Abstract
Chronic immunosuppressive therapy is often complicated by the development of both arterial hypertension and renal dysfunction. The principal aim of this study was to assess the effects of dual inhibition of renin–angiotensin system (RAS) and other antihypertensive treatment on blood pressure and renal function in normotensive and hypertensive Fawn-Hooded (FH) strains during chronic calcineurin inhibitor (CNI) administration. Combinations of perindopril (5 mg kg−1 per day) and losartan (50 mg kg−1 per day) or amlodipine (6 mg kg−1 per day) and metoprolol (80 mg kg−1 per day) were administered to normotensive (FHL) and hypertensive (FHH) rats, fed with diet containing tacrolimus (Tac; 12 mg kg−1 per day). Tac-induced arterial hypertension in both animal strains (FHL: 151±4; FHH: 198±6 mm Hg) was prevented by dual RAS inhibition (FHL: 132±3 mm Hg, P<0.05; FHH: 153±3 mm Hg, P<0.05) as well as by a combination of amlodipine and metoprolol (FHL: 136±3 mm Hg, P<0.05; FHH: 166±4 mm Hg, P<0.05). However, significant nephroprotection was observed only in animals on dual RAS inhibition where albuminuria was reduced in both FHL (51.1±3.9 vs. 68.3±4.5 μg per day; P<0.05) and FHH rats (13.1±0.3 vs. 18.8±0.7 mg per day; P<0.05). We also found Tac-induced enhancement in renal angiotensin II activity that was significantly reduced by dual RAS inhibition in both FHL (63.5±3.2 vs. 23.1±3.0 fmol g−1) and FHH (79.8±8.5 vs. 32.2±5.8 fmol g−1). In addition, histological analysis revealed that RAS inhibition noticeably diminished glomerulosclerosis and tubulo-interstitial injury. This study indicates that dual blockade of RAS significantly attenuates Tac-induced arterial hypertension and nephrotoxicity in FH rats and further supports the notion that RAS inhibitors display efficient renoprotective properties during CNI treatment.
Similar content being viewed by others
Introduction
The use of calcineurin inhibitors (CNIs) in transplantation of solid organs is commonly associated with development of arterial hypertension and nephrotoxicity.1, 2, 3, 4 The incidence of hypertension after transplantation remains high, despite considerable progress in immunosuppressive therapy. Several pathophysiological mechanisms, including direct vasoconstriction, impaired vasodilatation and sodium-retaining effects, have been proposed to underlie CNI-associated hypertension.3, 4 CNI-induced direct renal vasoconstrictive effects reduce glomerular filtration and renal blood flow, and the resulting hypoperfusion may subsequently activate the renin–angiotensin system (RAS), particularly in the kidney.2, 5, 6, 7 Moreover, recent studies have shown that CNIs increase the activity of sodium transport along the nephrons.4, 8, 9 As a result, chronic administration of CNIs could compromise normal renal function that contributes to increased incidence of arterial hypertension10 and activation of several systems involved in the progression of renal damage.2, 3, 4 However, the exact mechanisms underlying the development of CNI-induced hypertension and nephrotoxicity are not fully understood.
It is generally accepted that combination of antihypertensive drugs results in better blood pressure control and organoprotection, both in patients with various cardiovascular and renal diseases and in transplant recipients.11, 12, 13, 14 In addition, several studies have demonstrated that dual RAS combination therapy—angiotensin-converting enzyme inhibitors (ACEI) and angiotensin II (ANG II) receptor blocker (ARB)—shows further benefits both in hypertensive patients with chronic renal disease15 or with congestive heart failure.16 However, there is no evidence for the efficacy of dual RAS inhibition on Tac-induced hypertension and nephrotoxicity. Furthermore, there is also lacking comparison of renoprotective activity against CNIs with other combined antihypertensive regimen such as calcium channel blocker (CCB) together with beta blocker (BB).
The Fawn-Hooded (FH) rat strains were inbred and selected for high blood pressure and denoted accordingly as FH hypertensive (FHH) or FH low blood pressure (FHL) rats. In contrast to FHL, FHH rats develop early hypertension and exhibit the increased susceptibility to renal disease due to impaired autoregulatory efficiency of the kidney17, 18, 19 that leads to glomerular hypertension, hyperfiltration and proteinuria.18, 20, 21 Hypertension and proteinuria increase further with age,21, 22 and the progression of renal damage results in premature death from end-stage renal failure in these animals.20 This rat strain therefore represents a unique model of intrinsic human chronic kidney disease (CKD) more closely.23 Therefore, it is of clinical importance to evaluate the effect of long-term CNI treatment in CKD condition and assess the optimum antihypertensive therapy that exhibits sufficient renoprotection against CNI nephrotoxicity.
Thus, in the present study, we hypothesized that efficient antihypertensive treatment may prevent blood pressure increase and renal injury induced by chronic CNI treatment in a model of CKD and genetic hypertension. The specific aim of this study was to determine whether dual RAS blockade (ACEI and ARB combination) attenuates CNI-induced hypertension and nephrotoxicity in FH strains that were under chronic Tac medication. In addition, other antihypertensive treatment, a combination of CCB with BB, was also used to determine the possible attenuation of CNI effects in FH strains without affecting RAS.
Methods
The study was performed in male rats of two FH strains (normotensive FHL and hypertensive FHH animals) in accordance with the guidelines and practices established by the Institute for Clinical and Experimental Medicine Animal Care and Use Committee and in accordance with the law in the Czech Republic. Both FH strains were originally provided by RPE van Dokkum (The University of Groningen) to establish our colony. The animals used in the present study were housed in the institutional facility accredited by the Czech Association for Accreditation of Laboratory Animal Care.
