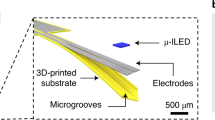
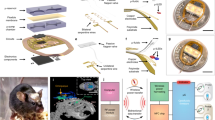
Abstract
This Protocol Extension describes the fabrication and technical procedures for implementing ultrathin, flexible optofluidic neural probe systems that provide targeted, wireless delivery of fluids and light into the brains of awake, freely behaving animals. As a Protocol Extension article, this article describes an adaptation of an existing Protocol that offers additional applications. This protocol serves as an extension of an existing Nature Protocol describing optoelectronic devices for studying intact neural systems. Here, we describe additional features of fabricating self-contained platforms that involve flexible microfluidic probes, pumping systems, microscale inorganic LEDs, wireless-control electronics, and power supplies. These small, flexible probes minimize tissue damage and inflammation, making long-term implantation possible. The capabilities include wireless pharmacological and optical intervention for dissecting neural circuitry during behavior. The fabrication can be completed in 1–2 weeks, and the devices can be used for 1–2 weeks of in vivo rodent experiments. To successfully carry out the protocol, researchers should have basic skill sets in photolithography and soft lithography, as well as experience with stereotaxic surgery and behavioral neuroscience practices. These fabrication processes and implementation protocols will increase access to wireless optofluidic neural probes for advanced in vivo pharmacology and optogenetics in freely moving rodents.
This protocol is an extension to: Nat. Protoc. 8, 2413–2428 (2013); doi:10.1038/nprot.2013.158; published online 07 November 2013
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Rajasethupathy, P., Ferenczi, E. & Deisseroth, K. Targeting neural circuits. Cell 165, 524–534 (2016).
Adamantidis, A.R., Zhang, F., Aravanis, A.M., Deisseroth, K. & de Lecea, L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 450, 420–424 (2007).
Arenkiel, B.R. et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 54, 205–218 (2007).
Armbruster, B.N., Li, X., Pausch, M.H., Herlitze, S. & Roth, B.L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. USA 104, 5163–5168 (2007).
Banghart, M., Borges, K., Isacoff, E., Trauner, D. & Kramer, R.H. Light-activated ion channels for remote control of neuronal firing. Nat. Neurosci. 7, 1381–1386 (2004).
Güler, A.D. et al. Transient activation of specific neurons in mice by selective expression of the capsaicin receptor. Nat. Commun. 3, 746 (2012).
Lima, S.Q. & Miesenböck, G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell 121, 141–152 (2005).
Zemelman, B.V., Lee, G.A., Ng, M. & Miesenböck, G. Selective photostimulation of genetically chARGed neurons. Neuron 33, 15–22 (2002).
Zemelman, B.V., Nesnas, N., Lee, G.A. & Miesenbock, G. Photochemical gating of heterologous ion channels: remote control over genetically designated populations of neurons. Proc. Natl. Acad. Sci. USA 100, 1352–1357 (2003).
Siuda, E.R. et al. Spatiotemporal control of opioid signaling and behavior. Neuron 86, 923–935 (2015).
Yizhar, O., Fenno, L.E., Davidson, T.J., Mogri, M. & Deisseroth, K. Optogenetics in neural systems. Neuron 71, 9–34 (2011).
Konermann, S. et al. Optical control of mammalian endogenous transcription and epigenetic states. Nature 500, 472–476 (2013).
Schindler, S.E. et al. Photo-activatable Cre recombinase regulates gene expression in vivo . Sci. Rep. 5, 13627 (2015).
Zhang, F. et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat. Protoc. 5, 439–456 (2010).
Cardin, J.A. et al. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat. Protoc. 5, 247–254 (2010).
Sparta, D.R. et al. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat. Protoc. 7, 12–23 (2011).
Sternson, S.M. & Roth, B.L. Chemogenetic tools to interrogate brain functions. Annu. Rev. Neurosci. 37, 387–407 (2014).
