Abstract
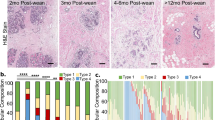
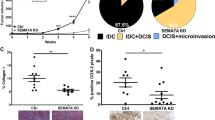
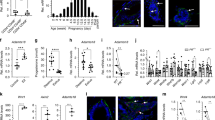
The prognosis of breast cancer in young women is influenced by reproductive history. Women diagnosed within 5 years postpartum have worse prognosis than nulliparous women or women diagnosed during pregnancy. Here we describe a mouse model of postpartum breast cancer that identifies mammary gland involution as a driving force of tumor progression. In this model, human breast cancer cells exposed to the involuting mammary microenvironment form large tumors that are characterized by abundant fibrillar collagen, high cyclooxygenase-2 (COX-2) expression and an invasive phenotype. In culture, tumor cells are invasive in a fibrillar collagen and COX-2–dependent manner. In the involuting mammary gland, inhibition of COX-2 reduces the collagen fibrillogenesis associated with involution, as well as tumor growth and tumor cell infiltration to the lung. These data support further research to determine whether women at high risk for postpartum breast cancer would benefit from treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) during postpartum involution.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lord, S.J. et al. Breast cancer risk and hormone receptor status in older women by parity, age of first birth, and breastfeeding: a case-control study. Cancer Epidemiol. Biomarkers Prev. 17, 1723–1730 (2008).
Lambe, M. et al. Transient increase in the risk of breast cancer after giving birth. N. Engl. J. Med. 331, 5–9 (1994).
Mathews, T.J. & Hamilton, B.E. Delayed Childbearing: More Women are Having their First Child Later in Life Vol. 21 (National Center for Health Statistics, Hyattsville, MD, 2009).
Lyons, T.R., Schedin, P.J. & Borges, V.F. Pregnancy and breast cancer: when they collide. J. Mammary Gland Biol. Neoplasia 14, 87–98 (2009).
Daling, J.R., Malone, K.E., Doody, D.R., Anderson, B.O. & Porter, P.L. The relation of reproductive factors to mortality from breast cancer. Cancer Epidemiol. Biomarkers Prev. 11, 235–241 (2002).
Whiteman, M.K. et al. Reproductive history and mortality after breast cancer diagnosis. Obstet. Gynecol. 104, 146–154 (2004).
Dodds, L. et al. Relationship of time since childbirth and other pregnancy factors to premenopausal breast cancer prognosis. Obstet. Gynecol. 111, 1167–1173 (2008).
Stensheim, H., Moller, B., van Dijk, T. & Fossa, S.D. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: a registry-based cohort study. J. Clin. Oncol. 27, 45–51 (2009).
Bemis, L.T. & Schedin, P. Reproductive state of rat mammary gland stroma modulates human breast cancer cell migration and invasion. Cancer Res. 60, 3414–3418 (2000).
Schedin, P., Mitrenga, T., McDaniel, S. & Kaeck, M. Mammary ECM composition and function are altered by reproductive state. Mol. Carcinog. 41, 207–220 (2004).
Clarkson, R.W., Wayland, M.T., Lee, J., Freeman, T. & Watson, C.J. Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast Cancer Res. 6, R92–R109 (2004).
Stein, T. et al. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. 6, R75–R91 (2004).
McDaniel, S.M. et al. Remodeling of the mammary microenvironment after lactation promotes breast tumor cell metastasis. Am. J. Pathol. 168, 608–620 (2006).
O'Brien, J. et al. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am. J. Pathol. 176, 1241–1255 (2010).
Schäfer, M. & Werner, S. Cancer as an overhealing wound: an old hypothesis revisited. Nat. Rev. Mol. Cell Biol. 9, 628–638 (2008).
Coussens, L.M. & Werb, Z. Inflammation and cancer. Nature 420, 860–867 (2002).
Schedin, P. Pregnancy-associated breast cancer and metastasis. Nat. Rev. Cancer 6, 281–291 (2006).
Wang, W. et al. Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Res. 62, 6278–6288 (2002).
Butcher, D.T., Alliston, T. & Weaver, V.M. A tense situation: forcing tumour progression. Nat. Rev. Cancer 9, 108–122 (2009).
Paszek, M.J. et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005).
Provenzano, P.P. et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 6, 11 (2008).
Broom, O.J., Massoumi, R. & Sjolander, A. α2β1 integrin signalling enhances cyclooxygenase-2 expression in intestinal epithelial cells. J. Cell Physiol. 209, 950–958 (2006).
