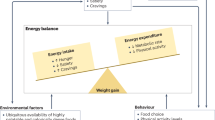
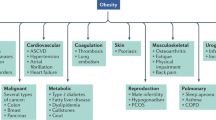
Abstract
Pediatric obesity is a serious medical condition associated with significant comorbidities during childhood and adulthood. Lifestyle modifications are essential for treating children with obesity, yet many have insufficient response to improve health with behavioral approaches alone. This review summarizes the relatively sparse data on pharmacotherapy for pediatric obesity and presents information on obesity medications in development. Most previously studied medications demonstrated, at best, modest effects on body weight and obesity-related conditions. It is to be hoped that the future will bring new drugs targeting specific obesity phenotypes that will allow clinicians to use etiology-specific, and therefore more effective, anti-obesity therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ogden CL, Carroll MD, Kit BK, Flegal KM . Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 2012; 307: 483–490.
World Health Organization. Obesity and Overweight Fact sheet Number 311. http://www.who.int/mediacentre/factsheets/fs311/en/index.html Accessed: 25 November 2011.
Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM . Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA 2010; 303: 242–249.
Rokholm B, Baker JL, Sorensen TI . The levelling off of the obesity epidemic since the year 1999 - a review of evidence and perspectives. Obes Rev 2010; 11: 835–846.
Sundblom E, Petzold M, Rasmussen F, Callmer E, Lissner L . Childhood overweight and obesity prevalences levelling off in Stockholm but socioeconomic differences persist. Int J Obes (Lond) 2008; 32: 1525–1530.
Salanave B, Peneau S, Rolland-Cachera MF, Hercberg S, Castetbon K . Stabilization of overweight prevalence in French children between 2000 and 2007. Int J Pediatr Obes 2009; 4: 66–72.
Peneau S, Salanave B, Maillard-Teyssier L, Rolland-Cachera MF, Vergnaud AC, Mejean C et al. Prevalence of overweight in 6- to 15-year-old children in central/western France from 1996 to 2006: trends toward stabilization. Int J Obes (Lond) 2009; 33: 401–407.
Olds T, Maher C, Zumin S, Peneau S, Lioret S, Castetbon K et al. Evidence that the prevalence of childhood overweight is plateauing: data from nine countries. Int J Pediatr Obes 2011; 6: 342–360.
Wang Y, Lobstein T . Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes 2006; 1: 11–25.
Kipping RR, Jago R, Lawlor DA . Obesity in children. Part 1: Epidemiology, measurement, risk factors, and screening. BMJ 2008; 337: a1824.
Mirmiran P, Sherafat-Kazemzadeh R, Jalali-Farahani S, Azizi F . Childhood obesity in the Middle East: a review. East Mediterr Health J 2010; 16: 1009–1017.
August GP, Caprio S, Fennoy I, Freemark M, Kaufman FR, Lustig RH et al. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab 2008; 93: 4576–4599.
Lee E . The world health organization's global strategy on diet, physical activity, and health: Turning strategy into action. Food Drug Law J 2005; 60: 569–601.
Freedman DS, Kahn HS, Mei Z, Grummer-Strawn LM, Dietz WH, Srinivasan SR et al. Relation of body mass index and waist-to-height ratio to cardiovascular disease risk factors in children and adolescents: the Bogalusa Heart Study. Am J Clin Nutr 2007; 86: 33–40.
Freedman DS, Katzmarzyk PT, Dietz WH, Srinivasan SR, Berenson GS . Relation of body mass index and skinfold thicknesses to cardiovascular disease risk factors in children: the Bogalusa Heart Study. Am J Clin Nutr 2009; 90: 210–216.
Young-Hyman D, Schlundt DG, Herman L, De Luca F, Counts D . Evaluation of the insulin resistance syndrome in 5- to 10-year-old overweight/obese African-American children. Diabetes Care 2001; 24: 1359–1364.
Csabi G, Torok K, Jeges S, Molnar D . Presence of metabolic cardiovascular syndrome in obese children. Eur J Pediatr 2000; 159: 91–94.
Daniels SR . Complications of obesity in children and adolescents. Int J Obes (Lond) 2009; 33 (Suppl 1): S60–S65.
Baker JL, Olsen LW, Sorensen TI . Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med 2007; 357: 2329–2337.
Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med 2011; 365: 1876–1885.
Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH . Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med 1992; 327: 1350–1355.
McGovern L, Johnson JN, Paulo R, Hettinger A, Singhal V, Kamath C et al. Clinical review: treatment of pediatric obesity: a systematic review and meta-analysis of randomized trials. J Clin Endocrinol Metab 2008; 93: 4600–4605.
Flynn MA, McNeil DA, Maloff B, Mutasingwa D, Wu M, Ford C et al. Reducing obesity and related chronic disease risk in children and youth: a synthesis of evidence with 'best practice' recommendations. Obes Rev 2006; 7 (Suppl 1): 7–66.
Oude Luttikhuis H, Baur L, Jansen H, Shrewsbury VA, O'Malley C, Stolk RP . Interventions for treating obesity in children. Cochrane Database Syst Rev 2009. CD001872.
Epstein LH, McCurley J, Valoski A, Wing RR . Growth in obese children treated for obesity. Am J Dis Child 1990; 144: 1360–1364.
Young KM, Northern JJ, Lister KM, Drummond JA, O'Brien WH . A meta-analysis of family-behavioral weight-loss treatments for children. Clin Psychol Rev 2007; 27: 240–249.
Whitlock EP, O'Conner EA, Williams SB, Beil TL, Lutz KW Effectiveness of Primary Care Interventions for Weight Management in Children and Adolescents. An Updated, Targeted Systematic Review for the US Preventive Services Task Force. Agency for Healthcare Research and Quality (US); Report No.: 10-05144-EF-1. Evidence Synthesis, No. 76. January 2010.
Fowler-Brown A, Kahwati LC . Prevention and treatment of overweight in children and adolescents. Am Fam Physician 2004; 69: 2591–2598.
Kalarchian MA, Levine MD, Arslanian SA, Ewing LJ, Houck PR, Cheng Y et al. Family-based treatment of severe pediatric obesity: randomized, controlled trial. Pediatrics 2009; 124: 1060–1068.
Epstein LH, Valoski A, Wing RR, McCurley J . Ten-year outcomes of behavioral family-based treatment for childhood obesity. Health Psychol 1994; 13: 373–383.
Seo DC, Sa J . A meta-analysis of obesity interventions among U.S. minority children. J Adolesc Health 2010; 46: 309–323.
Epstein LH, Paluch RA, Roemmich JN, Beecher MD . Family-based obesity treatment, then and now: twenty-five years of pediatric obesity treatment. Health Psychol 2007; 26: 381–391.
Spear BA, Barlow SE, Ervin C, Ludwig DS, Saelens BE, Schetzina KE et al. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics 2007; 120 (Suppl 4): S254–S288.
Barlow SE . Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007; 120 (Suppl 4): S164–S192.
Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 2011; 128 (Suppl 5): S213–S256.
Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med 2005; 353: 2111–2120.
Berkowitz RI, Wadden TA, Tershakovec AM, Cronquist JL . Behavior therapy and sibutramine for the treatment of adolescent obesity: a randomized controlled trial. JAMA 2003; 289: 1805–1812.
Yanovski JA . Intensive therapies for pediatric obesity. Pediatr Clin North Am 2001; 48: 1041–1053.
Daniels SR, Benuck I, Christakis DA, Dennison BA, Gidding SS, Gillman MW et al Overweight and Obesity. Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: The Report of the Expert Panel. NHLBI NIH Guidelines, NHLBI, DHHS: Bethesda, MD, 2011. pp 282–320.
