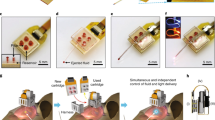
Abstract
This Protocol Extension describes the low-cost production of rapidly customizable optical neural probes for in vivo optogenetics. We detail the use of a 3D printer to fabricate minimally invasive microscale inorganic light-emitting-diode-based neural probes that can control neural circuit activity in freely behaving animals, thus extending the scope of two previously published protocols describing the fabrication and implementation of optoelectronic devices for studying intact neural systems. The 3D-printing fabrication process does not require extensive training and eliminates the need for expensive materials, specialized cleanroom facilities and time-consuming microfabrication techniques typical of conventional manufacturing processes. As a result, the design of the probes can be quickly optimized, on the basis of experimental need, reducing the cost and turnaround for customization. For example, 3D-printed probes can be customized to target multiple brain regions or scaled up for use in large animal models. This protocol comprises three procedures: (1) probe fabrication, (2) wireless module preparation and (3) implantation for in vivo assays. For experienced researchers, neural probe and wireless module fabrication requires ~2 d, while implantation should take 30–60 min per animal. Time required for behavioral assays will vary depending on the experimental design and should include at least 5 d of animal handling before implantation of the probe, to familiarize each animal to their handler, thus reducing handling stress that may influence the result of the behavioral assays. The implementation of customized probes improves the flexibility in optogenetic experimental design and increases access to wireless probes for in vivo optogenetic research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Experiments that support the findings of this protocol are described in our previous paper4. Further data are available from the corresponding authors upon request.
Code availability
The code and STL files used in this study are provided in Supplementary Data 1–3 and 7. Gerber and CAD files for the PCBs are provided in Supplementary Data 4. Code for the BLE SoC and smartphone app are provided in Supplementary Data 5 and 6 and are also available at https://github.com/juulee2011/3DPOPs_control_app_Android.git.
References
Deisseroth, K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 18, 1213–1225 (2015).
Adamantidis, A. et al. Optogenetics: 10 years after ChR2 in neurons—views from the community. Nat. Neurosci. 18, 1202–1212 (2015).
Janak, P. H. & Tye, K. M. From circuits to behaviour in the amygdala. Nature 517, 284–292 (2015).
Lee, J. et al. Rapidly customizable, scalable 3D-printed wireless optogenetic probes for versatile applications in neuroscience. Adv. Funct. Mater. 30, 2004285 (2020).
McCall, J. G. et al. Fabrication and application of flexible, multimodal light-emitting devices for wireless optogenetics. Nat. Protoc. 8, 2413–2428 (2013).
McCall, J. G. et al. Preparation and implementation of optofluidic neural probes for in vivo wireless pharmacology and optogenetics. Nat. Protoc. 12, 219–237 (2017).
Kim, T. et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340, 211–216 (2013).
Jeong, J.-W. et al. Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. Cell 162, 662–674 (2015).
Byun, S.-H. et al. Mechanically transformative electronics, sensors, and implantable devices. Sci. Adv. 5, eaay0418 (2019).
Poher, V. et al. Micro-LED arrays: a tool for two-dimensional neuron stimulation. J. Phys. Appl. Phys. 41, 094014 (2008).
Grossman, N. et al. Multi-site optical excitation using ChR2 and micro-LED array. J. Neural Eng. 7, 016004 (2010).
Gerhardt, K. P. et al. An open-hardware platform for optogenetics and photobiology. Sci. Rep. 6, 35363 (2016).
Aharoni, D., Khakh, B. S., Silva, A. J. & Golshani, P. All the light that we can see: a new era in miniaturized microscopy. Nat. Methods 16, 11–13 (2019).
Siegle, J. H. et al. Open Ephys: an open-source, plugin-based platform for multichannel electrophysiology. J. Neural Eng. 14, 045003 (2017).
Yang, Y. et al. Wireless multilateral devices for optogenetic studies of individual and social behaviors. Nat. Neurosci. 24, 1035–1045 (2021).
Shin, G. et al. Flexible near-field wireless optoelectronics as subdermal implants for broad applications in optogenetics. Neuron 93, 509–521.e3 (2017).
Park, S. I. et al. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat. Biotechnol. 33, 1280–1286 (2015).
