Chim. Acta 492, 49–67 (2003).
Cudjoe, E., Vuckovic, D., Hein, D. & Pawliszyn, J. Investigation of the effect of the extraction phase geometry on the performance of automated solid-phase microextraction. Anal. Chem. 81, 4226–4232 (2009).
Vuckovic, D., Cudjoe, E., Hein, D. & Pawliszyn, J. Automation of solid-phase microextraction in high-throughput format and applications to drug analysis. Anal. Chem. 80, 6870–6880 (2008).
Vatinno, R., Vuckovic, D., Zambonin, C.G. & Pawliszyn, J. Automated high-throughput method using solid-phase microextraction–liquid chromatography–tandem mass spectrometry for the determination of ochratoxin A in human urine. J. Chromatogr. A 1201, 215–221 (2008).
Alves, C., Santos-Neto, A.J., Fernandes, C., Rodrigues, J.C. & Lancas, F.M. Analysis of tricyclic antidepressant drugs in plasma by means of solid-phase microextraction–liquid chromatography–mass spectrometry. J. Mass Spec. 42, 1342–1347 (2007).
Volmer, D.A. & Hui, J.P.M. Rapid determination of corticosteroids in urine by combined solid phase microextraction/liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 11, 1926–1934 (1997).
Balakrishnan, V.K., Terry, K.A. & Toito, J. Determination of sulfonamide antibiotics in wastewater: a comparison of solid phase microextraction and solid phase extraction methods. J. Chromatogr. A 1131, 1–10 (2006).
Kataoka, H., Matsuura, E. & Mitani, K. Determination of cortisol in human saliva by automated in-tube solid-phase microextraction coupled with liquid chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 44, 160–165 (2007).
Musteata, F.M., Walles, M. & Pawliszyn, J. Fast assay of angiotensin 1 from whole blood by cation-exchange restricted-access solid-phase microextraction. Anal. Chim. Acta 537, 231–237 (2005).
Walles, M. et al. Verapamil drug metabolism studies by automated in-tube solid phase microextraction. J. Pharm. Biomed. Anal. 30, 307–319 (2002).
Kataoka, H., Narimatsu, S., Lord, H.L. & Pawliszyn, J. Automated in-tube solid-phase microextraction coupled with liquid chromatography/electrospray ionization mass spectrometry for the determination of b-blockers and metabolites in urine and serum samples. Anal. Chem. 71, 4237–4244 (1999).
Wu, J., Lord, H.L., Pawliszyn, J. & Kataoka, H. Polypyrrole-coated capillary in-tube solid phase microextraction coupled with liquid chromatography–electrospray ionization mass spectrometry for the determination of b-blockers in urine and serum samples. J. Microcolumn Sep. 12, 255–266 (2000).
Sagratini, G., Manes, J., Giardina, D., Damiani, P. & Pico, Y. Analysis of carbamate and phenylurea pesticide residues in fruit juices by solid-phase microextraction and liquid chromatography–mass spectrometry. J. Chromatogr. A 1147, 135–143 (2007).
Blasco, C., Font, G. & Pico, Y. Solid-phase microextraction–liquid chromatography–mass spectrometry applied to the analysis of insecticides in honey. Food Addit. Contam. Part A Chem. Anal., Control Expo. Risk Assess 25, 59–69 (2008).
Musteata, F.M. & Pawliszyn, J. Study of ligand–receptor binding using SPME: investigation of receptor, free, and total ligand concentrations. J. Proteome Res. 4, 789–800 (2005).
Heringa, M.B. & Hermens, J.L.M. Measurement of free concentrations using negligible depletion-solid phase microextraction (nd-SPME). Trends Analyt. Chem. 22, 575–587 (2003).
Heringa, M.B., Pastor, D., Algra, J., Vaes, W.H.J . & Hermens, J.L.M . Negligible depletion solid-phase microextraction with radiolabeled analytes to study free concentrations and protein binding: an example with [3H]estradiol. Anal. Chem. 74, 5993–5997 (2002).
Vuckovic, D. & Pawliszyn, J. Automated study of ligand–receptor binding using solid-phase microextraction. J. Pharm. Biomed. Anal. 50, 550–553 (2008).
Musteata, F.M., Pawliszyn, J., Qian, M.G., Wu, J. & Miwa, G.T. Determination of drug plasma protein binding by solid phase microextraction. J. Pharm. Sci. 95, 1712–1722 (2006).