Tye, K.M. & Deisseroth, K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat. Rev. Neurosci. 13, 251–266 (2012).
Zorzos, A.N., Boyden, E.S. & Fonstad, C.G. Multiwaveguide implantable probe for light delivery to sets of distributed brain targets. Opt. Lett. 35, 4133–4135 (2010).
Zorzos, A.N., Scholvin, J., Boyden, E.S. & Fonstad, C.G. Three-dimensional multiwaveguide probe array for light delivery to distributed brain circuits. Opt. Lett. 37, 4841–4843 (2012).
Kim, T. et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340, 211–216 (2013).
Al-Hasani, R. et al. Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron 87, 1063–1077 (2015).
Wu, F. et al. Monolithically integrated μLEDs on silicon neural probes for high-resolution optogenetic studies in behaving animals. Neuron 88, 1136–1148 (2015).
McAlinden, N., Gu, E., Dawson, M.D., Sakata, S. & Mathieson, K. Optogenetic activation of neocortical neurons in vivo with a sapphire-based micro-scale LED probe. Front. Neural Circuits 9, 25 (2015).
Stachniak, T.J., Ghosh, A. & Sternson, S.M. Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus→midbrain pathway for feeding behavior. Neuron 82, 797–808 (2014).
Park, S.I. et al. Ultraminiaturized photovoltaic and radio frequency powered optoelectronic systems for wireless optogenetics. J. Neural Eng. 12, 056002 (2015).
Montgomery, K.L. et al. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nat. Methods 12, 969–974 (2015).
Park, S.I. et al. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat. Biotechnol. 33, 1280–1286 (2015).
Ameli, R., Mirbozorgi, A., Neron, J.-L., LeChasseur, Y. & Gosselin, B. A wireless and batteryless neural headstage with optical stimulation and electrophysiological recording. in 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 5662–5665 (2013).
Lee, S.T. et al. A miniature, fiber-coupled, wireless, deep-brain optogenetic stimulator. IEEE Trans. Neural Syst. Rehabil. Eng. 23, 655–664 (2015).
Rossi, M.A. et al. A wirelessly controlled implantable LED system for deep brain optogenetic stimulation. Front. Integr. Neurosci. 9, 8 (2015).
Iwai, Y., Honda, S., Ozeki, H., Hashimoto, M. & Hirase, H. A simple head-mountable LED device for chronic stimulation of optogenetic molecules in freely moving mice. Neurosci. Res. 70, 124–127 (2011).
Wentz, C.T. et al. A wirelessly powered and controlled device for optical neural control of freely-behaving animals. J. Neural Eng. 8, 046021 (2011).
Siuda, E.R. et al. Optodynamic simulation of β-adrenergic receptor signalling. Nat. Commun. 6, 8480 (2015).
McCall, J.G. et al. Fabrication and application of flexible, multimodal light-emitting devices for wireless optogenetics. Nat. Protoc. 8, 2413–2428 (2013).
Jeong, J.-W. et al. Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. Cell 162, 662–674 (2015).
Parker, K.E., McCall, J.G. & Will, M.J. Basolateral amygdala opioids contribute to increased high-fat intake following intra-accumbens opioid administration, but not following 24-h food deprivation. Pharmacol. Biochem. Behav. 97, 262–266 (2010).
Boschi, G., Launay, N. & Rips, R. Implantation of an intracerebral cannula in the mouse. J. Pharmacol. Methods 6, 193–198 (1981).
Chefer, V.I., Thompson, A.C., Zapata, A. & Shippenberg, T.S. Overview of brain microdialysis. Curr. Protoc. Neurosci. Unit 7.1, supplement 47 (2009).
Ruigrok, T.J.H. & Apps, R. A light microscope-based double retrograde tracer strategy to chart central neuronal connections. Nat. Protoc. 2, 1869–1878 (2007).