The 5-month-old FHL and FHH males (320–350 g of body weight, n=78) were randomly divided into the experimental groups based on diet (standard 22% protein, 0.4% NaCl, with or without Tac 12 mg kg−1 per day; Astellas Pharma, Killorglin, Kerry, Ireland) and antihypertensive treatment: the dual long-term RAS blockade by ACEI (perindopril 5 mg kg−1 per day; Servier Laboratories, Suresnes Cedex, France) and ARB (losartan 50 mg kg−1 per day; Zentiva, Hlohovec, Slovakia) or a combination of CCB (amlodipine 6 mg kg−1 per day; Krka, d.d., Novo Mesto, Slovenia) with BB (metoprolol 80 mg kg−1 per day; Zentiva) were used for 4-month period in drinking water. Doses of antihypertensives were based on our previous studies24, 25, 26 and pilot studies where a maximal blood pressure reduction was achieved in hypertensive animals. Owing to a slight polydipsia in FHH rats, the concentration of the antihypertensives in the drinking water was adjusted to ensure equivalent doses in both FHH and FHL rats. Daily intake of Tac diet was not significantly different to standard diet intake in control animals, and body weight in Tac-treated animals was not significantly altered compared with nontreated groups. In addition, Tac concentrations in whole-blood samples were measured by chemiluminescent microparticle immunoassay (Abbot Laboratories, Abbott Park, IL, USA) and its daily intake resulted in 4.6–7.2 ng ml−1 concentrations in whole-blood samples. Antihypertensive treatment did not alter Tac blood concentration. There was no mortality during 4-month period in any experimental groups.
The experiments were performed in following six groups of FHL rats on standard or Tac diet:
-
1)
FHL-untreated control (n=6),
-
2)
FHL ACEI/ARB (n=6),
-
3)
FHL CCB/BB (n=6),
-
4)
FHL Tac (n=8),
-
5)
FHL Tac+ACEI/ARB (n=6),
-
6)
FHL Tac+CCB/BB (n=6);
and six groups of FHH animals on standard or Tac diet:
-
1)
FHH-untreated control (n=6),
-
2)
FHH ACEI/ARB (n=6),
-
3)
FHH CCB/BB (n=6);
-
4)
FHH Tac (n=8),
-
5)
FHH Tac+ACEI/ARB (n=8),
-
6)
FHH Tac+CCB/BB (n=6).
Systolic blood pressure (SBP) was monitored by tail-cuff plethysmography (MC 4000; Hatteras Instruments, Cary, NC, USA) regularly during the 4-month experimental period. In accordance with recommendations for blood pressure measurements in experimental animals,27 which is adequate for detecting intergroup differences in SBP over time, and therefore is optimal for long-term studies. This method is regularly used in our laboratory24, 25, 26 where a correlation between measurements by noninvasive plethysmography technique and direct blood pressure measurements was previously validated. Although the radiotelemetry system is preferred to obtain all blood pressure parameters in experimental animals, this approach is not suitable for such long-term studies.
Urine and blood collections were performed every 4 weeks of the treatment to determine albuminuria and plasma creatinine (PCr) concentration by commercially available kits (Assaypro LLC, St Charles, MO, USA; Cayman Chemical Company, Ann Arbor, MI, USA, respectively), as described previously.26, 28 In addition, changes in urinary angiotensinogen (AGT) and aldosterone were also assessed by specific sandwich enzyme-linked immunosorbent assay and radioimmunoassay kits, respectively, (IBL International GmbH, Hamburg, Germany; Immunotech, Marseille, France). At least twice before starting measurements, rats were accustomed to the procedure of tail-cuff SBP measurements and metabolic cages. At the end of experiments, animals were decapitated to collect whole blood and harvest the tissues for RAS peptide measurements and histological analysis. The full blood was collected into two separate pre-chilled test tubes with specific inhibitors. For ANG II assays, the blood samples were immediately mixed with the recommended reagents: 5 mM EDTA, 10 μM pepstatin A, 1.25 mM 1,10-phenanthroline, 20 μM enalapril malenate and cooled down in ice bath. Other blood samples were mixed only with cooled EDTA for plasma renin activity (PRA) assay. The kidneys were immediately removed and homogenized in cooled methanol. All samples had to be kept on ice and centrifuged (3000 g 10 min−1 4 °C−1). Kidney homogenates were purified by solid-phase extraction. All dried samples were stored at −20 °C until assay. Indirect PRA and ANG II levels were measured by radioimmunoassay kits (Cisbio Bioassays, Codolet, France; Euro-Diagnostica, Malmö, Sweden) as described previously.29
Histological evaluation was assessed by high-power field technique in the PAS-stained kidney slices. A total number of glomeruli in each kidney slice were examined on a semiquantitative scale, as described previously,24, 25, 26, 30 and the glomerulosclerosis index was calculated using the following formula:

where nx is the number of glomeruli in each grade of glomerulosclerosis (grade 0: non-sclerotic glomeruli; grade 1: sclerotic area of glomeruli up to 25% (minimal); grade 2: sclerotic area 25–50% (moderate); grade 3: sclerotic area 50–75% (severe); grade 4: sclerotic area 75–100% (total). Renal cortical tubulo-interstitial injury was evaluated as an inflammatory cell infiltration, tubular dilatation and/or atrophy, or interstitial fibrosis and was graded semiquantitatively using the following scale of lesions: grade 0, no abnormal findings; grade 1, mild (<25%); grade 2, moderate (25–50%); and grade 3, severe (>50%). The lesions were evaluated in at least 30 random and nonoverlapping fields in the renal cortex.25, 26
In another series of experiments (n=28), effects of Tac on AGT and renin gene expressions were evaluated. Total RNA was extracted from kidney and liver tissue using Trizol (Invitrogen, Prague, Czech Republic) according to the manufacturer’s directions. DNase I-treated total RNA (Fermentas, Thermoscientific, Waltham, MA, USA) was reverse transcribed and amplified using One Step SYBR PrimeScript RT-PCR Kit II (TAKARA BIO, Shiga, Japan) following the manufacturer’s recommendations with total volume 20 μl. All samples were analyzed in triplicates. The primers were designed by Primer3 software (NCBI, Bethesda, MD, USA). Primer sequences for AGT were: forward: 5′-TGTGACAGGGTGGAAGATGA-3′, reverse: 5′-ACTCCAGTGCTGGAAGTTGC-3′; for rat renin forward: 5′-GGCTGTTGATGGAGTCATCC-3′, reverse: 5′-AGCCGGCCTTGCTGAT-3′; for rat ACE forward: 5′-TCCTATTCCCGCTCATCTGC-3′, reverse: 5′-CCAGCCCTTCTGTACCATT-3′; for rat ACE 2 forward: 5′-GAATGCGACCATCAAGCGTC-3′, reverse: 5′-CAAGCCCAGAGCCTACGAT-3′; and for β-actin: forward: 5′-TGACTGACTACCTCATGAAGA-3′ and reverse: 5′-CACGTCACACTTCATGATG-3′; PCR amplifications were performed using the CFX96 Touch Real-Time PCR Detection System (Bio-Rad Laboratories, Prague, Czech Republic). The relative gene expression was calculated by the ΔΔCt method31 and results were expressed as the n-fold difference in gene expression relative to β-actin mRNA and control FHL group.