Zhou, S.N., Oakes, K.D., Servos, M.R. & Pawliszyn, J. Application of solid-phase microextraction for in vivo laboratory and field sampling of pharmaceuticals in fish. Env. Sci. Tech. 42, 6073–6079 (2008).
Musteata, F.M. & Pawliszyn, J. Assay of stability, free and total concentration of chlorhexidine in saliva by solid phase microextraction. J. Pharm. Biomed. Anal. 37, 1015–1024 (2005).
Hage, D.S. & Tweed, S.A. Recent advances in chromatographic and electrophoretic methods for the study of drug-protein interactions. J. Chromatogr. B 699, 499–525 (1997).
Musteata, F.M. & Pawliszyn, J. In vivo sampling with solid phase microextraction. J. Biochem. Biophys. Methods 70, 181–193 (2007).
Liu, Z., Li, F. & Yuesheng, H. Determination of unbound drug concentration and protein-drug binding fraction in plasma. Biomed. Chromatogr. 13, 262–266 (1999).
Bertucci, C. & Domenici, E. Reversible and covalent binding of drugs to human serum albumin: methodological approaches and physiological relevance. Curr. Med. Chem. 9, 1463–1481 (2002).
Seedher, N. & Bhatia, S. Mechanism of interaction of the non-steroidal antiinflammatory drugs meloxicam and nimesulfide with serum albumin. J. Pharm. Biomed. Anal. 39, 257–262 (2005).
Huang, S., Tsai, T., Yeh, P. & Tsai, T. Measurement of unbound ranitidine in blood and bile of anesthesized rats using microdialysis coupled to liquid chromatography and its pharmacokinetic application. J. Chromatogr. A 1073, 297–302 (2005).
Zhang, Y., Leonessa, F., Clarke, R. & Wainer, I.W. Development of an immobilized P-glycoprotein stationary phase for on-line liquid chromatographic determination of drug-binding affinities. J. Chromatogr. B 739, 33–37 (2000).
Ng, E.S.M., Chan, N.W.C., Lewis, D.F., Hindsgaul, O. & Schriemer, D.C. Frontal affinity chromatography–mass spectrometry. Nat. Protoc. 2, 1907–1917 (2007).
Ostergaard, J., Schou, C., Larsen, C. & Heegaard, N.H.H. Evaluation of capillary electrophoresis-frontal analysis for the study of low molecular weight drug–human serum albumin interactions. Electrophoresis 23, 2842–2853 (2002).
Martinez-Pla, J.J. et al. High-throughput capillary electrophoresis frontal analysis method for the study of drug interactions with human serum albumin at near physiological conditions. Electrophoresis 25, 3176–3185 (2004).
Berger, G. & Girault, G. Macromolecule-ligand binding studied by the Hummel and Dreyer method: current state of the methodology. J. Chromatogr. B 797, 51–61 (2003).
Moaddel, R., Lu, L., Baynham, M. & Wainer, I.W. Immobilized receptor- and transporter-based liquid chromatographic phases for on-line pharmacological and biochemical studies: a mini-review. J. Chromatogr. B: Anal. Techn. Biomed. Life Sci. 768, 41–53 (2002).
Wan, H. & Bergstroem, F. High throughput screening of drug-protein binding in drug discovery. J. Liq. Chromatogr. Rel. Technol. 30, 681–700 (2007).
Banker, M.J., Clark, T.H. & Williams, J.A. Development and validation of a 96-well equilibrium dialysis apparatus for measuring plasma protein binding. J. Pharm. Sci. 92, 967–974 (2003).
Fung, E.N., Chen, Y. & Lau, Y.Y. Semi-automatic high-throughput determination of plasma protein binding using a 96-well plate filtrate assembly and fast liquid chromatography tandem mass spectrometry. J. Chromatogr. B 795, 187–194 (2003).
Wan, H. & Rehngren, M. High-throughput screening of protein binding by equilibrium dialysis combined with liquid chromatography and mass spectrometry. J. Chromatogr. A 1102, 125–134 (2006).
Hutchinson, J.P., Setkova, L. & Pawliszyn, J. Automation of solid-phase microextraction on a 96-well plate format. J. Chromatogr. A 1149, 127–137 (2007).
Risticevic, S., Lord, H.L., Gorecki, T., Arthur, C.L. & Pawliszyn, J. Protocol for solid phase microextraction method development. Nat. Protoc. 5, 122–139 (2010).