Mann, J.R., Backlund, M.G. & DuBois, R.N. Mechanisms of disease: inflammatory mediators and cancer prevention. Nat. Clin. Pract. Oncol. 2, 202–210 (2005).
Thun, M.J., Namboodiri, M.M. & Heath, C.W. Jr. Aspirin use and reduced risk of fatal colon cancer. N. Engl. J. Med. 325, 1593–1596 (1991).
Elder, D.J. & Paraskeva, C. COX-2 inhibitors for colorectal cancer. Nat. Med. 4, 392–393 (1998).
Sheehan, K.M. et al. The relationship between cyclooxygenase-2 expression and colorectal cancer. J. Am. Med. Assoc. 282, 1254–1257 (1999).
Ristimäki, A. et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 62, 632–635 (2002).
Denkert, C. et al. Elevated expression of cyclooxygenase-2 is a negative prognostic factor for disease free survival and overall survival in patients with breast carcinoma. Cancer 97, 2978–2987 (2003).
Spizzo, G. et al. Correlation of COX-2 and Ep-CAM overexpression in human invasive breast cancer and its impact on survival. Br. J. Cancer 88, 574–578 (2003).
Howe, L.R. Inflammation and breast cancer. Cyclooxygenase/prostaglandin signaling and breast cancer. Breast Cancer Res. 9, 210 (2007).
Minn, A.J. et al. Genes that mediate breast cancer metastasis to lung. Nature 436, 518–524 (2005).
Bos, P.D. et al. Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1009 (2009).
Gauthier, M.L. et al. Abrogated response to cellular stress identifies DCIS associated with subsequent tumor events and defines basal-like breast tumors. Cancer Cell 12, 479–491 (2007).
Visscher, D.W. et al. Association between cyclooxygenase-2 expression in atypical hyperplasia and risk of breast cancer. J. Natl. Cancer Inst. 100, 421–427 (2008).
Kwan, M.L., Habel, L.A., Slattery, M.L. & Caan, B. NSAIDs and breast cancer recurrence in a prospective cohort study. Cancer Causes Control 18, 613–620 (2007).
Holmes, M.D. et al. Aspirin intake and survival after breast cancer. J. Clin. Oncol. 28, 1467–1472 (2010).
Liu, C.H. et al. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J. Biol. Chem. 276, 18563–18569 (2001).
Howe, L.R. et al. HER2/neu-induced mammary tumorigenesis and angiogenesis are reduced in cyclooxygenase-2 knockout mice. Cancer Res. 65, 10113–10119 (2005).
Larkins, T.L., Nowell, M., Singh, S. & Sanford, G.L. Inhibition of cyclooxygenase-2 decreases breast cancer cell motility, invasion and matrix metalloproteinase expression. BMC Cancer 6, 181 (2006).
Singh, B. et al. COX-2 involvement in breast cancer metastasis to bone. Oncogene 26, 3789–3796 (2007).
Miller, F.R., Santner, S.J., Tait, L. & Dawson, P.J. MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. J. Natl. Cancer Inst. 92, 1185–1186 (2000).
Hu, M. et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell 13, 394–406 (2008).
Allred, D.C. et al. Ductal carcinoma in situ and the emergence of diversity during breast cancer evolution. Clin. Cancer Res. 14, 370–378 (2008).
Sørlie, T. et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 98, 10869–10874 (2001).
van de Vijver, M.J. et al. A gene-expression signature as a predictor of survival in breast cancer. N. Engl. J. Med. 347, 1999–2009 (2002).
Provenzano, P.P. et al. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 4, 38 (2006).
Anders, C.K. et al. Breast carcinomas arising at a young age: unique biology or a surrogate for aggressive intrinsic subtypes? J. Clin. Oncol. 29, e18–e20 (2011).
Chang, H.Y. et al. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc. Natl. Acad. Sci. USA 102, 3738–3743 (2005).
Rodriguez, A.O. et al. Evidence of poorer survival in pregnancy-associated breast cancer. Obstet. Gynecol. 112, 71–78 (2008).
Lethaby, A.E., O'Neill, M.A., Mason, B.H., Holdaway, I.M. & Harvey, V.J. Overall survival from breast cancer in women pregnant or lactating at or after diagnosis. Auckland Breast Cancer Study Group. Int. J. Cancer 67, 751–755 (1996).
Bladström, A., Anderson, H. & Olsson, H. Worse survival in breast cancer among women with recent childbirth: results from a Swedish population-based register study. Clin. Breast Cancer 4, 280–285 (2003).
