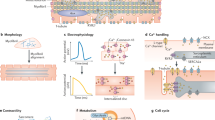
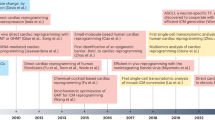
Abstract
Nuclear reprogramming of somatic cells with ectopic stemness factors to bioengineer pluripotent autologous stem cells signals a new era in regenerative medicine. The study of developmental biology has provided a roadmap for cardiac differentiation from embryonic tissue formation to adult heart muscle rejuvenation. Understanding the molecular mechanisms of stem-cell-derived cardiogenesis enables the reproducible generation, isolation, and monitoring of progenitors that have the capacity to recapitulate embryogenesis and differentiate into mature cardiac tissue. With the advent of induced pluripotent stem (iPS) cell technology, patient-specific stem cells provide a reference point to systematically decipher cardiogenic differentiation through discrete stages of development. Interrogation of iPS cells and their progeny from selected cohorts of patients is an innovative approach towards uncovering the molecular mechanisms of disease. Thus, the principles of cardiogenesis can now be applied to regenerative medicine in order to optimize personalized therapeutics, diagnostics, and discovery-based science for the development of novel clinical applications.
Key Points
-
Induced pluripotent stem (iPS) cells are autologous, somatic cells that have been modified to acquire an embryonic stem-cell-like capacity
-
iPS cells enable patient-specific examination of the molecular mechanisms of health and disease, and provide cell-based platforms for personalized diagnostics and therapeutics
-
iPS-cell-derived progeny have the potential to closely recapitulate natural cardiogenic differentiation
-
Nuclear reprogramming involves embryo-independent generation of iPS cells with ectopic stemness factors, which resets the fate of the cell to a self-sustainable pluripotent state
-
Various strategies are used for nuclear reprogramming, including genetic engineering with viruses, traceless systems, and genomic-free approaches
-
Bioengineered clones must undergo stringency testing to validate their functionality as iPS cells
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Meissner, A., Wernig, M. & Jaenisch, R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat. Biotechnol. 25, 1177–1181 (2007).
Park, I. H. et al. Disease-specific induced pluripotent stem cells. Cell 134, 877–886 (2008).
Jaenisch, R. & Young, R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 132, 567–582 (2008).
Murry, C. E. & Keller, G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132, 661–680 (2008).
Yamanaka, S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell 1, 39–49 (2007).
Park, I. H., Lerou, P. H., Zhao, R., Huo, H. & Daley, G. Q. Generation of human-induced pluripotent stem cells. Nat. Protoc. 3, 1180–1186 (2008).
Yamanaka, S. & Blau, H. M. Nuclear reprogramming to a pluripotent state by three approaches. Nature 465, 704–712 (2010).
Nelson, T., Behfar, A. & Terzic, A. Stem cells: biologics for regeneration. Clin. Pharmacol. Ther. 84, 620–623 (2008).
Nelson, T. J., Behfar, A. & Terzic, A. Strategies for therapeutic repair: the “R3” regenerative medicine paradigm. Clin. Transl. Sci. 1, 168–171 (2008).
Raya, A. et al. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature 460, 53–59 (2009).
Lee, G. et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature 461, 402–406 (2009).
Nelson, T. J., Behfar, A., Yamada, S., Martinez-Fernandez, A. & Terzic, A. Stem cell platforms for regenerative medicine. Clin. Transl. Sci. 2, 222–227 (2009).
Wu, J. C., Abraham, M. R. & Kraitchman, D. L. Current perspectives on imaging cardiac stem cell therapy. J. Nucl. Med. 51 (Suppl. 1), 128S–136S (2010).
Parker, A. et al. Diagnosis of post-transplant lymphoproliferative disorder in solid organ transplant recipients—BCSH and BTS Guidelines. Br. J. Haematol. 149, 675–692 (2010).
Martinez, O. M. & de Gruijl, F. R. Molecular and immunologic mechanisms of cancer pathogenesis in solid organ transplant recipients. Am. J. Transplant. 8, 2205–2211 (2008).