US Food and Drug Administration. Qualifying for Pediatric Exclusivity Under Section 505 A of the Federal Food, Drug, and Cosmetic Act: Frequently Asked Questions on Pediatric Exclusivity (505 A), The Pediatric ‘Rule,’ and their Interaction. Development & Approval Process (Drugs). http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm077915.htm Accessed: 30 March 2012.
Samanin R, Garattini S . Neurochemical mechanism of action of anorectic drugs. Pharmacol Toxicol 1993; 73: 63–68.
von Spranger J . Phentermine resinate in obesity. Clinical trial of Mirapront in adipose children. Munch Med Wochenschr 1965; 107: 1833–1834.
Andelman MB, Jones C, Nathan S . Treatment of obesity in underprivileged adolescents. Comparison of diethylpropion hydrochloride with placebo in a double-blind study. Clin Pediatr (Phila) 1967; 6: 327–330.
Dolecek R . Endocrine studies with mazindol in obese patients. Pharmatherapeutica 1980; 2: 309–316.
Golebiowska M, Chlebna-Sokol D, Kobierska I, Konopinska A, Malek M, Mastalska A et al. Clinical evaluation of Teronac (mazindol) in the treatment of obesity in children. Part II. Anorectic properties and side effects (author's transl). Przegl Lek 1981; 38: 355–358.
Golebiowska M, Chlebna-Sokol D, Mastalska A, Zwaigzne-Raczynska J . The clinical evaluation of teronac (Mazindol) in the treatment of children with obesity. Part I. Effect of the drug on somatic patterns and exercise capacity (author's transl). Przegl Lek 1981; 38: 311–314.
Komorowski JM, Zwaigzne-Raczynska J, Owczarczyk I, Golebiowska M, Zarzycki J . Effect of mazindol (teronac) on various hormonal indicators in children with simple obesity. Pediatr Pol 1982; 57: 241–246.
Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 2001; 39: 32–41.
Kaplan LM . Pharmacologic therapies for obesity. Gastroenterol Clin North Am 2010; 39: 69–79.
Rothman RB, Ayestas MA, Dersch CM, Baumann MH . Aminorex, fenfluramine, and chlorphentermine are serotonin transporter substrates. Implications for primary pulmonary hypertension. Circulation 1999; 100: 869–875.
Drug Enforcement Administration, Office of Diversion Control. List of scheduling actions controlled substances regulated chemicals, U.S. Department of Justice. http://www.deadiversion.usdoj.gov/schedules/index.html Accessed: 1 February 2012.
Altschuler S, Conte A, Sebok M, Marlin RL, Winick C . Three controlled trials of weight loss with phenylpropanolamine. Int J Obes 1982; 6: 549–556.
Kernan WN, Viscoli CM, Brass LM, Broderick JP, Brott T, Feldmann E et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med 2000; 343: 1826–1832.
Isojarvi JI, Turkka J, Pakarinen AJ, Kotila M, Rattya J, Myllyla VV . Thyroid function in men taking carbamazepine, oxcarbazepine, or valproate for epilepsy. Epilepsia 2001; 42: 930–934.
Haas JT, Miao J, Chanda D, Wang Y, Zhao E, Haas ME et al. Hepatic insulin signaling is required for obesity-dependent expression of SREBP-1c mRNA but not for feeding-dependent expression. Cell Metab 2012; 15: 873–884.
Lorber J . Obesity in childhood. A controlled trial of anorectic drugs. Arch Dis Child 1966; 41: 309–312.
Stewart DA, Bailey JD, Patell H . Tenuate dospan as an appetitie suppressant in the treatment of obese children. Appl Ther 1970; 12: 34–36.
Malecka-Tendera E, Koehler B, Muchacka M, Wazowski R, Trzciakowska A . Efficacy and safety of dexfenfluramine treatment in obese adolescents. Pediatr Pol 1996; 71: 431–436.
Bacon GE, Lowrey GH . A clinical trial of fenfluramine in obese children. Curr Ther Res Clin Exp 1967; 9: 626–630.
Goldstein DJ, Rampey AH, Enas GG, Potvin JH, Fludzinski LA, Levine LR . Fluoxetine: a randomized clinical trial in the treatment of obesity. Int J Obes Relat Metab Disord 1994; 18: 129–135.
Pedrinola F, Cavaliere H, Lima N, Medeiros-Neto G . Is DL-fenfluramine a potentially helpful drug therapy in overweight adolescent subjects? Obes Res 1994; 2: 1–4.
Pedrinola F, Sztejnsznajd C, Lima N, Halpern A, Medeiros-Neto G . The addition of dexfenfluramine to fluoxetine in the treatment of obesity: a randomized clinical trial. Obes Res 1996; 4: 549–554.
Rauh JL, Lipp R . Chlorphentermine as an anorexigenic agent in adolescent obesity. Report of its efficacy in a double-blind study of 30 teen-agers. Clin Pediatr (Phila) 1968; 7: 138–140.
Anon. Cardiac valvulopathy associated with exposure to fenfluramine or dexfenfluramine: U.S. Department of Health and Human Services interim public health recommendations, November 1997. MMWR Morb Mortal Wkly Rep 1997; 46: 1061–1066.
Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med 1997; 337: 581–588.
Abenhaim L, Moride Y, Brenot F, Rich S, Benichou J, Kurz X et al. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hypertension Study Group. N Engl J Med 1996; 335: 609–616.
Weintraub M, Hasday JD, Mushlin AI, Lockwood DH . A double-blind clinical trial in weight control. Use of fenfluramine and phentermine alone and in combination. Arch Intern Med 1984; 144: 1143–1148.
Mason PW, Krawiecki N, Meacham LR . The use of dextroamphetamine to treat obesity and hyperphagia in children treated for craniopharyngioma. Arch Pediatr Adolesc Med 2002; 156: 887–892.
Davis C, Fattore L, Kaplan AS, Carter JC, Levitan RD, Kennedy JL . The suppression of appetite and food consumption by methylphenidate: the moderating effects of gender and weight status in healthy adults. Int J Neuropsychopharmacol 2011; 1–7.
Greenhill LL, Findling RL, Swanson JM . A double-blind, placebo-controlled study of modified-release methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics 2002; 109: E39.
Wigal T, Greenhill L, Chuang S, McGough J, Vitiello B, Skrobala A et al. Safety and tolerability of methylphenidate in preschool children with ADHD. J Am Acad Child Adolesc Psychiatry 2006; 45: 1294–1303.
Klein-Schwartz W . Abuse and toxicity of methylphenidate. Curr Opin Pediatr 2002; 14: 219–223.
Ioannides-Demos LL, Piccenna L, McNeil JJ . Pharmacotherapies for obesity: past, current, and future therapies. J Obes 2011 2011; 179674.
Wald AB, Uli NK . Pharmacotherapy in pediatric obesity: current agents and future directions. Rev Endocr Metab Disord 2009; 10: 205–214.
Dunican KC, Desilets AR, Montalbano JK . Pharmacotherapeutic options for overweight adolescents. Ann Pharmacother 2007; 41: 1445–1455.
Godoy-Matos A, Carraro L, Vieira A, Oliveira J, Guedes EP, Mattos L et al. Treatment of obese adolescents with sibutramine: a randomized, double-blind, controlled study. J Clin Endocrinol Metab 2005; 90: 1460–1465.