Conte, W.L., Kamishina, H. & Reep, R.L. Multiple neuroanatomical tract-tracing using fluorescent Alexa Fluor conjugates of cholera toxin subunit B in rats. Nat. Protoc. 4, 1157–1166 (2009).
Spieth, S. et al. An intra-cerebral drug delivery system for freely moving animals. Biomed. Microdevices 14, 799–809 (2012).
Canales, A. et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo . Nat. Biotechnol. 33, 277–284 (2015).
Farra, R. et al. First-in-human testing of a wirelessly controlled drug delivery microchip. Sci. Transl. Med. 4, 122ra21–122ra21 (2012).
Hoare, T. et al. A magnetically triggered composite membrane for on-demand drug delivery. Nano Lett. 9, 3651–3657 (2009).
Hoare, T. et al. Magnetically triggered nanocomposite membranes: a versatile platform for triggered drug release. Nano Lett. 11, 1395–1400 (2011).
Timko, B.P. et al. Near-infrared-actuated devices for remotely controlled drug delivery. Proc. Natl. Acad. Sci. USA 111, 1349–1354 (2014).
Minev, I.R. et al. Biomaterials. Electronic dura mater for long-term multimodal neural interfaces. Science 347, 159–163 (2015).
Richards, N. & McMahon, S.B. Targeting novel peripheral mediators for the treatment of chronic pain. Br. J. Anaesth. 111, 46–51 (2013).
Scheib, J. & Höke, A. Advances in peripheral nerve regeneration. Nat. Rev. Neurol. 9, 668–676 (2013).
Banghart, M.R. & Sabatini, B.L. Photoactivatable neuropeptides for spatiotemporally precise delivery of opioids in neural tissue. Neuron 73, 249–259 (2012).
Kramer, R.H., Mourot, A. & Adesnik, H. Optogenetic pharmacology for control of native neuronal signaling proteins. Nat. Neurosci. 16, 816–823 (2013).
Tochitsky, I. et al. Restoring visual function to blind mice with a photoswitch that exploits electrophysiological remodeling of retinal ganglion cells. Neuron 81, 800–813 (2014).
Polosukhina, A. et al. Photochemical restoration of visual responses in blind mice. Neuron 75, 271–282 (2012).
Myers, M.W., Laughlin, C.A., Jay, F.T. & Carter, B.J. Adenovirus helper function for growth of adeno-associated virus: effect of temperature-sensitive mutations in adenovirus early gene region 2. J. Virol. 35, 65–75 (1980).
Sinn, P.L., Sauter, S.L. & McCray, P.B. Gene therapy progress and prospects: development of improved lentiviral and retroviral vectors – design, biosafety, and production. Gene Ther. 12, 1089–1098 (2005).
Beer, C., Meyer, A., Müller, K. & Wirth, M. The temperature stability of mouse retroviruses depends on the cholesterol levels of viral lipid shell and cellular plasma membrane. Virology 308, 137–146 (2003).
Kawai, A. & Takeuchi, K. Temperature-sensitivity of the replication of rabies virus (HEP-flury strain) in BHK-21 cells I. Alteration of viral RNA synthesis at the elevated temperature. Virology 186, 524–532 (1992).
Croughan, W.S. & Behbehani, A.M. Comparative study of inactivation of herpes simplex virus types 1 and 2 by commonly used antiseptic agents. J. Clin. Microbiol. 26, 213–215 (1988).
Petr, G. & Jiran, E. The effect of temperature on the hemagglutinin activity of the canine adenovirus (infectious canine hepatitis virus). Zentralbl Veterinärmed B 17, 569–581 (1970).
Merten, O.-W., Gény-Fiamma, C. & Douar, A.M. Current issues in adeno-associated viral vector production. Gene Ther. 12, S51–S61 (2005).
Resendez, S.L. et al. Visualization of cortical, subcortical and deep brain neural circuit dynamics during naturalistic mammalian behavior with head-mounted microscopes and chronically implanted lenses. Nat. Protoc. 11, 566–597 (2016).