Statistical analysis
All values are expressed as means±s.e.m. With Graph-Pad Prism software (Graph Pad Software, San Diego, CA, USA), statistical analysis was performed using Student’s t-test or analysis of variance followed by Bonferroni’s multiple comparison test, when appropriate. Values exceeding the 95% probability limits (P<0.05) were considered statistically significant.
Results
Blood pressure responses to Tac diet and antihypertensive treatment in FH strains
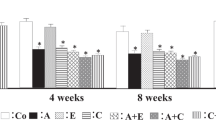
During 4-month period, SBP remained in FHL control rats within the normal range (133±3–137±4 mm Hg; Figure 1a), whereas FHH rats exhibited moderately increasing hypertension (175±3–184±5 mm Hg; Figure 1c). Dietary administration of Tac caused progressive increases in SBP in both FHL (133±2–151±4 mm Hg, P<0.05; Figure 1b) and FHH strain (174±3–198±6 mm Hg, P<0.05; Figure 1d) within 4-month period. In FHL, the dual RAS blockade prevented Tac-induced increases in SBP (131±2–132±3 mm Hg; Figure 1b). Moreover, it significantly lowered SBP in FHH rats (173±3–153±2 mm Hg; Figure 1d) to similar levels as in FHH group treated with ACEI and ARB without Tac (Figure 1c). The combination of CCB and BB also prevented Tac-induced increases in SBP in both FHL (132±3–136±3 mm Hg; Figure 1b) and FHH (174±3–166±4 mm Hg; Figure 1d), although the antihypertensive effect was less pronounced in FHH compared with RAS inhibition.
Time course of systolic blood pressure (SBP) in normotensive Fawn-Hooded (FHL; a and b) and hypertensive rats (FHH; c and d) and SBP response to combined antihypertensive treatment (angiotensin-converting enzyme inhibitors (ACEI) and angiotensin II receptor blocker (ARB), ACEI/ARB; or calcium channel blocker (CCB) and beta blocker (BB), CCB/BB) during standard diet or dietary administration of tacrolimus (Tac). Note the scale differences of y axis between a, b and c, d. *P<0.05 vs. basal, #P<0.05 vs. Tac group, &P<0.05 vs. control group.
Effects of Tac diet and antihypertensive treatment on albuminuria, PCr and ANG II concentrations in FH strains
Figure 2 shows that albuminuria was significantly different between FHL and FHH strains on standard diet. Dual RAS inhibition led to marked decrease of albuminuria in FHH rats (Figure 2c). In Tac-treated animals, combined RAS blockade prevented significant increase in albuminuria in FHL (Figure 2b) and resulted in significant reduction of albuminuria in FHH (Figure 2d). The combination of CCB and BB failed to reduce albuminuria not only in FHH on standard diet but also in both strains treated with Tac.
Time course of albuminuria in normotensive Fawn-Hooded (FHL; a and b) and hypertensive rats (FHH; c and d) and effects of combined antihypertensive treatment (angiotensin-converting enzyme inhibitor (ACEI)/angiotensin II receptor blocker (ARB) or calcium channel blocker (CCB)/beta blocker (BB)) during standard diet or dietary administration of tacrolimus (Tac) in these rats. Note the scale differences of y axis between a, b and c, d. *P<0.05 vs. basal, #P<0.05 vs. Tac group, &P<0.05 vs. control group.
In addition, PCr concentration was slightly but significantly increased by Tac (0.67±0.03–0.84±0.07 mg dl−1, P<0.05) in FHL. In contrast, Tac did not cause further significant PCr increase in FHH compared with control group (0.91±0.08 vs. 0.79±0.06 mg dl−1). RAS inhibition normalized PCr in FHL (0.64±0.03 mg dl−1) and significantly diminished PCr in FHH animals (0.72±0.04 mg dl−1) during Tac feeding. CCB and BB treatment also prevented noticeable PCr increases in FHL and FHH animals fed with Tac (0.68±0.04 and 0.74±0.05 mg dl−1, respectively).
As shown in Figure 3, there were no substantial differences in plasma and renal ANG II levels between 9-month-old normotensive FHL (30.7±3.2 fmol ml−1 and 47.6±2.8 fmol g−1, respectively) and FHH control rats (21.5±2.8 fmol ml−1 and 57.2±6.4 fmol g−1, respectively). Although circulating ANG II was not markedly elevated at the end of 4-month period of Tac administration (Figures 3a and c), renal ANG II was significantly enhanced in both FHL (63.5±3.2 fmol g−1; Figure 3b) and FHH (79.8±8.5 fmol g−1, Figure 3d) strains. These enhancements in renal ANG II level were significantly decreased by dual RAS inhibition in both FHL (23.1±3.0 fmol g−1) and FHH (32.2±5.8 fmol g−1) to a similar extent as observed in control groups treated with ACEI and ARB. On the other hand, CCB and BB treatment did not affect plasma and renal ANG II levels significantly either in control FHL or FHH animals, either on standard normal diet or on Tac (Figures 3a and c).
Angiotensin II (ANG II) levels in plasma and the kidney after 4-month period of tacrolimus (Tac) dietary administration and effects of combined antihypertensive treatment (angiotensin-converting enzyme inhibitor (ACEI)/angiotensin II receptor blocker (ARB) or calcium channel blocker (CCB)/beta blocker (BB)) in Fawn-Hooded control (FHL; a and b, respectively) and hypertensive (FHH; c and d, respectively) rats. ANG II levels were not significantly different in plasma, in contrast to increasing renal ANG II concentration owing to Tac treatment. ACEI/ARB combination markedly reduced ANG II in the kidney as it was expected. *P<0.05 vs. corresponding control groups, #P<0.05 vs. corresponding Tac group.