Violante-Ortiz R, Del-Rio-Navarro BE, Lara-Esqueda A, Perez P, Fanghanel G, Madero A et al. Use of sibutramine in obese Hispanic adolescents. Adv Ther 2005; 22: 642–649.
Berkowitz RI, Fujioka K, Daniels SR, Hoppin AG, Owen S, Perry AC et al. Effects of sibutramine treatment in obese adolescents: a randomized trial. Ann Intern Med 2006; 145: 81–90.
Garcia-Morales LM, Berber A, Macias-Lara CC, Lucio-Ortiz C, Del-Rio-Navarro BE, Dorantes-Alvarez LM . Use of sibutramine in obese mexican adolescents: a 6-month, randomized, double-blind, placebo-controlled, parallel-group trial. Clin Ther 2006; 28: 770–782.
Reisler G, Tauber T, Afriat R, Bortnik O, Goldman M . Sibutramine as an adjuvant therapy in adolescents suffering from morbid obesity. Isr Med Assoc J 2006; 8: 30–32.
Budd GM, Hayman LL, Crump E, Pollydore C, Hawley KD, Cronquist JL et al. Weight loss in obese African American and Caucasian adolescents: secondary analysis of a randomized clinical trial of behavioral therapy plus sibutramine. J Cardiovasc Nurs 2007; 22: 288–296.
Daniels SR, Long B, Crow S, Styne D, Sothern M, Vargas-Rodriguez I et al. Cardiovascular effects of sibutramine in the treatment of obese adolescents: results of a randomized, double-blind, placebo-controlled study. Pediatrics 2007; 120: e147–e157.
Danielsson P, Janson A, Norgren S, Marcus C . Impact sibutramine therapy in children with hypothalamic obesity or obesity with aggravating syndromes. J Clin Endocrinol Metab 2007; 92: 4101–4106.
Van Mil EG, Westerterp KR, Kester AD, Delemarre-van de Waal HA, Gerver WJ, Saris WH . The effect of sibutramine on energy expenditure and body composition in obese adolescents. J Clin Endocrinol Metab 2007; 92: 1409–1414.
Yanovski JA . Behavior therapy and sibutramine for the treatment of adolescent obesity. J Pediatr 2003; 143: 686.
Pischon T, Sharma AM . Recent developments in the treatment of obesity-related hypertension. Curr Opin Nephrol Hypertens 2002; 11: 497–502.
Torp-Pedersen C, Caterson I, Coutinho W, Finer N, Van Gaal L, Maggioni A et al. Cardiovascular responses to weight management and sibutramine in high-risk subjects: an analysis from the SCOUT trial. Eur Heart J 2007; 28: 2915–2923.
James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med 2010; 363: 905–917.
US Food and Drug Administration. Meridia (sibutramine): Market Withdrawal Due to Risk of SeriousCardiovascular Events. http://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm228830.htm Accessed: 20 October 2010.
Yanovski SZ, Yanovski JA . Obesity. N Engl J Med 2002; 346: 591–602.
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM . Positional cloning of the mouse obese gene and its human homologue. Nature 1994; 372: 425–432.
Havel PJ . Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol 2002; 13: 51–59.
Havel PJ, Townsend R, Chaump L, Teff K . High-fat meals reduce 24-h circulating leptin concentrations in women. Diabetes 1999; 48: 334–341.
Weigle DS, Cummings DE, Newby PD, Breen PA, Frayo RS, Matthys CC et al. Roles of leptin and ghrelin in the loss of body weight caused by a low fat, high carbohydrate diet. J Clin Endocrinol Metab 2003; 88: 1577–1586.
McDuffie JR, Riggs PA, Calis KA, Freedman RJ, Oral EA, DePaoli AM et al. Effects of exogenous leptin on satiety and satiation in patients with lipodystrophy and leptin insufficiency. J Clin Endocrinol Metab 2004; 89: 4258–4263.
Schwartz MW . Brain pathways controlling food intake and body weight. Exp Biol Med (Maywood) 2001; 226: 978–981.
Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 1995; 269: 543–546.
Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995; 269: 540–543.
Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P . Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks [see comments]. Science 1995; 269: 546–549.
Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 1999; 341: 879–884.
Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 2002; 110: 1093–1103.
Gibson WT, Farooqi IS, Moreau M, DePaoli AM, Lawrence E, O'Rahilly S et al. Congenital leptin deficiency due to homozygosity for the Delta133G mutation: report of another case and evaluation of response to four years of leptin therapy. J Clin Endocrinol Metab 2004; 89: 4821–4826.
Paz-Filho G, Wong ML, Licinio J . Ten years of leptin replacement therapy. Obes Rev 2011; 12: e315–e323.
Chong AY, Lupsa BC, Cochran EK, Gorden P . Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia 2010; 53: 27–35.
Chou SH, Chamberland JP, Liu X, Matarese G, Gao C, Stefanakis R et al. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci USA 2011; 108: 6585–6590.
Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 1999; 282: 1568–1575.
Moon HS, Matarese G, Brennan AM, Chamberland JP, Liu X, Fiorenza CG et al. Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance. Diabetes 2011; 60: 1647–1656.
Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL . Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab 2002; 87: 2391–2394.
Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 2005; 115: 3579–3586.
Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J . Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest 2008; 118: 2583–2591.
Goldsmith R, Joanisse DR, Gallagher D, Pavlovich K, Shamoon E, Leibel RL et al. Effects of experimental weight perturbation on skeletal muscle work efficiency, fuel utilization, and biochemistry in human subjects. Am J Physiol Regul Integr Comp Physiol 2010; 298: R79–R88.
Baldwin KM, Joanisse DR, Haddad F, Goldsmith RL, Gallagher D, Pavlovich KH et al. Effects of Weight Loss and Leptin on Skeletal Muscle in Human Subjects. Am J Physiol Regul Integr Comp Physiol 2011; 301: R1259–R1266.
Anderson JW, Greenway FL, Fujioka K, Gadde KM, McKenney J, O'Neil PM . Bupropion SR enhances weight loss: a 48-week double-blind, placebo- controlled trial. Obes Res 2002; 10: 633–641.
Billes SK, Cowley MA . Inhibition of dopamine and norepinephrine reuptake produces additive effects on energy balance in lean and obese mice. Neuropsychopharmacology 2007; 32: 822–834.
Li Z, Maglione M, Tu W, Mojica W, Arterburn D, Shugarman LR et al. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med 2005; 142: 532–546.
Jain AK, Kaplan RA, Gadde KM, Wadden TA, Allison DB, Brewer ER et al. Bupropion SR vs. placebo for weight loss in obese patients with depressive symptoms. Obes Res 2002; 10: 1049–1056.
Glod CA, Lynch A, Flynn E, Berkowitz C, Baldessarini RJ . Open trial of bupropion SR in adolescent major depression. J Child Adolesc Psychiatr Nurs 2003; 16: 123–130.
Becker EA, Shafer A, Anderson R . Weight changes in teens on psychotropic medication combinations at Austin State Hospital. Tex Med 2005; 101: 62–70.
Martin CK, Redman LM, Zhang J, Sanchez M, Anderson CM, Smith SR et al. Lorcaserin, a 5-HT(2C) receptor agonist, reduces body weight by decreasing energy intake without influencing energy expenditure. J Clin Endocrinol Metab 2011; 96: 837–845.
Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med 2010; 363: 245–256.
Fidler MC, Sanchez M, Raether B, Weissman NJ, Smith SR, Shanahan WR et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab 2011; 96: 3067–3077.
O'Neil PM, Smith SR, Weissman NJ, Fidler MC, Sanchez M, Zhang J et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring) 2012; 20: 1426–1436.