Cetin, A., Komai, S., Eliava, M., Seeburg, P.H. & Osten, P. Stereotaxic gene delivery in the rodent brain. Nat. Protoc. 1, 3166–3173 (2007).
Cui, G. et al. Deep brain optical measurements of cell type-specific neural activity in behaving mice. Nat. Protoc. 9, 1213–1228 (2014).
Carlezon, W.A. & Chartoff, E.H. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat. Protoc. 2, 2987–2995 (2007).
Kim, D.-H. et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 9, 511–517 (2010).
Tao, H. et al. Silk-based conformal, adhesive, edible food sensors. Adv. Mater. 24, 1067–1072 (2012).
Hwang, S.-W. et al. A physically transient form of silicon electronics. Science 337, 1640–1644 (2012).
Artificial cerebrospinal fluid (ACSF). Cold Spring Harb. Protoc. http://cshprotocols.cshlp.org/content/2011/9/pdb.rec065730.full?text_only=true (2011).
Ting, J., Daigle, T., Chen, Q. & Feng, G. in Patch-Clamp Methods and Protocols (eds. Martina, M. & Taverna, S.) 221–242 (New York: Springer, 2014).
Jeong, J.W. et al. Soft microfluidic neural probes for wireless drug delivery in freely behaving mice. in 2015 Transducers—2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS) 2264–2267 (2015).
Walf, A.A. & Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2, 322–328 (2007).
Noldus, L.P., Spink, A.J. & Tegelenbosch, R.A. EthoVision: a versatile video tracking system for automation of behavioral experiments. Behav. Res. Methods Instrum. Comput. 33, 398–414 (2001).
de Chaumont, F. et al. Computerized video analysis of social interactions in mice. Nat. Methods 9, 410–417 (2012).
McCall, J.G. et al. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron 87, 605–620 (2015).
Deacon, R.M.J. & Rawlins, J.N.P. T-maze alternation in the rodent. Nat. Protoc. 1, 7–12 (2006).
Cunningham, C.L., Gremel, C.M. & Groblewski, P.A. Drug-induced conditioned place preference and aversion in mice. Nat. Protoc. 1, 1662–1670 (2006).
Jeong, J.-W. et al. Soft materials in neuroengineering for hard problems in neuroscience. Neuron 86, 175–186 (2015).
Acknowledgements
This work is supported by the EUREKA National Institute on Drug Abuse (NIDA) grant R01DA037152 (to M.R.B.), National Institute of Mental Health grant F31 MH101956 (to J.G.M.), and NIDA grant K99DA038725 (to R.A.). We thank the Bruchas laboratory and the laboratory of R.W. Gereau IV for helpful discussions and support. We thank W.Z. Ray for supporting the facilities for the rat surgery. All biomedical aspects of the device work were supported by a National Security Science and Engineering Faculty Fellowship of Energy (to J.A.R.) and startup funding from the University of Colorado Boulder (to J.-W.J.). The LED development was enabled by funding from the US Department of Energy, Division of Materials Sciences, under award no. DE-FG02-07ER46471 (to J.A.R.), the National Institutes of Health Common Fund National Institute of Neurological Disorders and Stroke grant R01NS081707 (to J.A.R. and M.R.B.), and the Materials Research Laboratory and Center for Microanalysis of Materials (grant DE-FG02-07ER46453 to J.A.R.).
Author information
Authors and Affiliations
Contributions
J.G.M., R.Q., G.S., S.L., M.H.I., K.-I.J., Y.L., R.A., and J.-W.J. performed the experiments. J.G.M., M.R.B., J.-W.J., and J.A.R. developed the protocol. J.G.M., M.R.B., J.-W.J., and J.A.R. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.A.R. and M.R.B. are co-founders of Neurolux, a company that is making wireless optogenetic probes. The devices described here are not yet part of the company's current product portfolio, but we list this information here as a full disclosure.
