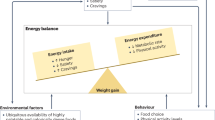
Abstract
Pediatric obesity is a highly prevalent chronic disease, which has traditionally been treated with lifestyle therapy alone. Yet for many youth, lifestyle intervention as a monotherapy is often insufficient for achieving clinically significant and durable BMI reduction. While metabolic/bariatric surgery achieves robust and long-lasting outcomes, it is neither widely accessible nor wanted by most pediatric patients and families. In the past 3 years, this treatment gap between lifestyle therapy and metabolic/bariatric surgery has been filled with a number of landmark clinical trials examining the safety and efficacy of anti-obesity medication (AOM) for use in children and adolescents. These trials include studies of liraglutide, phentermine/topiramate ER, semaglutide, and setmelanotide, all of which have led to FDA and/or EMA approval. Concurrent with this developing evidence base, in 2023, the American Academy of Pediatrics published their first Clinical Practice Guideline on the assessment and management of childhood obesity. The Guideline includes the recommendation that pediatric health care providers should offer AOM to youth ages ≥12 years with obesity. Recognizing that AOM use in the pediatric population will likely become the standard of care and to provide perspective on the recently generated data regarding new AOM, this narrative review summarizes the published randomized controlled trials (RCTs) from the past 10 years that examine AOM for the pediatric population. This report additionally includes RCTs examining AOM for special populations of pediatric obesity including monogenic obesity, Bardet Biedl syndrome, Prader Willi syndrome, and hypothalamic obesity. Finally, the clinical application of AOM for children and adolescents, as well as future directions and challenges are discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–42.
Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999-2016. Pediatrics. 2018;141:e20173459.
Woolford SJ, Sidell M, Li X, Else V, Young DR, Resnicow K, et al. Changes in body mass index among children and adolescents during the COVID-19 Pandemic. JAMA. 2021;326:1434–6.
Lartey ST, Jayawardene WP, Dickinson SL, Chen X, Gletsu-Miller N, Lohrmann DK. Evaluation of unintended consequences of COVID-19 pandemic restrictions and obesity prevalence among youths. JAMA Netw Open. 2023;6:e2323596.
Andes LJ, Cheng YJ, Rolka DB, Gregg EW, Imperatore G. Prevalence of prediabetes among adolescents and young adults in the United States, 2005-16. JAMA Pediatr. 2020;174:e194498.
Mann JP, Valenti L, Scorletti E, Byrne CD, Nobili V. Nonalcoholic fatty liver disease in children. Semin Liver Dis. 2018;38:1–13.
Mathew JL, Narang I. Sleeping too close together: obesity and obstructive sleep apnea in childhood and adolescence. Paediatr Respir Rev. 2014;15:211–8.
Griffiths LJ, Parsons TJ, Hill AJ. Self-esteem and quality of life in obese children and adolescents: a systematic review. Int J Pediatr Obes. 2010;5:282–304.
Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr. 2002;76:653–8.
Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. The relation of childhood BMI to adult adiposity: the Bogalusa Heart Study. Pediatrics. 2005;115:22–7.
Ryder JR, Jacobs DR Jr, Sinaiko AR, Kornblum AP, Steinberger J. Longitudinal changes in weight status from childhood and adolescence to adulthood. J Pediatr. 2019;214:187–92.e2.
Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120:S164–92.
McDonagh MS, Selph S, Ozpinar A, Foley C. Systematic review of the benefits and risks of metformin in treating obesity in children aged 18 years and younger. JAMA Pediatr. 2014;168:178–84.
Kirk S, Armstrong S, King E, Trapp C, Grow M, Tucker J, et al. Establishment of the Pediatric Obesity Weight Evaluation Registry: a national research collaborative for identifying the optimal assessment and treatment of pediatric obesity. Child Obes. 2016;13:9–17.
Ryder JR, Kaizer A, Rudser KD, Gross A, Kelly AS, Fox CK. Effect of phentermine on weight reduction in a pediatric weight management clinic. Int J Obes. 2017;41:90–3.
Fox CK, Marlatt KL, Rudser KD, Kelly AS. Topiramate for weight reduction in adolescents with severe obesity. Clin Pediatr. 2015;54:6.
Borzutzky C, King E, Fox CK, Stratbucker W, Tucker J, Yee JK, et al. Trends in prescribing anti-obesity pharmacotherapy for paediatric weight management: data from the POWER work group. Pediatr Obes. 2020;16:e12701.
Kumar S, King EC, Christison AL, Kelly AS, Ariza AJ, Borzutzky C, et al. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57–65.e4.
Johnston CA, Tyler C, Palcic JL, Stansberry SA, Gallagher MR, Foreyt JP. Smaller weight changes in standardized body mass index in response to treatment as weight classification increases. J Pediatr. 2011;158:624–7.
Danielsson P, Kowalski J, Ekblom O, Marcus C. Response of severely obese children and adolescents to behavioral treatment. Arch Pediatr Adolesc Med. 2012;166:1103–8.