Jaslow R FDA approves obesity pill Belviq for obese, overweight people with weight-related health problems. Accessed: 1 July 2012.
FDA. approves Belviq to treat some overweight or obese adults. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm309993.htm Accessed: 1 July 2012.
Astrup A, Madsbad S, Breum L, Jensen TJ, Kroustrup JP, Larsen TM . Effect of tesofensine on bodyweight loss, body composition, and quality of life in obese patients: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 372: 1906–1913.
Gilbert JA, Gasteyger C, Raben A, Meier DH, Astrup A, Sjodin A . The effect of tesofensine on appetite sensations. Obesity 2012; 20: 553–561.
Sjodin A, Gasteyger C, Nielsen AL, Raben A, Mikkelsen JD, Jensen JK et al. The effect of the triple monoamine reuptake inhibitor tesofensine on energy metabolism and appetite in overweight and moderately obese men. Int J Obes (Lond) 2010; 34: 1634–1643.
Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A . Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet 2007; 370: 1706–1713.
Van Gaal L, Pi-Sunyer X, Despres JP, McCarthy C, Scheen A . Efficacy and safety of rimonabant for improvement of multiple cardiometabolic risk factors in overweight/obese patients: pooled 1-year data from the Rimonabant in Obesity (RIO) program. Diabetes Care 2008; 31 (Suppl 2): S229–S240.
US Food and Drug Administration. Endocrine and Metabolic Drugs Advisory Committee Meeting. Sanofi Aventis: Zimulti (Rimonabant) Briefing Document - NDA 21-888. May 20, 2007. http://www.scribd.com/doc/1117189/US-Food-and-Drug-Administration-20074306b101sponsorbackgrounder Accessed: 30 March 2012.
Wathion N European Medicines Agency Public Statement on Acomplia (rimonabant) - Withdrawal of the Marketing Authorisation in European Union, Report Number: EMEA/39457/2009. http://www.ema.europa.eu/docs/en_GB/document_library/Public_statement/2009/11/WC500012189.pdf Accessed: 30 March 2012.
Heal DJ, Gosden J, Smith SL . Regulatory challenges for new drugs to treat obesity and comorbid metabolic disorders. Br J Clin Pharmacol 2009; 68: 861–874.
Koch L . Obesity: Taranabant no longer developed as an antiobesity agent. Nat Rev Endocrinol 2010; 6: 300.
Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, Jeong WI et al. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest 2008; 118: 3160–3169.
Nogueiras R, Veyrat-Durebex C, Suchanek PM, Klein M, Tschop J, Caldwell C et al. Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes 2008; 57: 2977–2991.
Nakata M, Yada T . Cannabinoids inhibit insulin secretion and cytosolic Ca2+ oscillation in islet beta-cells via CB1 receptors. Regul Pept 2008; 145: 49–53.
Ruby MA, Nomura DK, Hudak CS, Mangravite LM, Chiu S, Casida JE et al. Overactive endocannabinoid signaling impairs apolipoprotein E-mediated clearance of triglyceride-rich lipoproteins. Proc Natl Acad Sci USA 2008; 105: 14561–14566.
Kramer CK, Leitao CB, Pinto LC, Canani LH, Azevedo MJ, Gross JL . Efficacy and safety of topiramate on weight loss: a meta-analysis of randomized controlled trials. Obes Rev 2011; 12: e338–e347.
Narula PK, Rehan HS, Unni KE, Gupta N . Topiramate for prevention of olanzapine associated weight gain and metabolic dysfunction in schizophrenia: a double-blind, placebo-controlled trial. Schizophr Res 2010; 118: 218–223.
Glauser TA, Dlugos DJ, Dodson WE, Grinspan A, Wang S, Wu SC . Topiramate monotherapy in newly diagnosed epilepsy in children and adolescents. J Child Neurol 2007; 22: 693–699.
Ferraro D, Di Trapani G . Topiramate in the prevention of pediatric migraine: literature review. J Headache Pain 2008; 9: 147–150.
Lessig MC, Shapira NA, Murphy TK . Topiramate for reversing atypical antipsychotic weight gain. J Am Acad Child Adolesc Psychiatry 2001; 40: 1364.
Pavuluri MN, Janicak PG, Carbray J . Topiramate plus risperidone for controlling weight gain and symptoms in preschool mania. J Child Adolesc Psychopharmacol 2002; 12: 271–273.
Canitano R . Clinical experience with Topiramate to counteract neuroleptic induced weight gain in 10 individuals with autistic spectrum disorders. Brain Dev 2005; 27: 228–232.
Carter GT, Yudkowsky MP, Han JJ, McCrory MA . Topiramate for weight reduction in Duchenne muscular dystrophy. Muscle Nerve 2005; 31: 788–789.
Nathan PJ, O'Neill BV, Napolitano A, Bullmore ET . Neuropsychiatric adverse effects of centrally acting antiobesity drugs. CNS Neurosci Ther 2011; 17: 490–505.
Fountain NB . A pregnant pause to consider teratogenicity of topiramate. Epilepsy Curr 2009; 9: 36–38.
Roberts MD US Food and Drug Administration Endocrinologic and Metabolic Drugs Advisory Committee Clinical Briefing Document February 22, 2012. VIVUS, Inc. New Drug Application 22580: VI-0521 QNEXA (phentermine/topiramate). http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM292315.pdf Aaccessed: 30 March 2012.
Potes CS, Lutz TA . Brainstem mechanisms of amylin-induced anorexia. Physiol Behav 2010; 100: 511–518.
Hay DL, Christopoulos G, Christopoulos A, Sexton PM . Amylin receptors: molecular composition and pharmacology. Biochem Soc Trans 2004; 32 (Partt 5): 865–867.
Singh-Franco D, Perez A, Harrington C . The effect of pramlintide acetate on glycemic control and weight in patients with type 2 diabetes mellitus and in obese patients without diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 2011; 13: 169–180.
Maggs D, Shen L, Strobel S, Brown D, Kolterman O, Weyer C . Effect of pramlintide on A1C and body weight in insulin-treated African Americans and Hispanics with type 2 diabetes: a pooled post hoc analysis. Metabolism 2003; 52: 1638–1642.
Aronne L, Fujioka K, Aroda V, Chen K, Halseth A, Kesty NC et al. Progressive reduction in body weight after treatment with the amylin analog pramlintide in obese subjects: a phase 2, randomized, placebo-controlled, dose-escalation study. J Clin Endocrinol Metab 2007; 92: 2977–2983.
Smith SR, Aronne LJ, Burns CM, Kesty NC, Halseth AE, Weyer C . Sustained weight loss following 12-month pramlintide treatment as an adjunct to lifestyle intervention in obesity. Diabetes Care 2008; 31: 1816–1823.
Chase HP, Lutz K, Pencek R, Zhang B, Porter L . Pramlintide lowered glucose excursions and was well-tolerated in adolescents with type 1 diabetes: results from a randomized, single-blind, placebo-controlled, crossover study. J Pediatr 2009; 155: 369–373.
Kishiyama CM, Burdick PL, Cobry EC, Gage VL, Messer LH, McFann K et al. A pilot trial of pramlintide home usage in adolescents with type 1 diabetes. Pediatrics 2009; 124: 1344–1347.
Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 2001; 86: 5992.
Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS et al. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med 2002; 8: 643–644.
De Waele K, Ishkanian SL, Bogarin R, Miranda CA, Ghatei MA, Bloom SR et al. Long-acting octreotide treatment causes a sustained decrease in ghrelin concentrations but does not affect weight, behaviour and appetite in subjects with Prader-Willi syndrome. Eur J Endocrinol 2008; 159: 381–388.
Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 2009; 374: 1606–1616.
Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 2009; 32: 1224–1230.
Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia 2009; 52: 2046–2055.
Nauck MA, Ratner RE, Kapitza C, Berria R, Boldrin M, Balena R . Treatment with the human once-weekly glucagon-like peptide-1 analog taspoglutide in combination with metformin improves glycemic control and lowers body weight in patients with type 2 diabetes inadequately controlled with metformin alone: a double-blind placebo-controlled study. Diabetes Care 2009; 32: 1237–1243.
Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009; 32: 84–90.
Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373: 473–481.
Taylor K, Gurney K, Han J, Pencek R, Walsh B, Trautmann M . Exenatide once weekly treatment maintained improvements in glycemic control and weight loss over 2 years. BMC Endocr Disord 2011; 11: 9.
Rosenstock J, Klaff LJ, Schwartz S, Northrup J, Holcombe JH, Wilhelm K et al. Effects of exenatide and lifestyle modification on body weight and glucose tolerance in obese subjects with and without pre-diabetes. Diabetes Care 2010; 33: 1173–1175.
Kelly AS, Metzig AM, Rudser KD, Fitch AK, Fox CK, Nathan BM et al. Exenatide as a weight-loss therapy in extreme pediatric obesity: a randomized, controlled pilot study. Obesity (Silver Spring) 2012; 20: 364–370.
Mathis LL US Food and Drug Administration. Pediatric Advisory Committee Meeting March 22, 2010. Orlistat Update. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM205380.pdf Accessed: 30 March 2012.
McDuffie JR, Calis KA, Uwaifo GI, Sebring NG, Fallon EM, Hubbard VS et al. Three-month tolerability of orlistat in adolescents with obesity-related comorbid conditions. Obes Res 2002; 10: 642–650.
Zhi J, Moore R, Kanitra L . The effect of short-term (21-day) orlistat treatment on the physiologic balance of six selected macrominerals and microminerals in obese adolescents. J Am Coll Nutr 2003; 22: 357–362.
Norgren S, Danielsson P, Jurold R, Lotborn M, Marcus C . Orlistat treatment in obese prepubertal children: a pilot study. Acta Paediatr 2003; 92: 666–670.
Ozkan B, Bereket A, Turan S, Keskin S . Addition of orlistat to conventional treatment in adolescents with severe obesity. Eur J Pediatr 2004; 163: 738–741.
McDuffie JR, Calis KA, Uwaifo GI, Sebring NG, Fallon EM, Frazer TE et al. Efficacy of orlistat as an adjunct to behavioral treatment in overweight African American and Caucasian adolescents with obesity-related co-morbid conditions. J Pediatr Endocrinol Metab 2004; 17: 307–319.
Chanoine JP, Hampl S, Jensen C, Boldrin M, Hauptman J . Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. Jama 2005; 293: 2873–2883.
Maahs D, de Serna DG, Kolotkin RL, Ralston S, Sandate J, Qualls C et al. Randomized, double-blind, placebo-controlled trial of orlistat for weight loss in adolescents. Endocr Pract 2006; 12: 18–28.
Chanoine JP, Richard M . Early weight loss and outcome at one year in obese adolescents treated with orlistat or placebo. Int J Pediatr Obes 2011; 6: 95–101.
Yanovski JA, McDuffie JR, Salaita CS, Tanofsky-Kraff M, Sebring NG, Young-Hyman D et al. A randomized, placebo-controlled trial of the effects of orlistat on body weight and body composition in African American and Caucasian adolescents with obesity-related comorbid conditions. Obesity 2008; 16 (Suppl. 1): S63.
Umemura T, Ichijo T, Matsumoto A, Kiyosawa K . Severe hepatic injury caused by orlistat. Am J Med 2006; 119: e7.
Forrester MB . Pattern of orlistat exposures in children aged 5 years or less. J Emerg Med 2009; 37: 396–399.
O'Connor MB . An orlistat ‘overdose’ in a child. Ir J Med Sci 2010; 179: 315.
Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L . XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004; 27: 155–161.
McDuffie JR, Calis KA, Booth SL, Uwaifo GI, Yanovski JA . Effects of orlistat on fat-soluble vitamins in obese adolescents. Pharmacotherapy 2002; 22: 814–822.
FDA. Approves Orlistat for Over-the-Counter Use. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm108839.htm Accessed: 9 July 2012.
Kopelman P, Bryson A, Hickling R, Rissanen A, Rossner S, Toubro S et al. Cetilistat (ATL-962), a novel lipase inhibitor: a 12-week randomized, placebo-controlled study of weight reduction in obese patients. Int J Obes (Lond) 2007; 31: 494–499.
Kopelman P, Groot Gde H, Groot G, Rissanen A, Rossner S, Toubro S et al. Weight loss, HbA 1c reduction, and tolerability of cetilistat in a randomized, placebo-controlled phase 2 trial in obese diabetics: comparison with orlistat (xenical). Obesity 2010; 18: 108–115.
Kopelman P, Groot Gde H, Rissanen A, Rossner S, Toubro S, Palmer R et al. Weight loss, HbA1c reduction, and tolerability of cetilistat in a randomized, placebo-controlled phase 2 trial in obese diabetics: comparison with orlistat (Xenical). Obesity (Silver Spring) 2010; 18: 108–115.
Salvatore T, Giugliano D . Pharmacokinetic-pharmacodynamic relationships of Acarbose. Clin Pharmacokinet 1996; 30: 94–106.
Wang JS, Lin SD, Lee WJ, Su SL, Lee IT, Tu ST et al. Effects of acarbose versus glibenclamide on glycemic excursion and oxidative stress in type 2 diabetic patients inadequately controlled by metformin: a 24-week, randomized, open-label, parallel-group comparison. Clin Ther 2011; 33: 1932–1942.
Wolever TM, Chiasson JL, Josse RG, Hunt JA, Palmason C, Rodger NW et al. Small weight loss on long-term acarbose therapy with no change in dietary pattern or nutrient intake of individuals with non-insulin-dependent diabetes. Int J Obes Relat Metab Disord 1997; 21: 756–763.
Tugrul S, Kutlu T, Pekin O, Baglam E, Kiyak H, Oral O. . Clinical, endocrine, and metabolic effects of acarbose, a alpha-glucosidase inhibitor, in overweight and nonoverweight patients with polycystic ovarian syndrome. Fertil Steril 2008; 90: 1144–1148.
Hussey EK, Clark RV, Amin DM, Kipnes MS, O'Connor-Semmes RL, O'Driscoll EC et al. Single-dose pharmacokinetics and pharmacodynamics of sergliflozin etabonate, a novel inhibitor of glucose reabsorption, in healthy volunteers and patients with type 2 diabetes mellitus. J Clin Pharmacol 2010; 50: 623–635.
Hussey EK, Dobbins RL, Stoltz RR, Stockman NL, O'Connor-Semmes RL, Kapur A et al. Multiple-dose pharmacokinetics and pharmacodynamics of sergliflozin etabonate, a novel inhibitor of glucose reabsorption, in healthy overweight and obese subjects: a randomized double-blind study. J Clin Pharmacol 2010; 50: 636–646.
Komoroski B, Vachharajani N, Boulton D, Kornhauser D, Geraldes M, Li L et al. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther 2009; 85: 520–526.
Zhang L, Feng Y, List J, Kasichayanula S, Pfister M . Dapagliflozin treatment in patients with different stages of type 2 diabetes mellitus: effects on glycaemic control and body weight. Diabetes Obes Metab 2010; 12: 510–516.
Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S . Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab 2011; 13: 928–938.
Nauck MA, Del Prato S, Meier JJ, Duran-Garcia S, Rohwedder K, Elze M et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care 2011; 34: 2015–2022.
Bolinder J, Ljunggren O, Kullberg J, Johansson L, Wilding J, Langkilde AM et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012; 97: 1020–1031.
Grogan K FDA panel rejects B-MS/AZ's diabetes drug...but only just. Pharma Times Online. Published on-line 07/20/2011 http://www.pharmatimes.com/Article/11-07-20/FDA_panel_rejects_B-MS_AZ_s_diabetes_drug_but_only_just.aspx Accessed: 25 November 2011.
Mehnert H . Metformin, the rebirth of a biguanide: mechanism of action and place in the prevention and treatment of insulin resistance. Exp Clin Endocrinol Diabetes 2001; 109 (Suppl 2): S259–S264.
Hundal RS, Inzucchi SE . Metformin: new understandings, new uses. Drugs 2003; 63: 1879–1894.
Bestermann W, Houston MC, Basile J, Egan B, Ferrario CM, Lackland D et al. Addressing the global cardiovascular risk of hypertension, dyslipidemia, diabetes mellitus, and the metabolic syndrome in the southeastern United States, part II: treatment recommendations for management of the global cardiovascular risk of hypertension, dyslipidemia, diabetes mellitus, and the metabolic syndrome. Am J Med Sci 2005; 329: 292–305.
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403.
Rezvanian H, Hashemipour M, Kelishadi R, Tavakoli N, Poursafa P . A randomized, triple masked, placebo-controlled clinical trial for controlling childhood obesity. World J Pediatr 2010; 6: 317–322.
Freemark M, Bursey D . The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics 2001; 107: E55.
Wilson DM, Abrams SH, Aye T, Lee PD, Lenders C, Lustig RH et al. Metformin extended release treatment of adolescent obesity: a 48-week randomized, double-blind, placebo-controlled trial with 48-week follow-up. Arch Pediatr Adolesc Med 2010; 164: 116–123.
Yanovski JA, Krakoff J, Salaita CG, McDuffie JR, Kozlosky M, Sebring NG et al. Effects of metformin on body weight and body composition in obese insulin-resistant children: a randomized clinical trial. Diabetes 2011; 60: 477–485.
Fu JF, Liang L, Zou CC, Hong F, Wang CL, Wang XM et al. Prevalence of the metabolic syndrome in Zhejiang Chinese obese children and adolescents and the effect of metformin combined with lifestyle intervention. Int J Obes (Lond) 2007; 31: 15–22.
Atabek ME, Pirgon O . Use of metformin in obese adolescents with hyperinsulinemia: a 6-month, randomized, double-blind, placebo-controlled clinical trial. J Pediatr Endocrinol Metab 2008; 21: 339–348.
Clarson CL, Mahmud FH, Baker JE, Clark HE, McKay WM, Schauteet VD et al. Metformin in combination with structured lifestyle intervention improved body mass index in obese adolescents, but did not improve insulin resistance. Endocrine 2009; 36: 141–146.
Wiegand S, l'Allemand D, Hubel H, Krude H, Burmann M, Martus P et al. Metformin and placebo therapy both improve weight management and fasting insulin in obese insulin-resistant adolescents: a prospective, placebo-controlled, randomized study. Eur J Endocrinol 2010; 163: 585–592.
Legro RS . Impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women: do we need a new drug? J Clin Endocrinol Metab 2008; 93: 4218–4220.
Mastorakos G, Koliopoulos C, Deligeoroglou E, Diamanti-Kandarakis E, Creatsas G . Effects of two forms of combined oral contraceptives on carbohydrate metabolism in adolescents with polycystic ovary syndrome. Fertil Steril 2006; 85: 420–427.
Hoeger K, Davidson K, Kochman L, Cherry T, Kopin L, Guzick DS . The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J Clin Endocrinol Metab 2008; 93: 4299–4306.
Ibanez L, de Zegher F . Ethinylestradiol-drospirenone, flutamide-metformin, or both for adolescents and women with hyperinsulinemic hyperandrogenism: opposite effects on adipocytokines and body adiposity. J Clin Endocrinol Metab 2004; 89: 1592–1597.
Bridger T, MacDonald S, Baltzer F, Rodd C . Randomized placebo-controlled trial of metformin for adolescents with polycystic ovary syndrome. Arch Pediatr Adolesc Med 2006; 160: 241–246.
Allen HF, Mazzoni C, Heptulla RA, Murray MA, Miller N, Koenigs L et al. Randomized controlled trial evaluating response to metformin versus standard therapy in the treatment of adolescents with polycystic ovary syndrome. J Pediatr Endocrinol Metab 2005; 18: 761–768.
Arslanian SA, Lewy V, Danadian K, Saad R . Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J Clin Endocrinol Metab 2002; 87: 1555–1559.
Klein DJ, Cottingham EM, Sorter M, Barton BA, Morrison JA . A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry 2006; 163: 2072–2079.
Bjorkhem-Bergman L, Asplund AB, Lindh JD . Metformin for weight reduction in non-diabetic patients on antipsychotic drugs: a systematic review and meta-analysis. J Psychopharmacol 2011; 25: 299–305.
Reinstatler L, Qi YP, Williamson RS, Garn JV, Oakley GP . Association of biochemical B12 deficiency with metformin therapy and vitamin B12 supplements: the national health and nutrition examination survey, 1999–2006. Diabetes Care 2012; 35: 327–333.
Salpeter SR, Greyber E, Pasternak GA, Salpeter EE . Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev 2010; 4, CD002967.
Gambineri A, Patton L, De Iasio R, Cantelli B, Cognini GE, Filicori M et al. Efficacy of octreotide-LAR in dieting women with abdominal obesity and polycystic ovary syndrome. J Clin Endocrinol Metab 2005; 90: 3854–3862.
Haqq AM, Stadler DD, Rosenfeld RG, Pratt KL, Weigle DS, Frayo RS et al. Circulating ghrelin levels are suppressed by meals and octreotide therapy in children with Prader-Willi syndrome. J Clin Endocrinol Metab 2003; 88: 3573–3576.
Dietz J, Schwartz J . Growth hormone alters lipolysis and hormone-sensitive lipase activity in 3T3-F442A adipocytes. Metabolism 1991; 40: 800–806.
Snel YE, Doerga ME, Brummer RJ, Zelissen PM, Zonderland ML, Koppeschaar HP . Resting metabolic rate, body composition and related hormonal parameters in growth hormone-deficient adults before and after growth hormone replacement therapy. Eur J Endocrinol 1995; 133: 445–450.
Gregory JW, Greene SA, Jung RT, Scrimgeour CM, Rennie MJ . Changes in body composition and energy expenditure after six weeks' growth hormone treatment. Arch Dis Child 1991; 66: 598–602.
Hoos MB, Westerterp KR, Gerver WJ . Short-term effects of growth hormone on body composition as a predictor of growth. J Clin Endocrinol Metab 2003; 88: 2569–2572.
Eden Engstrom B, Burman P, Holdstock C, Karlsson FA . Effects of growth hormone (GH) on ghrelin, leptin, and adiponectin in GH-deficient patients. J Clin Endocrinol Metab 2003; 88: 5193–5198.
Hoybye C, Hilding A, Jacobsson H, Thoren M . Growth hormone treatment improves body composition in adults with Prader-Willi syndrome. Clin Endocrinol (Oxf) 2003; 58: 653–661.