Integrated supplementary information
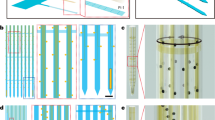
Supplementary Figure 1 Fabrication processes for the ultrathin, flexible microfluidic probe.
(A) Immerse a glass substrate in Pt inhibition solution to activate its surface for easy release of PDMS. (B) Rinse the glass substrate with methanol and bake on a hot plate at 70°C. The glass has amine layer on its surface, which prevents PDMS polymerization. (C) Flip the glass substrate in preparation for PDMS press-casting. (D) Prepare a microfluidic channel mold on a silicon wafer using UV-curable epoxy (SU-8 10). (E) Make anti-stiction surface treatment on the mold wafer by evaporating anti-stiction agent (TMCS) in a completely enclosed wafer box. (F) Cast PDMS on the mold. (G) Press and clip the PDMS-casted mold with the glass substrate and cure it at 70°C for 1 hour. (H) Release PDMS glass substrate from the mold, which has ~20-μm-thick microfluidic channel patterns (channel cross-section = 10 μm ×10 μm). (I–J) Prepare a thin flat PDMS layer on the PC sheet for bonding with the patterned PDMS layer in (H). The thickness of PDMS layer can be controlled by adjusting the spinning speed: 2000 rpm for 60 sec results in 20-μm-thick flat PDMS layer. (M) Treat the surface of both PDMS layer in (H) and (L) with oxygen plasma and bond together. This forms ultrathin PDMS microfluidic channels. (N) Remove PC from the bonded PDMS. (O, P) Release the bonded PDMS layer from the glass substrate. The amine group formed on the glass substrate facilitates delamination of PDMS without damage.
Supplementary Figure 3 Tools used in fabrication of microfluidic neural probes.
(A) Custom-designed blade tool to create a small (500 μm wide) microfluidic probe (Supplementary Data 2). (B) 3D printed alignment tool for punching (Supplementary Data 3). (C) Punch (Harris Uni-Core, 0.50 mm).
Supplementary Figure 4 Schematic diagram showing the operation principle of microfluidic devices for fluid delivery.
By turning on a heater in the device, the thermally expandable layer becomes expanded and pumps out fluid in the reservoir.
Supplementary Figure 7 Circuit diagrams for IR wireless control modules.
(A) Wireless receiver module. (B) Wireless remote controller.
Supplementary information
Combo PDF
Supplementary Figures 1–7 and the Supplementary Note. (PDF 789 kb)
Supplementary Data 1
AutoCAD file for the design of heaters and microfluidic channels. (ZIP 126 kb)
Supplementary Data 2
SolidWorks part document for the custom-designed blade tool to create a 500-μm-wide microfluidic probe. (ZIP 82 kb)
Supplementary Data 3
STL file for the punch alignment tool. (ZIP 8 kb)
Supplementary Data 4
STL file for the device case. (ZIP 47 kb)
Supplementary Data 5
STL file for the case lid. (ZIP 5 kb)
Rights and permissions
About this article
Cite this article
McCall, J., Qazi, R., Shin, G. et al. Preparation and implementation of optofluidic neural probes for in vivo wireless pharmacology and optogenetics. Nat Protoc 12, 219–237 (2017). https://doi.org/10.1038/nprot.2016.155
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.155
This article is cited by
-
Wearable and Implantable Light-Emitting Diodes and Their Biomedical Applications
Korean Journal of Chemical Engineering (2024)
-
Customizable, wireless and implantable neural probe design and fabrication via 3D printing
Nature Protocols (2023)
-
Multifunctional microelectronic fibers enable wireless modulation of gut and brain neural circuits
Nature Biotechnology (2023)
-
Self-assembled ultraflexible probes for long-term neural recordings and neuromodulation
Nature Protocols (2023)
-
Porosity-based heterojunctions enable leadless optoelectronic modulation of tissues
Nature Materials (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.