Silva, J. & Smith, A. Capturing pluripotency. Cell 132, 532–536 (2008).
Campbell, K. H., McWhir, J., Ritchie, W. A. & Wilmut, I. Sheep cloned by nuclear transfer from a cultured cell line. Nature 380, 64–66 (1996).
Beyhan, Z., Iager, A. E. & Cibelli, J. B. Interspecies nuclear transfer: implications for embryonic stem cell biology. Cell Stem Cell 1, 502–512 (2007).
Byrne, J. A. et al. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature 450, 497–502 (2007).
French, A. J. et al. Development of human cloned blastocysts following somatic cell nuclear transfer with adult fibroblasts. Stem Cells 26, 485–493 (2008).
Yamanaka, S. Pluripotency and nuclear reprogramming. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 2079–2087 (2008).
Yamanaka, S. A fresh look at iPS cells. Cell 137, 13–17 (2009).
Yamanaka, S. Elite and stochastic models for induced pluripotent stem cell generation. Nature 460, 49–52 (2009).
Dimos, J. T. et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 321, 1218–1221 (2008).
Ebert, A. D. et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 457, 277–280 (2009).
Takahashi, K., Okita, K., Nakagawa, M. & Yamanaka, S. Induction of pluripotent stem cells from fibroblast cultures. Nat. Protoc. 2, 3081–3089 (2007).
Aasen, T. et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat. Biotechnol. 26, 1276–1284 (2008).
Eminli, S. et al. Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nat. Genet. 41, 968–976 (2009).
Sun, N. et al. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc. Natl Acad. Sci. USA 106, 15720–15725 (2009).
Marion, R. M. et al. Telomeres acquire embryonic stem cell characteristics in induced pluripotent stem cells. Cell Stem Cell 4, 141–154 (2009).
Deng, J. et al. Targeted bisulfite sequencing reveals changes in DNA methylation associated with nuclear reprogramming. Nat. Biotechnol. 27, 353–360 (2009).
Mikkelsen, T. S. et al. Dissecting direct reprogramming through integrative genomic analysis. Nature 454, 49–55 (2008).
Silva, J. et al. Nanog is the gateway to the pluripotent ground state. Cell 138, 722–737 (2009).
Yoshida, Y., Takahashi, K., Okita, K., Ichisaka, T. & Yamanaka, S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell 5, 237–241 (2009).
Huangfu, D. et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat. Biotechnol. 26, 795–797 (2008).
Lin, T. et al. A chemical platform for improved induction of human iPSCs. Nat. Methods 6, 805–808 (2009).
Banito, A. et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 23, 2134–2139 (2009).
Utikal, J. et al. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature 460, 1145–1148 (2009).
Marión, R. M. et al. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature 460, 1149–1153 (2009).
Li, H. et al. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature 460, 1136–1139 (2009).
Kawamura, T. et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature 460, 1140–1144 (2009).
Hong, H. et al. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature 460, 1132–1135 (2009).
Smith, Z. D., Nachman, I., Regev, A. & Meissner, A. Dynamic single-cell imaging of direct reprogramming reveals an early specifying event. Nat. Biotechnol. 28, 521–526 (2010).
Hanna, J. et al. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature 462, 595–601 (2009).
Hotta, A. & Ellis, J. Retroviral vector silencing during iPS cell induction: an epigenetic beacon that signals distinct pluripotent states. J. Cell. Biochem. 105, 940–948 (2008).
Stadtfeld, M., Nagaya, M., Utikal, J., Weir, G. & Hochedlinger, K. Induced pluripotent stem cells generated without viral integration. Science 322, 945–949 (2008).
Okita, K., Nakagawa, M., Hyenjong, H., Ichisaka, T. & Yamanaka, S. Generation of mouse induced pluripotent stem cells without viral vectors. Science 322, 949–953 (2008).