Effects of Tac diet and antihypertensive treatment on renal injury in FH strains
At the end of experimental protocol, histological analysis of glomerulosclerosis and tubulo-interstitial injury revealed marked differences in renal lesions between FHL and FHH strains (Figure 4), as expected in this model of CKD. Tac administration significantly worsened glomerular sclerotic lesions in both FH strains. However, only dual RAS blockade but not CCB and BB treatment exhibited substantial renoprotective effects against Tac-induced renal injury in FHL rats (Figures 4a and b). Similar findings were revealed in FHH strain, although the magnitude of changes was higher (Figures 4c and d). Again, noticeable tubulo-interstitial injury in Tac-treated rats, such as tubular dilatation, vacuolization or atrophy, interstitial infiltrate and fibrosis, was attenuated only by dual RAS blockade. Characteristic glomerular and tubulo-interstitial lesions are presented in representative histological slices of the kidney tissue from FHL and FHH groups (Supplementary Figures).
Glomerulosclerosis index (GSI) and tubulo-interstitial injury (TII) after 4-month period of tacrolimus (Tac) dietary administration and effects of combined antihypertensive treatment (angiotensin-converting enzyme inhibitor (ACEI)/angiotensin II receptor blocker (ARB) or calcium channel blocker (CCB)/beta blocker (BB)) in normotensive (FHL; a and b, respectively) and hypertensive (FHH; c and d, respectively) rats. Note the scale differences of y axis in figures. *P<0.05 vs. corresponding control groups, #P<0.05 vs. corresponding Tac groups, &P<0.05 vs. corresponding groups without Tac.
Effects of Tac diet on PRA, renin, AGT, ACE and ACE 2 mRNA expression and urinary excretion of AGT and aldosterone in FH strains
At the end of 4-month period, Tac administration did not affect significantly PRA in either animal strain (Figure 5a). In contrast, Tac induced upregulations of renin (Figure 5b) and AGT mRNA expression in the kidney (Figure 5d). AGT mRNA expression in the liver was unaltered in both strains (Figure 5c). In addition, there was slightly but significantly increased urinary AGT excretion in both Tac-treated FHL (68±3 vs. 43±2 ng per day; P<0.05) and FHH rats (1580±98 vs. 1204±42 ng per day; P<0.05) when compared with untreated control animals. On the other hand, urinary aldosterone levels were not markedly altered by Tac treatment either in FHL (83±11 vs. 67±8 ng per day; NS) or FHH rats (102±13 vs. 86±10 ng per day; no significance) in comparison with untreated control FH strains.
Plasma renin activity (PRA; a), expression of renin mRNA in the kidney (b), angiotensinogen (AGT) mRNA in the liver (c) and in the kidney (d) in untreated and tacrolimus-treated (Tac) normotensive (FHL) and hypertensive (FHH) rats after 4-month experimental period. Tac increased PRA and upregulated renin and AGT expression in the kidney in both strains. #P<0.05 vs. FHL groups, *P<0.05 vs. corresponding untreated control groups.
ACE mRNA expression in the kidney remained unaltered at the end of experiment; however, compared with control group, Tac-induced decreases of ACE 2 mRNA expression in the kidney (by 50%) were completely ameliorated by dual RAS inhibition.
Discussion
The present investigation focused on studies of pathophysiology and treatment of arterial hypertension and renal dysfunction during chronic CNI medication. Our major findings can be summarized as follows: (1) long-term treatment with Tac resulted in development of hypertension and renal injury in normotensive FHL rats, and worsened hypertension and progression of CKD in FHH strain, particularly because of enhanced renal RAS activity; (2) dual blockade of RAS by ACEI and ARB during 4-month period of Tac treatment displayed efficient antihypertensive and renoprotective properties not only in FHL rats but especially in FHH strain; and (3) potent antihypertensive combination of CCB and BB failed to prevent Tac-induced renal injury in both FH strains.
Our observations may indicate that renoprotection in this animal model cannot be simply attributed to blood pressure reduction by antihypertensive treatment as shown also in different animal models.32, 33 It can be argued that dual RAS blockade exhibited a more profound antihypertensive effect in comparison with CCB/BB combination; however, a substantial blood pressure decrease during treatment with CCB/BB was not accompanied by appreciable renoprotection in the present study. Therefore, besides barotrauma other factors also have a significant role in nephrotoxicity of CNIs.1, 2, 3, 4 One of the possible explanations could be Tac-induced enhancement in renal ANG II levels. This hypothesis is supported by documented activation of RAS by CNIs, administered either acutely or chronically.2 This observation has been described especially during cyclosporine treatment1, 4, 6 while less evidence is available for Tac administration.34 The recent study by Kidokoro et al.7 clearly showed the favorable diminishing effect of ARB on Tac-induced oxidative stress and subsequent inflammatory changes in the kidney. Although the authors suggested that Tac leads to overexpression of AT1 receptors in the glomerulus that might be responsible for CNI-nephrotoxic action, there is no compelling evidence on the RAS activity as a primary cause of chronic CNI nephrotoxicity. To elucidate the above hypotheses, our study focused on the activity of the RAS during Tac treatment. We also attempted to distinguish Tac effect on circulating and intrarenal RAS. In this respect, it is important to emphasize that we did not find any significant changes in PRA and plasma ANG II levels at the end of experimental period. On the other hand, observed increases in renal ANG II levels both in FHL and FHH rats treated with Tac diet strongly suggest the role of enhanced intrarenal RAS activity in CNI-induced nephrotoxicity. These results were further supported by higher urinary excretion of AGT and by upregulation of renin and AGT mRNA expression in the kidney. The most important and original observation of the present study is that both CNI-induced hypertension and nephrotoxicity could be prevented by dual RAS blockade. This is true not only for normotensive controls but also for hypertensive animals with progressive CKD. As alternative antihypertensive regimen without the effect on RAS failed to prevent Tac-induced nephrotoxicity and dual RAS blockade was shown (besides its antihypertensive effects) to reduce efficiently ANG II levels in the kidneys of FH rats, this appears to be the main underlying mechanism responsible for the renoprotective action of RAS inhibition. We are aware of the limitation of this present study to describe the exact mechanism by which Tac increases renal RAS activity. However, the previous studies2, 7, 9, 34 showed how Tac alters renal physiological function causing renin release, endothelial dysfunction and sodium retention, and what mechanisms such oxidative stress and inflammatory process are involved in renal injury. On the basis of above observation, it seems that enhanced activity of renal RAS has a crucial role in the pathophysiology of CNI-induced hypertension and nephrotoxicity at least in our model, although the role of aldosterone remains still unresolved because ANG II-induced stimulation of its production could be contemporaneously attenuated by Tac in our present study.35