Kalarchian MA, Levine MD, Arslanian SA, Ewing LJ, Houck PR, Cheng Y, et al. Family-based treatment of severe pediatric obesity: randomized, controlled trial. Pediatrics. 2009;124:1060–8.
Levine MD, Ringham RM, Kalarchian MA, Wisniewski L, Marcus MD. Is family-based behavioral weight control appropriate for severe pediatric obesity? Int J Eat Disord. 2001;30:318–28.
Grossman DC, Bibbins-Domingo K, Curry SJ, Barry MJ, Davidson KW, Doubeni CA, et al. Screening for obesity in children and adolescents: us preventive services task force recommendation statement. JAMA. 2017;317:2417–26.
O’Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the us preventive services task force. JAMA. 2017;317:2427–44.
Tsai WS, Inge TH, Burd RS. Bariatric surgery in adolescents: recent national trends in use and in-hospital outcome. Arch Pediatr Adolesc Med. 2007;161:217–21.
Campbell EG, Alasmar A, Lawrence R, Kurpius-Brock M, DeCamp M, Kovar A, et al. Barriers to metabolic bariatric surgery in adolescents: results of a qualitative study. Surg Obes Relat Dis. 2022;18:794–802.
Hampl SE, Hassink SG, Skinner AC, Armstrong SC, Barlow SE, Bolling CF, et al. Clinical practice guideline for the evaluation and treatment of children and adolescents with obesity. Pediatrics. 2023;151:e2022060640.
Drucker DJ. GLP-1 physiology informs the pharmacotherapy of obesity. Mol Metab. 2022;57:101351.
van Bloemendaal L, Ten Kulve JS, la Fleur SE, Ijzerman RG, Diamant M. Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol. 2014;221:T1–16.
Müller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt PR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab. 2019;30:72–130.
Kelly AS, Rudser KD, Nathan BM, Fox CK, Metzig AM, Coombes BJ, et al. The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial. JAMA Pediatr. 2013;167:355–60.
Weghuber D, Forslund A, Ahlström H, Alderborn A, Bergström K, Brunner S, et al. A 6-month randomized, double-blind, placebo-controlled trial of weekly exenatide in adolescents with obesity. Pediatr Obes. 2020;15:e12624.
Fox CK, Clark JM, Rudser KD, Ryder JR, Gross AC, Nathan BM, et al. Exenatide for weight-loss maintenance in adolescents with severe obesity: A randomized, placebo-controlled trial. Obesity. 2022;30:1105–15.
Chanoine JP, Hampl S, Jensen C, Boldrin M, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA. 2005;293:2873–83.
Kelly AS, Auerbach P, Barrientos-Perez M, Gies I, Hale PM, Marcus C, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med. 2020;382:2117–28.
Bensignor MO, Bramante CT, Bomberg EM, Fox CK, Hale PM, Kelly AS, et al. Evaluating potential predictors of weight loss response to liraglutide in adolescents with obesity: a post hoc analysis of the randomized, placebo-controlled SCALE teens trial. Pediatr Obes. 2023;18:e13061.
Weghuber D, Barrett T, Barrientos-Pérez M, Gies I, Hesse D, Jeppesen OK, et al. Once-weekly semaglutide in adolescents with obesity. N Engl J Med. 2022;387:2245–57.
Kelly AS, Arslanian S, Hesse D, Iversen AT, Körner A, Schmidt S, et al. Reducing BMI below the obesity threshold in adolescents treated with once-weekly subcutaneous semaglutide 2.4 mg. Obesity. 2023;31:2139–49.
Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Brandt ML, Xanthakos SA, et al. Five-year outcomes of gastric bypass in adolescents as compared with adults. N Engl J Med. 2019;380:2136–45.
Borzutzky C, King E, Fox CK, Stratbucker W, Tucker J, Yee JK, et al. Trends in prescribing anti-obesity pharmacotherapy for paediatric weight management: data from the POWER work group. Pediatr Obes. 2021;16:e12701.
Berman C, Naguib M, Hegedus E, Vidmar AP. Topiramate for weight management in children with severe obesity. Child Obes. 2023;19:219–25.
Delgado TC. Glutamate and GABA in appetite regulation. Front Endocrino. 2013;4:103.
Stanley BG, Urstadt KR, Charles JR, Kee T. Glutamate and GABA in lateral hypothalamic mechanisms controlling food intake. Physiol Behav. 2011;104:40–6.
Fox CK, Kaizer AM, Rudser KD, Nathan BM, Gross AC, Sunni M, et al. Meal replacements followed by topiramate for the treatment of adolescent severe obesity: A pilot randomized controlled trial. Obesity. 2016;24:2553–61.
Kelly AS, Bensignor MO, Hsia DS, Shoemaker AH, Shih W, Peterson C, et al. Phentermine/Topiramate for the treatment of adolescent obesity. NEJM Evid. 2022;1:EVIDoa2200014.
Wabitsch M, Farooqi S, Flück CE, Bratina N, Mallya UG, Stewart M, et al. Natural history of obesity due to POMC, PCSK1, and LEPR deficiency and the Impact of Setmelanotide. J Endocr Soc. 2022;6:bvac057.