Cudjoe, E. & Pawliszyn, J. A new approach to the application of solid phase extraction disks with LC–MS/MS for the analysis of drugs on a 96-well plate format. J. Pharm. Biomed. Anal. 50, 556–562 (2008).
Hennion, M.C. Solid-phase extraction: method development, sorbents, and coupling with liquid chromatography. J. Chromatogr. A 856, 3–54 (1999).
Mullett, W.M. & Pawliszyn, J. Direct determination of benzodiazepines in biological fluids by restricted-access solid-phase microextraction. Anal. Chem. 74, 1081–1087 (2002).
Lord, H. & Pawliszyn, J. Microextraction of drugs. J. Chromatogr. A 902, 17–63 (2000).
Zhang, X., Es-Haghi, A., Musteata, F.M., Ouyang, G. & Pawliszyn, J. Quantitative in vivo microsampling for pharmacokinetic studies based on an integrated solid-phase microextraction system. Anal. Chem. 79, 4507–4513 (2007).
Musteata, F.M., Musteata, M.L. & Pawliszyn, J. Fast in vivo microextraction: a new tool for clinical analysis. Clin. Chem. 52, 708–715 (2006).
Zhou, S., Song, Q., Tang, Y. & Naidong, W. Critical review of development, validation, and transfer for high throughput bioanalytical LC–MS/MS methods. Curr. Pharm. Anal. 1, 3–14 (2005).
Watson, J.T. & Sparkman, O.D. Liquid chromatography/mass spectrometry. In Introduction to Mass Spectrometry: Instrumentation, Applications, and Strategies for Data Interpretation edn. 4 (eds. Watson, J.T. & Sparkman, O.D.) 639–688 (John Wiley & Sons, Ltd., Chichester, West Sussex, England, 2007).
Food and Drug Administration. Bioanalytical method validation. Guidance for Industry (2001).
Yuan, H. & Pawliszyn, J. Application of solid-phase microextraction in the determination of diazepam binding to human serum albumin. Anal. Chem. 73, 4410–4416 (2001).
Kramer, N.I., van Eijkeren, J.C.H. & Hermens, J.L.M. Influence of albumin on sorption kinetics in solid-phase microextraction: consequences for chemical analyses and uptake processes. Anal. Chem. 79, 6941–6948 (2007).
Lord, H.L., Rajabi, M., Safari, S. & Pawliszyn, J. A study of the performance characteristics of immunoaffinity solid phase microextraction probes for extraction of a range of benzodiazepines. J. Pharm. Biomed. Anal. 44, 506–519 (2007).
Lord, H.L., Rajabi, M., Safari, S. & Pawliszyn, J. Development of immunoaffinity solid phase microextraction probes for analysis of sub ng/ml concentrations of 7-aminoflunitrazepam. J. Pharm. Biomed. Anal. 40, 769–780 (2006).
Hu, X., Pan, J., Hu, Y., Huo, Y. & Li, G. Preparation and evaluation of solid-phase microextraction fibre based on molecularly imprinted polymers for trace analysis of tetracyclines in complicated samples. J. Chromatogr. A 1188, 97–107 (2008).
Hu, X., Hu, Y. & Li, G. Development of novel molecularly imprinted solid-phase microextraction fibre and its application for the determination of triazines in complicated samples coupled with high-performance liquid chromatography. J. Chromatogr. A 1147, 1–9 (2007).
Turiel, E., Tadeo, J.L. & Martin-Esteban, A. Molecularly imprinted polymeric fibres for solid-phase microextraction. Anal. Chem. 79, 3099–3104 (2007).
Koster, E.H.M., Crescenzi, C., den Hoedt, W., Ensing, K. & de Jong, G.J. Fibres coated with molecularly imprinted polymers for solid-phase microextraction. Anal. Chem. 73, 3140–3145 (2001).
Mullett, W.M., Martin, P. & Pawliszyn, J. In-tube molecularly imprinted polymer solid-phase microextraction for the selective determination of propranolol. Anal. Chem. 73, 2383–2389 (2001).
Xu, R.N., Fan, L., Rieser, M.J. & El-Shourbagy, T.A. Recent advances in high-throughput quantitative bioanalysis by LC–MS/MS. J. Pharm. Biomed. Anal. 44, 342–355 (2007).