Chang, C. W. et al. Polycistronic lentiviral vector for “hit and run” reprogramming of adult skin fibroblasts to induced pluripotent stem cells. Stem Cells 27, 1042–1049 (2009).
Kaji, K. et al. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature 458, 771–775 (2009).
Woltjen, K. et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 458, 766–770 (2009).
Yu, J. et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science 324, 797–801 (2009).
Zhou, H. et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 4, 381–384 (2009).
Kim, D. et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 4, 472–476 (2009).
Schenke-Layland, K. et al. Reprogrammed mouse fibroblasts differentiate into cells of the cardiovascular and hematopoietic lineages. Stem Cells 26, 1537–1546 (2008).
Martinez-Fernandez, A. et al. iPS programmed without c-MYC yield proficient cardiogenesis for functional heart chimerism. Circ. Res. 105, 648–656 (2009).
Mauritz, C. et al. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation 118, 507–517 (2008).
Narazaki, G. et al. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation 118, 498–506 (2008).
Zhang, J. et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 104, e30–e41 (2009).
Tashiro, K. et al. Efficient adipocyte and osteoblast differentiation from mouse induced pluripotent stem cells by adenoviral transduction. Stem Cells 27, 1802–1811 (2009).
Niwa, A. et al. Orderly hematopoietic development of induced pluripotent stem cells via Flk-1+ hemoangiogenic progenitors. J. Cell. Physiol. 221, 367–377 (2009).
Senju, S. et al. Characterization of dendritic cells and macrophages generated by directed differentiation from mouse induced pluripotent stem cells. Stem Cells 27, 1021–1031 (2009).
Maehr, R. et al. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc. Natl Acad. Sci. USA 106, 15768–15773 (2009).
Tateishi, K. et al. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J. Biol. Chem. 283, 31601–31607 (2008).
Zhang, D. et al. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 19, 429–438 (2009).
Si-Tayeb, K. et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 51, 297–305 (2010).
Song, Z. et al. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 19, 1233–1242 (2009).
Buchholz, D. E. et al. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells 27, 2427–2434 (2009).
Meyer, J. S. et al. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc. Natl Acad. Sci. USA 106, 16698–16703 (2009).
Osakada, F. et al. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J. Cell Sci. 122, 3169–3179 (2009).
Karumbayaram, S. et al. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells 27, 806–811 (2009).
Smith, K. P., Luong, M. X. & Stein, G. S. Pluripotency: toward a gold standard for human ES and iPS cells. J. Cell. Physiol. 220, 21–29 (2009).
Nelson, T. J., Martinez-Fernandez, A. & Terzic, A. KCNJ11 knockout morula re-engineered by stem cell diploid aggregation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 269–276 (2009).
Nagy, A., Nagy, K. & Gertsenstein, M. Production of mouse chimeras by aggregating pluripotent stem cells with embryos. Methods Enzymol. 476, 123–149 (2010).
Zhao, X. Y. et al. iPS cells produce viable mice through tetraploid complementation. Nature 461, 86–90 (2009).
Stadtfeld, M. et al. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature 465, 175–181 (2010).
Hanna, J. et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 318, 1920–1923 (2007).
Xu, D. et al. Phenotypic correction of murine hemophilia A using an iPS cell-based therapy. Proc. Natl Acad. Sci. USA 106, 808–813 (2009).
Wernig, M. et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc. Natl Acad. Sci. USA 105, 5856–5861 (2008).
Nelson, T. J. et al. Repair of acute myocardial infarction with human stemness factors induced pluripotent stem cells. Circulation 120, 408–416 (2009).
Ye, Z. et al. Human-induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood 114, 5473–5480 (2009).
Chien, K. R., Domian, I. J. & Parker, K. K. Cardiogenesis and the complex biology of regenerative cardiovascular medicine. Science 322, 1494–1497 (2008).
Slack, J. M. Origin of stem cells in organogenesis. Science 322, 1498–1501 (2008).
Garry, D. J. & Olson, E. N. A common progenitor at the heart of development. Cell 127, 1101–1104 (2006).