The nephrotoxic effects of CNIs are likely complex and vary between the different nephron segments.2, 36 In our study, we also confirmed that chronic Tac administration led to the progression of glomerular and tubulo-interstitial morphological changes. The signaling mechanisms underlying these alterations still remain unclear. However, the activation of the RAS has been demonstrated as common and important integrated mechanism contributing to acute and chronic CNI nephrotoxicity described both in animal or human studies.2, 7, 36 The previous investigations strongly suggested a local renal activation of RAS by CNI-induced renal ischemia.5, 34 Such direct enhancement of renal RAS alters even more renal vascular resistance and tubular sodium transport, and interacts with other important vasoactive systems.2, 3, 4, 5 Our supportive findings also indicate that enhanced RAS activity contributes significantly to Tac-induced renal damage in FH strains, as the dual RAS blockade completely prevented any progression of renal morphological changes during Tac dietary administration. ANG II, a well-known renal growth factor that modulates cell growth and extracellular matrix synthesis and degradation, has been involved in the progression of glomerulosclerosis and interstitial fibrosis.1, 37 The kidney is a unique organ in terms of the tissue concentrations of ANG II, which are much greater than the concentrations in the circulation.38 The major fraction of ANG II is generated locally from AGT delivered to the kidney as well as from AGT produced by tubular cells.38 This local RAS generation is significantly augmented by CNI6 and therefore only RAS inhibition effectively blocks this phenomenon. The explanation for the intrarenal RAS enhancement by CNI including Tac could be also mediated by local accumulation of CNI in the kidney.39 Thus, the changes in circulating RAS during chronic CNI administration may not be crucial for the nephrotoxicity as the changes in renal RAS. Therefore, ANG II not only contributes to alteration of renal hemodynamics, but has pleiotropic and proinflammatory effects that are mainly mediated by AT1 receptors and induction of transforming growth factor-β.40, 41 For all above reasons, it appears that inappropriate activation of the RAS in the kidney is not only important for its hemodynamic contribution to acute CNI nephrotoxicity1, 2, 5 but also promotes directly chronic CNI nephrotoxicity.2
The rationale for combined therapy with ACEI and ARB is based on the different mechanism of action of these two drugs within the RAS. Although ACEI cannot completely inhibit formation of ANG II owing to alternative production via other non-ACE-dependent pathway, it displays several other beneficial effects and remains widely used antihypertensive agent.11, 12 In contrast, ARB completely abolishes the action of ANG II through blockade of the AT1 receptor but increases circulating ANG II level. Many other studies10, 11, 12, 13, 14, 15, 16, 24, 42 showed that the additive effect of combination of ACEI and ARBs on slowing the progression of many cardiovascular and renal diseases. Therefore, our aim was to achieve the maximal RAS inhibition with substantial antihypertensive effect to delineate RAS-dependent pathway in Tac-induced hypertension and nephrotoxicity in our experimental model. This study indicates that the major pathophysiological mechanisms of CNI would be an inappropriate activation of renal RAS. There is continuous discussion regarding efficiency of monotherapy inhibiting the RAS at different levels in comparison with complete RAS inhibition in multiorgan protection. It can be assumed that the efficiency of ACEI or ARBs alone would fully depend on inhibition of renal RAS to the same level as the dual RAS therapy. However, we are aware that during dual RAS blockade, the serious adverse effects such as acute renal failure, hyperkalemia and symptomatic hypotension can occur more frequently. In ONTARGET study,43 the combination RAS therapy reduced proteinuria more than monotherapy; however, declining renal function occurred especially in diabetic patients. This observation was further evaluated and confirmed by the recent ONTARGET revision and the trial VA NEPHRON-D.44, 45 Overall, the recommendation of the dual RAS blockade remains controversial for their uncertainty concerning adverse effects and outcomes that may limit applicability to clinical practice.46 This potential risk during combined RAS inhibition could also exist in patients chronically treated with CNI. However, our study suggests that the dual RAS blockade could still provide beneficial effects, in particular proteinuric renal disease including CNI-induced nephrotoxicity. During this therapeutic approach, the close examination of patients is required to achieve the optimization of treatment that should provide an adequate reduction of proteinuria and blood pressure without any decline in renal function. This should lead to the efficient renoprotection during chronic CNI treatment but further clinical evaluation is needed to address this specific issue more conclusively.
It is important to emphasize that dual ACEI and ARB therapy in doses used in our study exhibited maximum antihypertensive effect in FHH rats. This potentiated effect seemed to be very suitable approach in CKD model with maximal inhibition of intrarenal RAS activity. It has been shown18, 21 and we also confirmed in our preliminary study that RAS monotherapy is not efficiently renoprotective in older FHH rats with previously established kidney damage, despite lowering blood pressure. Moreover, the previous studies12, 13, 42 already demonstrated the effectiveness of RAS inhibition monotherapy compared with dual RAS blockade in renoprotection. Our data confirm high efficiency of dual RAS blockade in prevention of Tac-induced hypertension and renal damage in FH strains as compared with RAS-independent antihypertensive treatment. These findings are in good agreement with other studies showing the absence of renoprotective effects of amlodipine or other CCB despite their substantial antihypertensive effect and/or an improvement in renal function.47, 48, 49, 50 One explanation might be that amlodipine treatment fails to reduce glomerular pressure in hypertensive rats.48 Although the combination of CCB and BB is commonly recommended for hypertension control in clinical practice, especially in patients with coronary heart disease,11 the present study has shown that nephroprotection of amlodipine with metoprolol remain very inconclusive. Moreover, it is likely that renoprotection of the combined CCB and RAS inhibitor therapy would be attributed mostly to the RAS inhibition rather than CCB action that could even oppose the ANG II-decreasing effect in the kidney.49 In agreement, combination of CCB and BB reveal a lack of renoprotection particularly because of the inability to diminish renal RAS activity in the present study.