Park, S. I. et al. Ultraminiaturized photovoltaic and radio frequency powered optoelectronic systems for wireless optogenetics. J. Neural Eng. 12, 056002 (2015).
Noh, K. N. et al. Miniaturized, battery-free optofluidic systems with potential for wireless pharmacology and optogenetics. Small 14, 1702479 (2018).
Kim, C. Y. et al. Soft subdermal implant capable of wireless battery charging and programmable controls for applications in optogenetics. Nat. Commun. 12, 535 (2021).
Qazi, R. et al. Wireless optofluidic brain probes for chronic neuropharmacology and photostimulation. Nat. Biomed. Eng. 3, 655–669 (2019).
Qazi, R., Kim, C. Y., Byun, S.-H. & Jeong, J.-W. Microscale inorganic LED based wireless neural systems for chronic in vivo optogenetics. Front. Neurosci. 12, 764 (2018).
Sparta, D. R. et al. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat. Protoc. 7, 12–23 (2011).
Dagnew, R. et al. CerebraLux: a low-cost, open-source, wireless probe for optogenetic stimulation. Neurophotonics 4, 045001 (2017).
Tye, K. M. et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362 (2011).
Pisanello, F. et al. Dynamic illumination of spatially restricted or large brain volumes via a single tapered optical fiber. Nat. Neurosci. 20, 1180–1188 (2017).
Pisanello, M. et al. Tailoring light delivery for optogenetics by modal demultiplexing in tapered optical fibers. Sci. Rep. 8, 4467 (2018).
Wu, F. et al. Monolithically integrated μLEDs on silicon neural probes for high-resolution optogenetic studies in behaving animals. Neuron 88, 1136–1148 (2015).
Burton, A. et al. Wireless, battery-free subdermally implantable photometry systems for chronic recording of neural dynamics. Proc. Natl Acad. Sci. USA 117, 2835–2845 (2020).
Implantable Biomedical Microsystems - 1st Edition. https://www.elsevier.com/books/implantable-biomedical-microsystems/bhunia/978-0-323-26208-8
Lu, L. et al. Wireless optoelectronic photometers for monitoring neuronal dynamics in the deep brain. Proc. Natl Acad. Sci. USA 115, E1374–E1383 (2018).
Yin, M. et al. Wireless neurosensor for full-spectrum electrophysiology recordings during free behavior. Neuron 84, 1170–1182 (2014).
Burton, A. et al. Wireless, battery-free, and fully implantable electrical neurostimulation in freely moving rodents. Microsyst. Nanoeng. 7, 1–12 (2021).
Lee, W. et al. Microfabrication and in vivo performance of a microdialysis probe with embedded membrane. Anal. Chem. 88, 1230–1237 (2016).
Mickle, A. D. et al. A wireless closed loop system for optogenetic peripheral neuromodulation. Nature 565, 361–365 (2019).
Zhang, Y. et al. Battery-free, fully implantable optofluidic cuff system for wireless optogenetic and pharmacological neuromodulation of peripheral nerves. Sci. Adv. 5, eaaw5296 (2019).
Scharf, R. et al. Depth-specific optogenetic control in vivo with a scalable, high-density μLED neural probe. Sci. Rep. 6, 28381 (2016).
Shim, E., Chen, Y., Masmanidis, S. & Li, M. Multisite silicon neural probes with integrated silicon nitride waveguides and gratings for optogenetic applications. Sci. Rep. 6, 22693 (2016).
Zorzos, A. N., Scholvin, J., Boyden, E. S. & Fonstad, C. G. Three-dimensional multiwaveguide probe array for light delivery to distributed brain circuits. Opt. Lett. 37, 4841–4843 (2012).
Delcasso, S., Denagamage, S., Britton, Z. & Graybiel, A. M. HOPE: hybrid-drive combining optogenetics, pharmacology and electrophysiology. Front. Neural Circuits 12, (2018).
Kim, C. K. et al. Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain. Nat. Methods 13, 325–328 (2016).
Kim, K. et al. HectoSTAR microLED optoelectrodes for large-scale, high-precision in invo opto-electrophysiology. Preprint at https://doi.org/10.1101/2020.10.09.334227 (2020).
Wentz, C. T. et al. A wirelessly powered and controlled device for optical neural control of freely-behaving animals. J. Neural Eng. 8, 046021 (2011).