Gupta, P.B. et al. Systemic stromal effects of estrogen promote the growth of estrogen receptor-negative cancers. Cancer Res. 67, 2062–2071 (2007).
Levental, K.R. et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 (2009).
Wilgus, T.A. et al. The impact of cyclooxygenase-2 mediated inflammation on scarless fetal wound healing. Am. J. Pathol. 165, 753–761 (2004).
Conklin, M.W. et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 178, 1221–1232 (2011).
Howe, L.R. et al. Celecoxib, a selective cyclooxygenase 2 inhibitor, protects against human epidermal growth factor receptor 2 (HER-2)/neu-induced breast cancer. Cancer Res. 62, 5405–5407 (2002).
Krause, S., Maffini, M.V., Soto, A.M. & Sonnenschein, C. A novel 3D in vitro culture model to study stromal-epithelial interactions in the mammary gland. Tissue Eng. Part C Methods 14, 261–271 (2008).
Liang, C.C., Park, A.Y. & Guan, J.L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2, 329–333 (2007).
Acknowledgements
We are grateful to K. Polyak (Harvard Medical School) and M. Hu for providing MCF10DCIS parental cells and advice, C. Ambrosone, L. Hines, A. Thor and S. Edgerton for human tissue acquisition, M. Garcia and M. Skokan for FISH analysis, M. Lucia and R. Wilson for assistance with quantitative immunohistochemistry analysis, O. Maller, K. Bell, S. Jindal, K. Hedman, D. Powell, N. DeWaele and Y. Kwarteng for technical assistance, and S. Sillau for advanced statistical analyses. We thank K. Polyak, A. Thorburn, M. Moss and Nature Medicine reviewers for critical evaluation of the manuscript, and we gratefully acknowledge the subjects for their contribution to this research. This work was supported by Department of Defense Synergistic Idea Award BC060531, Komen Foundation grant KG090629, Mary Kay Ash Foundation grant 078-08, AMC cancer fund, and University of Colorado Cancer Center grants to P.S. and V.B., Department of Defense Award BC074970 to P.J.K., American Cancer Society New England Division Postdoctoral Fellowship Spin Odyssey PF-08-257-01-CSM to T.R.L., Department of Defense Postdoctoral grant BC087579 to A.M., and Department of Defense Predoctoral grant BC073482 to J.O.
Author information
Authors and Affiliations
Contributions
T.R.L. developed the postpartum mouse model, and designed and performed the in vivo celecoxib, two-dimensional cell culture, protein expression, three-dimensional collagen, celecoxib, COX-2 knockdown and human DCIS studies, and data analyses. J.O. designed and performed the in vivo ibuprofen experiments, the collagen western blot quantification, the three-dimensional gelatin assay and data analyses. P.J.K. and M.W.C. performed quantitative SHG collagen imaging and collagen fiber orientation. K.W.E. provided critical guidance for the SHG imaging. A.M. generated GFP-expressing MCF10DCIS cells and provided MCF10DCIS cells with stable knockdown of COX-2. A.-C.T. performed the human outcome analyses. V.B. and T.R.L. were responsible for regulatory oversight of human tissue acquisition and V.B. and P.S. for human tissue acquisition. P.S. and V.B. were responsible for hypothesis development, conceptual design and all data analysis and interpretation. T.R.L., J.O. and P.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Tables 1 and 2 and Supplementary Methods (PDF 788 kb)
Rights and permissions
About this article
Cite this article
Lyons, T., O'Brien, J., Borges, V. et al. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med 17, 1109–1115 (2011). https://doi.org/10.1038/nm.2416
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2416
This article is cited by
-
Toward Characterizing Lymphatic Vasculature in the Mammary Gland During Normal Development and Tumor-Associated Remodeling
Journal of Mammary Gland Biology and Neoplasia (2024)
-
Aiphanol, a multi-targeting stilbenolignan, potently suppresses mouse lymphangiogenesis and lymphatic metastasis
Acta Pharmacologica Sinica (2023)
-
Immune cells are increased in normal breast tissues of BRCA1/2 mutation carriers
Breast Cancer Research and Treatment (2023)
-
Gestational Breast Cancer – a Review of Outcomes, Pathophysiology, and Model Systems
Journal of Mammary Gland Biology and Neoplasia (2023)
-
Clinical characteristics and pathologic complete response (pCR) rate after neoadjuvant chemotherapy in postpartum women with breast cancer
Journal of Cancer Research and Clinical Oncology (2023)