Carrel AL, Myers SE, Whitman BY, Allen DB . Benefits of long-term GH therapy in Prader-Willi syndrome: a 4-year study. J Clin Endocrinol Metab 2002; 87: 1581–1585.
Myers SE, Davis A, Whitman BY, Santiago JV, Landt M . Leptin concentrations in Prader-Willi syndrome before and after growth hormone replacement. Clin Endocrinol (Oxf) 2000; 52: 101–105.
Shadid S, Jensen MD . Effects of growth hormone administration in human obesity. Obes Res 2003; 11: 170–175.
Bell J, Parker KL, Swinford RD, Hoffman AR, Maneatis T, Lippe B . Long-term safety of recombinant human growth hormone in children. J Clin Endocrinol Metab 2010; 95: 167–177.
Lammer C, Weimann E . [Changes in carbohydrate metabolism and insulin resistance in patients with Prader-Willi Syndrome (PWS) under growth hormone therapy]. Wien Med Wochenschr 2007; 157: 82–88.
Hauffa BP, Knaup K, Lehmann N, Neudorf U, Nagel B . Effects of growth hormone therapy on cardiac dimensions in children and adolescents with Prader-Willi syndrome. Horm Res Paediatr 2011; 75: 56–62.
Miller J, Silverstein J, Shuster J, Driscoll DJ, Wagner M . Short-term effects of growth hormone on sleep abnormalities in Prader-Willi syndrome. J Clin Endocrinol Metab 2006; 91: 413–417.
Festen DA, de Weerd AW, van den Bossche RA, Joosten K, Hoeve H, Hokken-Koelega AC . Sleep-related breathing disorders in prepubertal children with Prader-Willi syndrome and effects of growth hormone treatment. J Clin Endocrinol Metab 2006; 91: 4911–4915.
Genotropin (somatropin [rDNA origin] for injection). http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm153317.htm Accessed: 10 Jyly 2012.
US Food and Drug Administration.. MedWatch The FDA Safety Information and Adverse Event Reporting Program. Recombinant Human Growth Hormone (somatropin): Ongoing Safety Review - Possible Increased Risk of Death. Posted 12/22/2010. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm237969.htm Accessed: 30 March 2012.
Bray GA, Greenway FL . Current and potential drugs for treatment of obesity. Endocr Rev 1999; 20: 805–875.
Krotkiewski M . Thyroid hormones and treatment of obesity. Int J Obes Relat Metab Disord 2000; 24 (Suppl 2): S116–S119.
Bhasin S, Wallace W, Lawrence JB, Lesch M . Sudden death associated with thyroid hormone abuse. Am J Med 1981; 71: 887–890.
Baxter JD, Webb P, Grover G, Scanlan TS . Selective activation of thyroid hormone signaling pathways by GC-1: a new approach to controlling cholesterol and body weight. Trends Endocrinol Metab 2004; 15: 154–157.
Berkenstam A, Kristensen J, Mellstrom K, Carlsson B, Malm J, Rehnmark S et al. The thyroid hormone mimetic compound KB2115 lowers plasma LDL cholesterol and stimulates bile acid synthesis without cardiac effects in humans. Proc Natl Acad Sci USA 2008; 105: 663–667.
Larsen TM, Toubro S, van Baak MA, Gottesdiener KM, Larson P, Saris WH et al. Effect of a 28-d treatment with L-796568, a novel beta(3)-adrenergic receptor agonist, on energy expenditure and body composition in obese men. Am J Clin Nutr 2002; 76: 780–788.
Redman LM, de Jonge L, Fang X, Gamlin B, Recker D, Greenway FL et al. Lack of an effect of a novel beta3-adrenoceptor agonist, TAK-677, on energy metabolism in obese individuals: a double-blind, placebo-controlled randomized study. J Clin Endocrinol Metab 2007; 92: 527–531.
Buemann B, Toubro S, Astrup A . Effects of the two beta3-agonists, ZD7114 and ZD2079 on 24 hour energy expenditure and respiratory quotient in obese subjects. Int J Obes Relat Metab Disord 2000; 24: 1553–1560.
Astrup A . Thermogenic drugs as a strategy for treatment of obesity. Endocrine 2000; 13: 207–212.
Boozer CN, Daly PA, Homel P, Solomon JL, Blanchard D, Nasser JA et al. Herbal ephedra/caffeine for weight loss: a 6-month randomized safety and efficacy trial. Int J Obes Relat Metab Disord 2002; 26: 593–604.
Greenway FL, Bray GA . Treatment of hypothalamic obesity with caffeine and ephedrine. Endocr Pract 2008; 14: 697–703.
Molnar D, Torok K, Erhardt E, Jeges S . Safety and efficacy of treatment with an ephedrine/caffeine mixture. The first double-blind placebo-controlled pilot study in adolescents. Int J Obes Relat Metab Disord 2000; 24: 1573–1578.
McBride BF, Karapanos AK, Krudysz A, Kluger J, Coleman CI, White CM . Electrocardiographic and hemodynamic effects of a multicomponent dietary supplement containing ephedra and caffeine: a randomized controlled trial. JAMA 2004; 291: 216–221.
Pasman WJ, Westerterp-Plantenga MS, Saris WH . The effectiveness of long-term supplementation of carbohydrate, chromium, fibre and caffeine on weight maintenance. Int J Obes Relat Metab Disord 1997; 21: 1143–1151.
Daly PA, Krieger DR, Dulloo AG, Young JB, Landsberg L . Ephedrine, caffeine and aspirin: safety and efficacy for treatment of human obesity. Int J Obes Relat Metab Disord 1993; 17 (Suppl 1): S73–S78.
Hackman RM, Havel PJ, Schwartz HJ, Rutledge JC, Watnik MR, Noceti EM et al. Multinutrient supplement containing ephedra and caffeine causes weight loss and improves metabolic risk factors in obese women: a randomized controlled trial. Int J Obes (Lond) 2006; 30: 1545–1556.
Toubro S, Astrup AV, Breum L, Quaade F . Safety and efficacy of long-term treatment with ephedrine, caffeine and an ephedrine/caffeine mixture. Int J Obes Relat Metab Disord 1993; 17 (Suppl 1): S69–S72.
Norregaard J, Jorgensen S, Mikkelsen KL, Tonnesen P, Iversen E, Sorensen T et al. The effect of ephedrine plus caffeine on smoking cessation and postcessation weight gain. Clin Pharmacol Ther 1996; 60: 679–686.
Belza A, Frandsen E, Kondrup J . Body fat loss achieved by stimulation of thermogenesis by a combination of bioactive food ingredients: a placebo-controlled, double-blind 8-week intervention in obese subjects. Int J Obes (Lond) 2007; 31: 121–130.
Greenway FL . The safety and efficacy of pharmaceutical and herbal caffeine and ephedrine use as a weight loss agent. Obes Rev 2001; 2: 199–211.
Weintraub M . Long-term weight control: the National Heart, Lung, and Blood Institute funded multimodal intervention study. Clin Pharmacol Ther 1992; 51: 581–585.
Weintraub M, Sundaresan PR, Schuster B, Averbuch M, Stein EC, Cox C et al. Long-term weight control study. IV (weeks 156 to 190). The second double-blind phase. Clin Pharmacol Ther 1992; 51: 608–614.
Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377: 1341–1352.
Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr 2012; 95: 297–308.
Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring) 2011; 2: 330–342.
Lazarus R, Baur L, Webb K, Blyth F . Adiposity and body mass indices in children: Benn's index and other weight for height indices as measures of relative adiposity. Int J Obes Relat Metab Disord 1996; 20: 406–412.