Laflamme, M. A. & Murry, C. E. Regenerating the heart. Nat. Biotechnol. 23, 845–856 (2005).
Wu, S. M., Chien, K. R. & Mummery, C. Origins and fates of cardiovascular progenitor cells. Cell 132, 537–543 (2008).
Mummery, C. et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation 107, 2733–2740 (2003).
Schneider, V. A. & Mercola, M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 15, 304–315 (2001).
Marvin, M. J., Di Rocco, G., Gardiner, A., Bush, S. M. & Lassar, A. B. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 15, 316–327 (2001).
Olson, E. N. Development. The path to the heart and the road not taken. Science 291, 2327–2378 (2001).
Pashmforoush, M. et al. Nkx2-5 pathways and congenital heart disease; loss of ventricular myocyte lineage specification leads to progressive cardiomyopathy and complete heart block. Cell 117, 373–386 (2004).
Ishiwata, T., Nakazawa, M., Pu, W. T., Tevosian, S. G. & Izumo, S. Developmental changes in ventricular diastolic function correlate with changes in ventricular myoarchitecture in normal mouse embryos. Circ. Res. 93, 857–865 (2003).
Zhou, B. et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454, 109–113 (2008).
Christoffels, V. M. et al. Tbx18 and the fate of epicardial progenitors. Nature 458, E8–E9 (2009).
Moskowitz, I. P. et al. A molecular pathway including Id2, Tbx5, and Nkx2-5 required for cardiac conduction system development. Cell 129, 1365–1376 (2007).
Zhao, Y. et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell 129, 303–317 (2007).
Rentschler, S. et al. Neuregulin-1 promotes formation of the murine cardiac conduction system. Proc. Natl Acad. Sci. USA 99, 10464–10469 (2002).
Siedner, S. et al. Developmental changes in contractility and sarcomeric proteins from the early embryonic to the adult stage in the mouse heart. J. Physiol. 548, 493–505 (2003).
MacLellan, W. R. & Schneider, M. D. Genetic dissection of cardiac growth control pathways. Annu. Rev. Physiol. 62, 289–319 (2000).
Gai, H. et al. Generation and characterization of functional cardiomyocytes using induced pluripotent stem cells derived from human fibroblasts. Cell Biol. Int. 33, 1184–1193 (2009).
Kuzmenkin, A. et al. Functional characterization of cardiomyocytes derived from murine induced pluripotent stem cells in vitro. FASEB J. 23, 4168–4180 (2009).
Martinez-Fernandez, A., Nelson, T. J., Ikeda, Y. & Terzic, A. c-MYC independent nuclear reprogramming favors cardiogenic potential of induced pluripotent stem cells. J. Cardiovasc. Transl. Res. 3, 13–23 (2010).
Zwi, L. et al. Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation 120, 1513–1523 (2009).
Moretti, A. et al. Mouse and human induced pluripotent stem cells as a source for multipotent Isl1+ cardiovascular progenitors. FASEB J. 24, 700–711 (2010).
Yokoo, N. et al. The effects of cardioactive drugs on cardiomyocytes derived from human induced pluripotent stem cells. Biochem. Biophys. Res. Commun. 387, 482–488 (2009).
Pfannkuche, K. et al. Cardiac myocytes derived from murine reprogrammed fibroblasts: intact hormonal regulation, cardiac ion channel expression and development of contractility. Cell. Physiol. Biochem. 24, 73–86 (2009).
Moretti, A. et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N. Engl. J. Med. doi:10.1056/NEJMoa0908679.
Carvajal-Vergara, X. et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature 465, 808–812 (2010).
Hansen, A. et al. Development of a drug screening platform based on engineered heart tissue. Circ. Res. 107, 35–44 (2010).
Ieda, M. et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142, 375–386 (2010).
van Laake, L. W. et al. Reporter-based isolation of induced pluripotent stem cell- and embryonic stem cell-derived cardiac progenitors reveals limited gene expression variance. Circ. Res. 107, 340–347 (2010).