Immunosuppressive effect of CNI may be applicable in patients after transplantation to prevent the rejection. CNIs including Tac are also often used for their anti-inflammatory effects in the treatment of some autoimmune diseases.51 Beneficial effects of Tac in the treatment of nephrotic syndrome have resulted in more effective reduction of proteinuria than cyclosporine. Chronic immunosuppression has been also shown to attenuate hypertension and renal damage in different animal models. These effects are most likely mediated by the suppression of cytokines release and decreases of oxidative stress in the kidney.52, 53, 54 On the other hand, major concerns with CNIs are related to their propensity to induce arterial hypertension and nephrotoxicity over the long-term follow-up. This scenario typically occurs in post-transplant patients. Therefore, organo-protective approaches need to be evaluated to prevent CNI-induced renal injury not only in patients with intact kidney function but mainly in patients with incipient renal insufficiency. Taken together, our data suggest that RAS blockade is essential for the optimum treatment of CNI-induced hypertension and nephrotoxicity. Particularly, dual RAS inhibition seems to provide a maximal antihypertensive and renoprotective effects. Although the previous study55, 56 indicated that ANG II upregulates ACE through AT1 receptor and both ACEI and ARB can block the ACE enhancement, we did not observe any significant change in renal ACE mRNA expression at the end of experiment. On the other hand, Tac-induced significant decreases of ACE 2 mRNA expression in the kidney were completely ameliorated by dual RAS inhibition. In our preliminary study, we observed that used dual RAS blockade markedly increased ANG 1–7. Thus, we recognize that ANG 1–7 could be partially involved in the renoprotection of the RAS blockade in the present study. However, it needs further study to evaluate the exact role of ANG 1–7, ACE or ACE 2 activities and also differences in cortical and medullary RAS activity in CNI-induced hypertension and nephrotoxicity.
In conclusion, our findings provide pathophysiological background for new therapeutic strategies aiming at prevention of progression of CNI-induced hypertension and nephrotoxicity in order to lower the incidence of end-stage organ damage. The present study suggests that inappropriate intrarenal activation of RAS by CNI is a crucial pathophysiological mechanism underlying chronic CNI nephrotoxicity. It also indicates that dual blockade of the RAS efficiently attenuates Tac-induced arterial hypertension and nephrotoxicity. This effect can be observed not only in normotensive FHL but especially in FHH rats with spontaneous progression of hypertension and renal injury. Therefore, inhibition of RAS seems to be essential therapy for alleviation of nephrotoxic effects of chronic CNI medication. Treatment regimen should optimally involve RAS inhibition by both ACEI and ARB, as this combination potentiates renoprotective effect of these antihypertensive agents.
References
Burdmann EA, Andoh TF, Nast CC, Evan A, Connors BA, Coffman TM, Lindsley J, Bennett WM . Prevention of experimental cyclosporin-induced interstitial fibrosis by losartan and enalapril. Am J Physiol 1995; 269: F491–F499.
Naesens M, Kuypers DR, Sarwal M . Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 2009; 4: 481–508.
Chatzikyrkou C, Menne J, Gwinner W, Schmidt BM, Lehner F, Blume C, Schwarz A, Haller H, Schiffer M . Pathogenesis and management of hypertension after kidney transplantation. J Hypertens 2011; 29: 2283–2294.
Hoorn EJ, Walsh SB, McCormick JA, Zietse R, Unwin RJ, Ellison DH . Pathogenesis of calcineurin inhibitor-induced hypertension. J Nephrol 2012; 25: 269–275.
Lassila M . Interaction of cyclosporine A and the renin-angiotensin system; new perspectives. Curr Drug Metab 2002; 3: 61–71.
Nishiyama A, Kobori H, Fukui T, Zhang GX, Yao L, Rahman M, Hitomi H, Kiyomoto H, Shokoji T, Kimura S, Kohno M, Abe Y . Role of angiotensin II and reactive oxygen species in cyclosporine A-dependent hypertension. Hypertension 2003; 42: 754–760.
Kidokoro K, Satoh M, Nagasu H, Sakuta T, Kuwabara A, Yorimitsu D, Nishi Y, Tomita N, Sasaki T, Kashihara N . Tacrolimus induces glomerular injury via endothelial dysfunction caused by reactive oxygen species and inflammatory change. Kidney Blood Press Res 2012; 35: 549–557.
Damiano S, Scanni R, Ciarcia R, Florio S, Capasso G . Regulation of sodium transporters in the kidney during cyclosporine treatment. J Nephrol 2010; 23 (Suppl 16): S191–S198.
Hoorn EJ, Walsh SB, McCormick JA, Fürstenberg A, Yang CL, Roeschel T, Paliege A, Howie AJ, Conley J, Bachmann S, Unwin RJ, Ellison DH . The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med 2011; 17: 1304–1309.
Hosková L, Viklický O, Málek I, Podzimková M, Hegarová M, Pirk J, Vítko S, Kautzner J . Ischaemic heart disease is a risk factor for renal failure after heart transplantation. Int J Cardiol 2008; 123: 358–360.
Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, Caulfield MJ, Cifkova R, Clément D, Coca A, Dominiczak A, Erdine S, Fagard R, Farsang C, Grassi G, Haller H, Heagerty A, Kjeldsen SE, Kiowski W, Mallion JM, Manolis A, Narkiewicz K, Nilsson P, Olsen MH, Rahn KH, Redon J, Rodicio J, Ruilope L, Schmieder RE, Struijker-Boudier HA, van Zwieten PA, Viigimaa M, Zanchetti A . European Society of Hypertension. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens 2009; 27: 2121–2158.