Clement K, van den Akker E, Argente J, Bahm A, Chung WK, Connors H, et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020;8:960–70.
Aliferis K, Hellé S, Gyapay G, Duchatelet S, Stoetzel C, Mandel JL, et al. Differentiating Alström from Bardet-Biedl syndrome (BBS) using systematic ciliopathy genes sequencing. Ophthalmic Genet. 2012;33:18–22.
Haqq AM, Chung WK, Dollfus H, Haws RM, Martos-Moreno GA, Poitou C, et al. Efficacy and safety of setmelanotide, a melanocortin-4 receptor agonist, in patients with Bardet-Biedl syndrome and Alstrom syndrome: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial with an open-label period. Lancet Diabetes Endocrinol. 2022;10:859–68.
Khan MJ, Gerasimidis K, Edwards CA, Shaikh MG. Mechanisms of obesity in Prader-Willi syndrome. Pediatr Obes. 2018;13:3–13.
Diene G, Angulo M, Hale PM, Jepsen CH, Hofman PL, Hokken-Koelega A, et al. Liraglutide for weight management in children and adolescents with Prader-Willi syndrome and obesity. J Clin Endocrinol Metab. 2022;108:4–12.
McCandless SE, Yanovski JA, Miller J, Fu C, Bird LM, Salehi P, et al. Effects of MetAP2 inhibition on hyperphagia and body weight in Prader-Willi syndrome: A randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2017;19:1751–61.
Abuzzahab MJ, Roth CL, Shoemaker AH. Hypothalamic obesity: prologue and promise. Horm Res Paediatr. 2019;91:128–36.
Roth CL, Perez FA, Whitlock KB, Elfers C, Yanovski JA, Shoemaker AH, et al. A phase 3 randomized clinical trial using a once-weekly glucagon-like peptide-1 receptor agonist in adolescents and young adults with hypothalamic obesity. Diabetes Obes Metab. 2021;23:363–73.
Brauner R, Serreau R, Souberbielle JC, Pouillot M, Grouazel S, Recasens C, et al. Diazoxide in children with obesity after hypothalamic-pituitary lesions: a randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2016;101:4825–33.
Gross A, Kaizer A, Kelly AS, Rudser KD, Ryder JR, Borzutzky CR, et al. Long and short of it: early response predicts longer-term outcomes in pediatric weight management. Obesity. 2019;27:272–9.
Bomberg EM, Ryder JR, Brundage RC, Straka RJ, Fox CK, Gross AC, et al. Precision medicine in adult and pediatric obesity: a clinical perspective. Ther Adv Endocrinol Metab. 2019;10:2042018819863022.
Ryder JR, Kaizer AM, Jenkins TM, Kelly AS, Inge TH, Shaibi GQ. Heterogeneity in Response to treatment of adolescents with severe obesity: the need for precision obesity medicine. Obesity. 2019;27:288–94.
U.S. Department of Health and Human Services FaDA, Center for Drug Evaluation and Research (CDER) Guidance for Industry Developing Products for Weight Management Rockville, MD 2007 [Available from: https://www.fda.gov/downloads/Drugs/Guidances/ucm071612.pdf.
Reinehr T, Andler W. Changes in the atherogenic risk factor profile according to degree of weight loss. Arch Dis Child. 2004;89:419–22.
Frattarelli DA, Galinkin JL, Green TP, Johnson TD, Neville KA, Paul IM, et al. Off-label use of drugs in children. Pediatrics. 2014;133:563–7.
Smith J, Hegedus E, Naguib M, Goldman V, Moss L, Vidmar AP. Parental perceptions of medication use for the treatment of obesity in youth. Child Obes. 2022;19;428–33.
Del Prato S, Gallwitz B, Holst JJ, Meier JJ. The incretin/glucagon system as a target for pharmacotherapy of obesity. Obes Rev. 2022;23:e13372.
Hammoud R, Drucker DJ. Beyond the pancreas: contrasting cardiometabolic actions of GIP and GLP1. Nat Rev Endocrinol. 2023;19:201–16.
Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205–16.
Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US Youth and adults by sex and age, 2007-2008 to 2015-2016. Jama. 2018;319:1723–5.
Author information
Authors and Affiliations
Contributions
CKF was responsible for designing the review protocol, screening potentially eligible studies, assimilating results, and writing the manuscript. SJR, JLR, and ASK were responsible for assimilating results, making tables, and writing the manuscript. NTM was responsible for conducting the literature search and writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
CKF receives research support for serving as a site principal investigator from Novo Nordisk and Eli Lilly. Compensation is paid directly to her institution. ASK engages in unpaid consulting and educational activities for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Vivus; receives donated drug/placebo from Novo Nordisk and Vivus for National Institute of Diabetes and Digestive and Kidney Diseases-funded clinical trials. SJR receives research support for serving as a site co-investigator from Novo Nordisk and Eli Lilly. Compensations is paid directly to her institution. The other authors have no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fox, C.K., Kelly, A.S., Reilly, J.L. et al. Current and future state of pharmacological management of pediatric obesity. Int J Obes (2024). https://doi.org/10.1038/s41366-024-01465-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41366-024-01465-y