Ameli, R., Mirbozorgi, A., Neron, J.-L., LeChasseur, Y. & Gosselin, B. A wireless and batteryless neural headstage with optical stimulation and electrophysiological recording. In 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 5662–5665 (2013).
Hashimoto, M., Hata, A., Miyata, T. & Hirase, H. Programmable wireless light-emitting diode stimulator for chronic stimulation of optogenetic molecules in freely moving mice. Neurophotonics 1, 011002–011002 (2014).
Montgomery, K. L. et al. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nat. Methods 12, 969–974 (2015).
de Groot, A. et al. NINscope, a versatile miniscope for multi-region circuit investigations. eLife 9, e49987 (2020).
Barbera, G., Liang, B., Zhang, L., Li, Y. & Lin, D.-T. A wireless miniScope for deep brain imaging in freely moving mice. J. Neurosci. Methods 323, 56–60 (2019).
Jacob, A. D. et al. A compact head-mounted endoscope for in vivo calcium imaging in freely behaving mice. Curr. Protoc. Neurosci. 84, e51 (2018).
Qazi, R. et al. Scalable and modular wireless-network infrastructure for large-scale behavioural neuroscience. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-021-00814-w (2021).
Barbera, G. et al. An open source motorized swivel for in vivo neural and behavioral recordings. MethodsX 7, 101167 (2020).
Andrews, C. D. & Hutson, P. H. A reliable multichannel commutator for making electrical contact with conscious, freely moving rats. J. Neurosci. Methods 5, 73–76 (1982).
Fee, M. S. & Leonardo, A. Miniature motorized microdrive and commutator system for chronic neural recording in small animals. J. Neurosci. Methods 112, 83–94 (2001).
Roh, M., McHugh, T. J. & Lee, K. A video based feedback system for control of an active commutator during behavioral physiology. Mol. Brain 8, 61 (2015).
Shansky, R. M. & Murphy, A. Z. Considering sex as a biological variable will require a global shift in science culture. Nat. Neurosci. https://doi.org/10.1038/s41593-021-00806-8 (2021).
Miller, L. R. et al. Considering sex as a biological variable in preclinical research. FASEB J. 31, 29–34 (2017).
Butler, T. R., Karkhanis, A. N., Jones, S. R. & Weiner, J. L. Adolescent social isolation as a model of heightened vulnerability to comorbid alcoholism and anxiety disorders. Alcohol. Clin. Exp. Res. 40, 1202–1214 (2016).
Ma, X. et al. Social isolation-induced aggression potentiates anxiety and depressive-like behavior in male mice subjected to unpredictable chronic mild stress. PloS ONE 6, e20955 (2011).
Oehler, J., Jähkel, M. & Schmidt, J. Neuronal transmitter sensitivity after social isolation in rats. Physiol. Behav. 41, 187–191 (1987).
Tuboly, G., Benedek, G. & Horvath, G. Selective disturbance of pain sensitivity after social isolation. Physiol. Behav. 96, 18–22 (2009).
Tomova, L. et al. Acute social isolation evokes midbrain craving responses similar to hunger. Nat. Neurosci. 23, 1597–1605 (2020).
Matthews, G. A. et al. Dorsal raphe dopamine neurons represent the experience of social isolation. Cell 164, 617–631 (2016).
Nonogaki, K., Nozue, K. & Oka, Y. Social isolation affects the development of obesity and type 2 diabetes in mice. Endocrinology 148, 4658–4666 (2007).
Yizhar, O., Fenno, L. E., Davidson, T. J., Mogri, M. & Deisseroth, K. Optogenetics in neural systems. Neuron 71, 9–34 (2011).
Marcotte, M. et al. Handling techniques to reduce stress in mice. JoVE J. Vis. Exp. https://doi.org/10.3791/62593 (2021).
Gouveia, K. & Hurst, J. L. Improving the practicality of using non-aversive handling methods to reduce background stress and anxiety in laboratory mice. Sci. Rep. 9, 20305 (2019).
Sensini, F. et al. The impact of handling technique and handling frequency on laboratory mouse welfare is sex-specific. Sci. Rep. 10, 17281 (2020).