Volpe M, Danser AH, Menard J, Waeber B, Mueller DN, Maggioni AP, Ruilope LM . Inhibition of the rennin-angiotension-aldosterone system: is there room for dual blockade in the cardiorenal continuum? J Hypertens 2012; 30: 647–654.
Wilkinson-Berka JL, Gibbs NJ, Cooper ME, Skinner SL, Kelly DJ . Renoprotective and anti-hypertensive effects of combined valsartan and perindopril in progressive diabetic nephropathy in the transgenic (mRen-2)27 rat. Nephrol Dial Transplant 2001; 16: 1343–1349.
Hernández D, Muriel A, Abraira V, Pérez G, Porrini E, Marrero D, Zamora J, González-Posada JM, Delgado P, Rufino M, Torres A . Renin-angiotensin system blockade and kidney transplantation: a longitudinal cohort study. Nephrol Dial Transplant 2012; 27: 417–422.
Ruilope LM, Aldigier JC, Ponticelli C, Oddou-Stock P, Botteri F, Mann JF . Safety of the combination of valsartan and benazepril in patients with chronic renal disease. European Group for the Investigation of Valsartan in Chronic Renal Disease. J Hypertens 2000; 18: 89–95.
McMurray JJ, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, Olofsson B, Yusuf S, Pfeffer MA, CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003; 362: 767–771.
Simons JL, Provoost AP, Anderson S, Troy JL, Rennke HG, Sandstrom DJ, Brenner BM . Pathogenesis of glomerular injury in the fawn-hooded rat: early glomerular capillary hypertension predicts glomerular sclerosis. J Am Soc Nephrol 1993; 3: 1775–1782.
Verseput GH, Provoost AP, Braam BB, Weening JJ, Koomans HA . Angiotensin-converting enzyme inhibition in the prevention and treatment of chronic renal damage in the hypertensive fawn-hooded rat. J Am Soc Nephrol 1997; 8: 249–259.
van Dokkum RP, Sun CW, Provoost AP, Jacob HJ, Roman RJ . Altered renal hemodynamics and impaired myogenic responses in the fawn-hooded rat. Am J Physiol 1999; 276: R855–R863.
de Keijzer MH, Provoost AP, Molenaar JC . Proteinuria is an early marker in the development of progressive renal failure in hypertensive fawn-hooded rats. J Hypertens 1989; 7: 525–528.
Verseput GH, Koomans HA, Braam B, Weening JJ, Provoost AP . ACE inhibition delays development of terminal renal failure in the presence of severe albuminuria. Am J Kidney Dis 2000; 35: 202–210.
Ochodnický P, Henning RH, Buikema HJ, de Zeeuw D, Provoost AP, van Dokkum RP . Renal vascular dysfunction precedes the development of renal damage in the hypertensive Fawn-Hooded rat. Am J Physiol Renal Physiol 2010; 298: F625–F633.
Schulz A, Kreutz R . Mapping genetic determinants of kidney damage in rat models. Hypertens Res 2012; 35: 675–694.
Vanourková Z, Kramer HJ, Husková Z, Vanecková I, Opocenský M, Chábová VC, Tesar V, Skaroupková P, Thumová M, Dohnalová M, Mullins JJ, Cervenka L . AT1 receptor blockade is superior to conventional triple therapy in protecting against end-organ damage in Cyp1a1-Ren-2 transgenic rats with inducible hypertension. J Hypertens 2006; 24: 2465–2472.
Kujal P, Chábová VČ, Vernerová Z, Walkowska A, Kompanowska-Jezierska E, Sadowski J, Vaňourková Z, Husková Z, Opočenský M, Skaroupková P, Schejbalová S, Kramer HJ, Rakušan D, Malý J, Netuka I, Vaněčková I, Kopkan L, Cervenka L . Similar renoprotection after renin-angiotensin-dependent and -independent antihypertensive therapy in 5/6-nephrectomized Ren-2 transgenic rats: are there blood pressure-independent effects? Clin Exp Pharmacol Physiol 2010; 37: 1159–1169.
Vaněčková I, Kujal P, Husková Z, Vaňourková Z, Vernerová Z, Certíková Chábová V, Skaroupková P, Kramer HJ, Tesař V, Cervenka L . Effects of combined endothelin a receptor and renin-angiotensin system blockade on the course of end-organ damage in 5/6 nephrectomized ren-2 hypertensive rats. Kidney Blood Press Res 2012; 35: 382–392.
Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE . Recommendations for blood pressure measurements in humans and experimental animals. Part 2: blood pressure measurement in experimental animals: a statement for professionals from the subcommittee of professional and public education of the American Heart Association council on high blood pressure research. Hypertension 2005; 45: 299–310.
Sochman J, Peregrin JH, Bürgelová M, Kopkan L, Kramer HJ, Cervenka L . N-acetylcysteine attenuates iodine contrast agent-induced nephropathy in 5/6-nephrectomized rats. Kidney Blood Press Res 2010; 33: 149–156.
Honetschlägerová Z, Z Husková Z, Vaňourková Z, Sporková A, Kramer HJ, Hwang SH, Tsai HJ, Hammock BD, Imig JD, Červenka L, Kopkan L . Renal mechanisms contributing to the antihypertensive action of soluble epoxide hydrolase inhibition in Ren-2 transgenic rats with inducible hypertension. J Physiol 2011; 589: 207–219.
Honetschlägerová Z, Sporková A, Kopkan L, Husková Z, Hwang SH, Hammock BD, Imig JD, Kramer HJ, Kujal P, Vernerová Z, Chábová VC, Tesař V, Cervenka L . Inhibition of soluble epoxide hydrolase improves the impaired pressure-natriuresis relationship and attenuates the development of hypertension and hypertension-associated end-organ damage in Cyp1a1-Ren-2 transgenic rats. J Hypertens 2011; 29: 1590–1601.
Schmittgen TD, Livak KJ . Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008; 3: 1101–1108.
Ashek A, Menzies RI, Mullins LJ, Bellamy CO, Harmar AJ, Kenyon CJ, Flatman PW, Mullins JJ, Bailey MA . Activation of thiazide-sensitive co-transport by angiotensin II in the cyp1a1-Ren2 hypertensive rat. PLoS One 2012; 7: e36311.