Matuszewski, B.K. Standard line slopes as a measure of a relative matrix effect in quantitative HPLC–MS bioanalysis. J. Chromatogr. B: Anal. Techn. Biomed. Life Sci. 830, 293–300 (2006).
Matuszewski, B.K., Constanzer, M.L. & Chavez-Eng, C.M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC–MS/MS. Anal. Chem. 75, 3019–3030 (2003).
Xie, W., Pawliszyn, J., Mullett, W.M. & Matuszewski, B.K. Comparison of solid-phase microextraction and liquid–liquid extraction in 96-well format for the determination of a drug compound in human plasma by liquid chromatography with tandem mass spectrometric detection. J. Pharm. Biomed. Anal. 45, 599–608 (2007).
Zambonin, C.G. & Aresta, A. SPME-LC with UV detection to study delorazepam–serum albumin interactions. J. Pharm. Biomed. Anal. 29, 895–900 (2002).
Artola-Garciano, E., Vaes, W.H.J. & Hermens, J.L.M. Validation of negligible depletion solid-phase microextraction as a tool to determine tissue/blood partition coefficients for semivolatile and nonvolatile organic chemicals. Toxicol. Appl. Pharmacol. 166, 138–144 (2000).
Musteata, F.M. & Pawliszyn, J. Determination of free concentration of paclitaxel in liposome formulations. J. Pharm. Pharm. Sci. 9, 231–237 (2006).
Prosen, H., Fingler, S., Zupancic-Kralj, L. & Drevenkar, V. Partitioning of selected environmental pollutants into organic matter as determined by solid-phase microextraction. Chemosphere 66, 1580–1589 (2007).
Kosa, T., Maruyama, T. & Otagiri, M. Species differences of serum albumins: I. Drug binding sites. Pharm. Res. 14, 1607–1612 (1997).
Muller, W.E. & Wollert, U. Characterization of the binding of benzodiazepines to human serum albumin. Naunyn Schmiederbergs Arch. Pharmacol. 280, 229–237 (1973).
Muller, W.E. & Wollert, U. Influence of pH on the benzodiazepine–human serum albumin complex. Circular dichroism studies. Naunyn Schmiederbergs Arch. Pharmacol. 283, 67–82 (1974).
Acknowledgements
The authors thank the Natural Sciences and Engineering Research Council of Canada and Supelco for financial support. The authors thank Dietmar Hein (PAS Technology) for collaboration and the design and development of the Concept 96 autosampler.
Author information
Authors and Affiliations
Contributions
D.V. developed fiber coatings and evaluated performance of Concept 96 using fiber geometry for LC-MS applications, developed high-throughput applications, performed first automated ligand-binding study and wrote the manuscript. E.C. developed and evaluated thin-film configuration and corresponding coatings of the device. F.M.M. developed the theory and experimental workflow for ligand-receptor binding studies regardless of the degree of depletion. J.P. developed the initial idea for this project and supervised the activities in all stages of instrument and experimental design.
Corresponding author
Supplementary information
Supplementary Methods
A detailed step-by-step procedure to generate in vitro multi-point ligand-receptor binding isotherms using SPME/TFME and a PAS Concept 96 (PDF 59 kb)
Rights and permissions
About this article
Cite this article
Vuckovic, D., Cudjoe, E., Musteata, F. et al. Automated solid-phase microextraction and thin-film microextraction for high-throughput analysis of biological fluids and ligand–receptor binding studies. Nat Protoc 5, 140–161 (2010). https://doi.org/10.1038/nprot.2009.180
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.180
This article is cited by
-
Bioanalytical Applications of Microextraction Techniques: A Review of Reviews
Chromatographia (2020)
-
Development of a thin-film solid-phase microextraction (TF-SPME) method coupled to liquid chromatography and tandem mass spectrometry for high-throughput determination of steroid hormones in white sucker fish plasma
Analytical and Bioanalytical Chemistry (2020)
-
High-throughput analysis using non-depletive SPME: challenges and applications to the determination of free and total concentrations in small sample volumes
Scientific Reports (2018)
-
Screening of multiclass pesticide residues in honey by SPE-GC/MSD: a pilot study
Environmental Monitoring and Assessment (2018)
-
Re-exploring the high-throughput potential of microextraction techniques, SPME and MEPS, as powerful strategies for medical diagnostic purposes. Innovative approaches, recent applications and future trends
Analytical and Bioanalytical Chemistry (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.