Acknowledgements
We thank J. Yea, S. Zhang, J. Bilbily and J. Xiao for their help with the initial probe validation. This paper is based on research that has been conducted as part of the KAIST-funded Global Singularity Research Program. This work was also supported by the National Research Foundation of Korea (grant nos. NRF-2021R1A2C4001483 and NRF-2020M3A9G8018572, J.-W.J.), the National Institutes of Health (R01NS117899, R21DA055047, J.G.M.) and the Brain & Behavior Research Foundation (NARSAD YI – 28565, J.G.M.).
Author information
Authors and Affiliations
Contributions
K.E.P., J.L., J.R.K., J.-W.J. and J.G.M. conceived the project and designed the detailed experimental protocol. K.E.P., J.L., J.R.K., C.K., C.Y.K., R.Q. and K.-I.J. performed the experiments. K.E.P., J.L., J.R.K., J.-W.J. and J.G.M. wrote the paper. J.-W.J. and J.G.M. acquired funding and supervised the project. K.E.P., J.L. and J.R.K. contributed equally to this work. J.-W.J. and J.G.M. are co-senior authors.
Corresponding authors
Ethics declarations
Competing interests
J.-W.J. and J.G.M. share two US patents (US 10,617,300 B2 and 11,160,489 B2) for injectable and implantable cellular-scale electronic devices, but do not earn income related to these patents. The other authors have no conflicts of interest.
Peer review
Peer review information
Nature Protocols thanks Ying Fang, Suk-Won Hwang, Chong Xie and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Related links
Key reference using this protocol
Lee, J. et al. Adv. Funct. Mater. 30, 2004285 (2020): https://doi.org/10.1002/adfm.202004285
This protocol is an extension to: Nat. Protoc. 12, 219–237 (2017): https://doi.org/10.1038/nprot.2016.155
Extended data
Extended Data Fig. 1 Technical drawing with dimensions for 3D-printed stereotaxic adapter for cannula holder.
a,b, Top (a) and side (b) view of 3D-printed stereotaxic adapter.
Extended Data Fig. 2 Bulk preparation of Metabond quick set cement kit.
a, Selected components of the Metabond kit used to secure the implant in its position. b, Add one scoop of Metabond radiopaque L-powder to the chilled ceramic dish. c, Add four drops of Quick Base liquid. d, Add one drop of Catalyst liquid. e, Mix cement preparation with rounded spatula. f, Use spatula to apply it directly to the skull surface.
Extended Data Fig. 3 Technical drawing with dimensions for a 5-mm-long unilateral 3D-POP and a probe holder.
a, Top view (left) and cross-section view (right) of 3D-POP. b, Top view (left) and cross-section view (right) of probe holder.
Extended Data Fig. 4 Technical drawing with dimensions for a supporter and a stencil mask used for PDMS screen printing.
a, Top view (left) and cross-section view (right) of the supporter. b, Top view of stencil mask.
Extended Data Fig. 5 Circuit diagram for the BLE control circuit.
The circuit includes a BLE SoC, a voltage regulator, indicator LEDs (red, green) and connectors for the integration of a lithium polymer (LiPo) battery in a 3D-POP. The voltage regulator converts fluctuating input voltage from the LiPo battery into constant output voltage (3 V) and supplies it to the BLE SoC. The BLE SoC enables wireless control by smartphone and regulates the photostimulation condition of 3D-POPs. Indicator LEDs help to recognize the communication status of the wireless system.
Extended Data Fig. 6 Preparation of the behavioral assay for optogenetic stimulation.
Preparation for wireless operation. Integrate a 3D-POP and the BLE wireless control module. Check LED operation using the smartphone app. b,c, Preparation for a wired operation: solder wires to a male pin connector (b), and connect a signal generator to a 3D-POP through the wire and apply electrical pulse signals for testing (c).
Supplementary information
Supplementary Data 1
STL file for the stereotaxic adapter
Supplementary Data 2
STL files for the 1D probe
Supplementary Data 3
STL files for PDMS screen printing
Supplementary Data 4
Gerber and CAD files for the wireless control circuit
Supplementary Data 5
Program code for the BLE SoC
Supplementary Data 6
Source codes for the smartphone application
Supplementary Data 7
STL files for the 3 × 3 probe
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parker, K.E., Lee, J., Kim, J.R. et al. Customizable, wireless and implantable neural probe design and fabrication via 3D printing. Nat Protoc 18, 3–21 (2023). https://doi.org/10.1038/s41596-022-00758-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00758-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.