Srinivas, G., Anversa, P. & Frishman, W. H. Cytokines and myocardial regeneration: a novel treatment option for acute myocardial infarction. Cardiol. Rev. 17, 1–9 (2009).
Reinecke, H., Minami, E., Zhu, W. Z. & Laflamme, M. A. Cardiogenic differentiation and transdifferentiation of progenitor cells. Circ. Res. 103, 1058–1071 (2008).
Bartunek, J. et al. Delivery of biologics in cardiovascular regenerative medicine. Clin. Pharmacol. Ther. 85, 548–552 (2009).
Blin, G. et al. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J. Clin. Invest. 120, 1125–1139 (2010).
Alper, J. Geron gets green light for human trial of ES cell-derived product. Nat. Biotechnol. 27, 213–214 (2009).
Li, J. Y., Christophersen, N. S., Hall, V., Soulet, D. & Brundin, P. Critical issues of clinical human embryonic stem cell therapy for brain repair. Trends Neurosci. 31, 146–153 (2008).
Olson, E. N. A decade of discoveries in cardiac biology. Nat. Med. 10, 467–474 (2004).
Behfar, A. et al. Guided stem cell cardiopoiesis: discovery and translation. J. Mol. Cell. Cardiol. 45, 523–529 (2008).
Behfar, A. et al. Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. J. Exp. Med. 204, 405–420 (2007).
Chiriac, A., Nelson, T. J., Faustino, R. S., Behfar, A. & Terzic, A. Cardiogenic induction of pluripotent stem cells streamlined through a conserved SDF-1/VEGF/BMP2 integrated network. PLoS ONE 5, e9943 (2010).
Nelson, T. J. et al. CXCR4+/FLK-1+ biomarkers select a cardiopoietic lineage from embryonic stem cells. Stem Cells 26, 1464–1473 (2008).
Moretti, A. et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 127, 1151–1165 (2006).
Kattman, S. J., Huber, T. L. & Keller, G. M. Multipotent Flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev. Cell 11, 723–732 (2006).
Yang, L. et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 453, 524–528 (2008).
Takeuchi, J. K. & Bruneau, B. G. Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature 459, 708–711 (2009).
Padin-Iruegas, M. E. et al. Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation 120, 876–887 (2009).
Terzic, A. & Nelson, T. J. Regenerative medicine advancing health care 2020. J. Am. Coll. Cardiol. 55, 2254–2257 (2010).
Yoshida, Y. & Yamanaka, S. Recent stem cell advances: induced pluripotent stem cells for disease modeling and stem cell-based regeneration. Circulation 122, 80–87 (2010).
Author information
Authors and Affiliations
Contributions
T. J. Nelson and A. Terzic contributed to discussion of content for the article, researched data to include in the manuscript, wrote the manuscript, reviewed and edited the manuscript before submission, and revised the manuscript in response to the peer-reviewers' comments. A. Martinez-Fernandez contributed to discussion of content for the article, researched data to include in the manuscript, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Nelson, T., Martinez-Fernandez, A. & Terzic, A. Induced pluripotent stem cells: developmental biology to regenerative medicine. Nat Rev Cardiol 7, 700–710 (2010). https://doi.org/10.1038/nrcardio.2010.159
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2010.159
This article is cited by
-
The use of induced pluripotent stem cells in domestic animals: a narrative review
BMC Veterinary Research (2020)
-
Real-time Measurement of Biomagnetic Vector Fields in Functional Syncytium Using Amorphous Metal
Scientific Reports (2015)
-
Induced Pluripotent Stem Cells as a new Strategy for Osteogenesis and Bone Regeneration
Stem Cell Reviews and Reports (2015)
-
Sequential introduction of reprogramming factors reveals a time-sensitive requirement for individual factors and a sequential EMT–MET mechanism for optimal reprogramming
Nature Cell Biology (2013)
-
A blueprint for engineering cell fate: current technologies to reprogram cell identity
Cell Research (2013)