FDA. approves weight-management drug Qsymia. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm312468.htm Accessed: 18 July 2012.
Gadde KM, Yonish GM, Foust MS, Wagner HR . Combination therapy of zonisamide and bupropion for weight reduction in obese women: a preliminary, randomized, open-label study. J Clin Psychiatry 2007; 68: 1226–1229.
Greenway FL, Whitehouse MJ, Guttadauria M, Anderson JW, Atkinson RL, Fujioka K et al. Rational design of a combination medication for the treatment of obesity. Obesity (Silver Spring) 2009; 17: 30–39.
Hjalmarsen A, Aasebo U, Birkeland K, Sager G, Jorde R . Impaired glucose tolerance in patients with chronic hypoxic pulmonary disease. Diabetes Metab 1996; 22: 37–42.
Orexigen Therapeutics Inc. CONTRAVE (Naltrexone SR/Bupropion SR combination). Endocrinologic and Metabolic Drugs Advisory Committee briefing document. NDA 200063 http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM235672.pdf Accessed: 1 February 2012.
Wadden TA, Foreyt JP, Foster GD, Hill JO, Klein S, O'Neil PM et al. Weight loss with naltrexone SR/Bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring) 2011; 19: 110–120.
Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2010; 376: 595–605.
Wadden TA, Foreyt JP, Foster GD, Hill JO, Klein S, O'Neil PM et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring) 2011; 19: 110–120.
Padwal R . Contrave, a bupropion and naltrexone combination therapy for the potential treatment of obesity. Curr Opin Investig Drugs 2009; 10: 1117–1125.
Tran PT, Thomas A . Summary Minutes of the Endocrinologic and Metabolic Drugs Advisory Committee. U.S. Food and Drug Administration Center for Drug Evaluation and Research. U.S. Food and Drug Administration Silver Spring, Maryland, 2010. pp 1–8, http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/ucm191113.htm Accessed: 5 November 2011.
Ware C . FDA Won't Approve Weight Loss Drug Contrave-Agency Asks for More Studies to Check for Heart Attack Risk. WebMDhttp://www.webmd.com/diet/news/20110201/fda-wont-approve-weight-loss-drug-contrave Accessed: 1 February 2012.
Orexigen Therapeutics, Inc. Orexigen Announces Agreement From the FDA on a Special Protocol Assessment for the Contrave Outcomes Trial. http://ir.orexigen.com/phoenix.zhtml?c=207034&p=irol-newsArticle&ID=1656731&highlight= Accessed: 6 February 2012.
Ravussin E, Smith SR, Mitchell JA, Shringarpure R, Shan K, Maier H et al. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring) 2009; 17: 1736–1743.
Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci USA 2008; 105: 7257–7262.
Roth JD, Trevaskis JL, Wilson J, Lei C, Athanacio J, Mack C et al. Antiobesity effects of the beta-cell hormone amylin in combination with phentermine or sibutramine in diet-induced obese rats. Int J Obes (Lond) 2008; 32: 1201–1210.
Aronne LJ, Halseth AE, Burns CM, Miller S, Shen LZ . Enhanced weight loss following coadministration of pramlintide with sibutramine or phentermine in a multicenter trial. Obesity (Silver Spring) 2010; 18: 1739–1746.
Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 2010; 42: 937–948.
den Hoed M, Ekelund U, Brage S, Grontved A, Zhao JH, Sharp SJ et al. Genetic susceptibility to obesity and related traits in childhood and adolescence: influence of loci identified by genome-wide association studies. Diabetes 2010; 59: 2980–2988.
Zhao J, Bradfield JP, Zhang H, Sleiman PM, Kim CE, Glessner JT et al. Role of BMI-associated loci identified in GWAS meta-analyses in the context of common childhood obesity in European Americans. Obesity (Silver Spring) 2011; 19: 2436–2439.
Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, Caulfield MJ et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens 2009; 27: 2121–2158.
Skelton JA, Beech BM . Attrition in paediatric weight management: a review of the literature and new directions. Obes Rev 2011; 12: e273–e281.
Schulz KF, Altman DG, Moher D . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Bmj 2010; 340: c332.
Cole TJ, Faith MS, Pietrobelli A, Heo M . What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z-score or BMI centile? Eur J Clin Nutr 2005; 59: 419–425.
Berkey CS, Colditz GA . Adiposity in adolescents: change in actual BMI works better than change in BMI z score for longitudinal studies. Ann Epidemiol 2007; 17: 44–50.
Haddock CK, Poston WS, Dill PL, Foreyt JP, Ericsson M . Pharmacotherapy for obesity: a quantitative analysis of four decades of published randomized clinical trials. Int J Obes Relat Metab Disord 2002; 26: 262–273.
Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc 2007; 107: 1755–1767.
Marre M, Shaw J, Brandle M, Bebakar WM, Kamaruddin NA, Strand J et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU). Diabet Med 2009; 26: 268–278.
Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009; 374: 39–47.
Choy M, Lam S . Sitagliptin: a novel drug for the treatment of type 2 diabetes. Cardiol Rev 2007; 15: 264–271.
Perez-Monteverde A, Seck T, Xu L, Lee MA, Sisk CM, Williams-Herman DE et al. Efficacy and safety of sitagliptin and the fixed-dose combination of sitagliptin and metformin vs. pioglitazone in drug-naive patients with type 2 diabetes. Int J Clin Pract 2011; 65: 930–938.
Sloth B, Davidsen L, Holst JJ, Flint A, Astrup A . Effect of subcutaneous injections of PYY1-36 and PYY3-36 on appetite, ad libitum energy intake, and plasma free fatty acid concentration in obese males. Am J Physiol Endocrinol Metab 2007; 293: E604–E609.
Gantz I, Erondu N, Mallick M, Musser B, Krishna R, Tanaka WK et al. Efficacy and safety of intranasal peptide YY3-36 for weight reduction in obese adults. J Clin Endocrinol Metab 2007; 92: 1754–1757.
Bailey CJ, Gross JL, Pieters A, Bastien A, List JF . Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 375: 2223–2233.
Acknowledgements
The conduct of this research was supported by Intramural Research Program Grant 1ZIAHD000641 from the NICHD (to JA Yanovski).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
RS-K and SZY declare no conflict of interest. JAY is a Commissioned Officer in the United States Public Health Service, Department of Health and Human Services. JAY was the principal investigator for NICHD-sponsored clinical studies using metformin, orlistat and betahistine, and has received orlistat and matching placebo from Roche Pharmaceuticals and betahistine and matching placebo plus research support for clinical research studies from Obecure.
Additional information
Supplementary Information accompanies the paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Sherafat-Kazemzadeh, R., Yanovski, S. & Yanovski, J. Pharmacotherapy for childhood obesity: present and future prospects. Int J Obes 37, 1–15 (2013). https://doi.org/10.1038/ijo.2012.144
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2012.144
Keywords
This article is cited by
-
Signaling pathways in obesity: mechanisms and therapeutic interventions
Signal Transduction and Targeted Therapy (2022)
-
The promise of new anti-obesity therapies arising from knowledge of genetic obesity traits
Nature Reviews Endocrinology (2022)
-
Pediatric Obesity, Hypertension, Lipids
Current Treatment Options in Pediatrics (2020)
-
Severe Obesity in the Pediatric Population: Current Concepts in Clinical Care
Current Obesity Reports (2019)
-
Pediatric Obesity: Endocrinologic and Genetic Etiologies and Management
Current Cardiovascular Risk Reports (2019)