Maitland K, Bridges L, Davis WP, Loscalzo J, Pointer MA . Different effects of angiotensin receptor blockade on end-organ damage in salt-dependent and salt-independent hypertension. Circulation 2006; 114: 905–911.
Stillman IE, Andoh TF, Burdmann EA, Bennett WM, Rosen S . FK506 nephrotoxicity: morphologic and physiologic characterization of a rat model. Lab Invest 1995; 73: 794–803.
Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, Corrêa JW, Gagnon AM, Gomez-Sanchez CE, Gomez-Sanchez EP, Sorisky A, Ooi TC, Ruzicka M, Burns KD, Touyz RM . Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension 2012; 59: 1069–1078.
Randhawa PS, Starzl TE, Demetris AJ . Tacrolimus (FK506)-associated renal pathology. Adv Anat Pathol 1997; 4: 265–276.
Rüster C, Wolf G . Angiotensin II as a morphogenic cytokine stimulating renal fibrogenesis. J Am Soc Nephrol 2011; 22: 1189–1199.
Kobori H, Nangaku M, Navar LG, Nishiyama A . The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 2007; 59: 251–287.
Naesens M, Lerut E, de Jonge H, Van Damme B, Vanrenterghem Y, Kuypers DR . Donor age and renal P-glycoprotein expression associate with chronic histological damage in renal allografts. J Am Soc Nephrol 2009; 20: 2468–2480.
Mezzano SA, Ruiz-Ortega M, Egido J . Angiotensin II and renal fibrosis. Hypertension 2001; 38: 635–638.
Ozawa Y, Kobori H, Suzaki Y, Navar LG . Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol 2007; 292: F330–F339.
Taal MW, Brenner BM . Renoprotective benefits of RAS inhibition: from ACEI to angiotensin II antagonists. Kidney Int 2000; 57: 1803–1817.
Mann JFE, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsärinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Koon KT, Yusuf S . Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomized, double-blind, controlled trial. Lancet 2008; 372: 547–553.
Mann JFE, Anderson C, Gao P, Gerstein HC, Boehm M, Ryden L, Sleight P, Teo KK, Yusuf S, the ONTARGET Investigators. Dual inhibition of the rennin-angiotensin system in high- risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens 2013; 31: 414–421.
Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, Leehey DJ, McCullough PA, O'Connor T, Palevsky PM, Reilly RF, Seliger SL, Warren SR, Watnick S, Peduzzi P, Guarino P, VA NEPHRON-D Investigators. Combined Angiotensin inhibition for the treatment of diabetic nephropathy. New Engl J Med 2013; 369: 1892–1903.
ESH/ESC Task Force for the Management of Arterial Hypertension. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC task force for the management of arterial hypertension. J Hypertens 2013; 31: 1925–1938.
Dieperink H, Hansen HV, Kemp M, Leyssac PP, Starklint H, Kemp E . Antagonist capacity of felodipine on cyclosporin A nephrotoxicity in the rat. Nephrol Dial Transplant 1992; 7: 1124–1129.
Dworkin LD, Tolbert E, Recht PA, Hersch JC, Feiner H, Levin RI . Effects of amlodipine on glomerular filtration, growth, and injury in experimental hypertension. Hypertension 1996; 27: 245–250.
Mackie FE, Meyer TW, Campbell DJ . Effects of antihypertensive therapy on intrarenal angiotensin and bradykinin levels in experimental renal insufficiency. Kidney Int 2002; 61: 555–563.
Park HC, Xu ZG, Choi S, Goo YS, Kang SW, Choi KH, Ha SK, Lee HY, Han DS . Effect of losartan and amlodipine on proteinuria and transforming growth factor-beta1 in patients with IgA nephropathy. Nephrol Dial Transplant 2003; 18: 1115–1121.
Ponticelli C, Glassock RJ . Glomerular diseases: membranous nephropathy-a modern view. Clin J Am Soc Nephrol 2014; 9: 609–616.
Schweda F, Liebl R, Riegger GA, Krämer BK . Tacrolimus treatment for steroid- and cyclosporin-resistant minimal-change nephrotic syndrome. Nephrol Dial Transplant 1997; 12: 2433–2435.
Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC . Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol 2002; 161: 1679–1693.
De Miguel C, Lund H, Mattson DL . High dietary protein exacerbates hypertension and renal damage in Dahl SS rats by increasing infiltrating immune cells in the kidney. Hypertension 2011; 57: 269–274.
Prieto MC, Gonzalez AA, Navar LG . Evolving concepts on regulation and function of renin in distal nephron. Pflugers Arch 2013; 465: 121–132.
Motawi TK, El-Maraghy SA, Senousy MA . Angiotensin-converting enzyme inhibition and angiotensin AT1 receptor blockade downregulate angiotensin-converting enzyme expression and attenuate renal injury in streptozotocin-induced diabetic rats. J Biochem Mol Toxicol 2013; 27: 378–387.
Acknowledgements
This study was supported by the project of the Ministry of Health, Czech Republic—conceptual development of research organization (‘Institute for Clinical and Experimental Medicine, IN 00023001’) and by Internal Grant Agency of the Ministry of Health of the Czech Republic No. NT/11269-5 and No. NT/11262-6. ZH is also partially supported by the Internal Grant Agency of the Ministry of Health of the Czech Republic No. NT/12171–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Hypertension Research website
Supplementary information
Rights and permissions
About this article
Cite this article
Hošková, L., Málek, I., Kautzner, J. et al. Tacrolimus-induced hypertension and nephrotoxicity in Fawn-Hooded rats are attenuated by dual inhibition of renin–angiotensin system. Hypertens Res 37, 724–732 (2014). https://doi.org/10.1038/hr.2014.79
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2014.79
Keywords
This article is cited by
-
Augmented circadian rhythm of the intrarenal renin–angiotensin systems in anti-thymocyte serum nephritis rats
Hypertension Research (2016)
-
Association between renal iron accumulation and renal interstitial fibrosis in a rat model of chronic kidney disease
Hypertension Research (2015)