Abstract
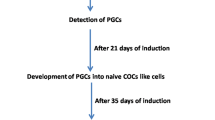
Mammalian fetal ovaries contain numerous primordial germ cells (PGCs), although few mature oocytes are obtained from females, owing to apoptosis and follicle atresia. The regulatory mechanisms underlying oogenesis/folliculogenesis remain unknown. Development of methods for obtaining mature oocytes from PGCs in fetal ovaries in vitro could contribute to clarifying these mechanisms. The failure of follicle assembly has been found to be the most challenging aspect in conventional culture conditions. Recently, we established novel culture conditions that enable successful follicle assembly, sustaining interactions between the oocyte and somatic cells, and, in turn, promoting oocyte growth and maturation. Mature oocytes were differentiated from PGCs after a 1-month culture period. A hundred mouse offspring were obtained from approximately a thousand mature oocytes, indicating that oocytes that were differentiated from PGCs in vitro acquired totipotency after fertilization. Here we provide a detailed protocol for using this in vitro system. This in vitro system will potentially provide a novel platform for studying oogenesis and preservation of female germ cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Jones, K.T. Meiosis in oocytes: predisposition to aneuploidy and its increased incidence with age. Hum. Reprod. Update 14, 143–158 (2008).
Obata, Y. et al. Disruption of primary imprinting during oocyte growth leads to the modified expression of imprinted genes during embryogenesis. Development 125, 1553–1560 (1998).
Ebert, K.M., Liem, H. & Hecht, N.B. Mitochondrial DNA in the mouse preimplantation embryo. J. Reprod. Fertil. 82, 145–149 (1988).
Howlett, S.K. & Bolton, V.N. Sequence and regulation of morphological and molecular events during the first cell cycle of mouse embryogenesis. J. Embryol. Exp. Morphol. 87, 175–206 (1985).
McLaren, A. Meiosis and differentiation of mouse germ cells. Sym. Soc. Exp. Biol. 38, 7–23 (1984).
Romero, S. & Smitz, J. Epiregulin can effectively mature isolated cumulus-oocyte complexes, but fails as a substitute for the hCG/epidermal growth factor stimulus on cultured follicles. Reproduction 137, 997–1005 (2009).
Kreeger, P.K., Deck, J.W., Woodruff, T.K. & Shea, L.D. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials 27, 714–723 (2006).
Whitten, W.K. & Biggers, J.D. Complete development in vitro of the pre-implantation stages of the mouse in a simple chemically defined medium. J. Reprod. Fertil. 17, 399–401 (1968).
Biggers, J.D., Moore, B.D. & Whittingham, D.G. Development of mouse embryos in vivo after cultivation from two-cell ova to blastocysts in vitro. Nature 206, 734–735 (1965).
Brinster, R.L. & Biggers, J.D. In-vitro fertilization of mouse ova within the explanted fallopian tube. J. Reprod. Fertil. 10, 277–279 (1965).
Morohaku, K. et al. Complete in vitro generation of fertile oocytes from mouse primordial germ cells. Proc. Natl. Acad. Sci. USA 113, 9021–9026 (2016).
Morohaku, K., Hirao, Y. & Obata, Y. Developmental competence of oocytes grown in vitro: has it peaked already? J. Reprod. Dev. 62, 1–5 (2016).
Sato, T. et al. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 471, 504–507 (2011).
Shen, W. et al. In vitro development of mouse fetal germ cells into mature oocytes. Reproduction 134, 223–231 (2007).
Obata, Y., Kono, T. & Hatada, I. Gene silencing: maturation of mouse fetal germ cells in vitro. Nature 418, 497 (2002).
Zhang, Z.P. et al. Growth of mouse oocytes to maturity from premeiotic germ cells in vitro. PLoS One 7, e41771 (2012).
O'Brien, M.J., Pendola, J.K. & Eppig, J.J. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol. Reprod. 68, 1682–1686 (2003).
Eppig, J.J. & O'Brien, M.J. Development in vitro of mouse oocytes from primordial follicles. Biol. Reprod. 54, 197–207 (1996).
Chen, Y., Breen, K. & Pepling, M.E. Estrogen can signal through multiple pathways to regulate oocyte cyst breakdown and primordial follicle assembly in the neonatal mouse ovary. J. Endocrinol. 202, 407–417 (2009).
Chen, Y., Jefferson, W.N., Newbold, R.R., Padilla-Banks, E. & Pepling, M.E. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology 148, 3580–3590 (2007).
Hirao, Y. et al. In vitro growth and development of bovine oocyte-granulosa cell complexes on the flat substratum: effects of high polyvinylpyrrolidone concentration in culture medium. Biol. Reprod. 70, 83–91 (2004).
Mochida, N. et al. Live births from isolated primary/early secondary follicles following a multistep culture without organ culture in mice. Reproduction 146, 37–47 (2013).
Eppig, J.J. & Schroeder, A.C. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol. Reprod. 41, 268–276 (1989).
Hikabe, O. et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature 539, 299–303 (2016).
Hayashi, K., Hikabe, O., Obata, Y. & Hirao, Y. Reconstitution of mouse oogenesis in a dish from pluripotent stem cells. Nat. Protoc. http://dx.doi.org/10.1038/nprot.2017.070.
Pfender, S. et al. Live imaging RNAi screen reveals genes essential for meiosis in mammalian oocytes. Nature 524, 239–242 (2015).
Brigid Hogan, F.C. & Elizabeth, Lacy Manipulating the Mouse Embryo (Cold Spring Harbor Laboratory, 1986).
Acknowledgements
The authors thank K. Hayashi at Kyushu University, T. Kono at Tokyo University of Agriculture, and V. Selvaraj at Cornell University for providing their opinions. The authors also thank R. Tanimoto and S. Ikuta for technical help. We are grateful to members of the Animal Life Science Research Center at Tokyo University of Agriculture for their contributions to the animal care. This work was supported by Grants-in-Aid for Scientific Research (26450449 and 25114008) to Y.O. and by a MEXT-Supported Program for the Strategic Research Foundation at Private Universities (S0801025).
Author information
Authors and Affiliations
Contributions
K.M., Y.H., and Y.O. developed this protocol. K.M. and Y.O. organized the figures and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Procedures for switching the medium of organ culture on days 5 and 11.
(a) New 6-well plates are prepared with fresh organ culture medium with or without ICI on day 5 or 11, respectively. (b) Figures show each step of the medium switching procedure. 1) A cultured Transwell-COL membrane insert is removed from an old well using sterilized forceps. 2) Old medium on the Transwell-COL membrane insert is aspirated using a pipette. 3) The Transwell-COL membrane insert is placed in a well containing 2 mL fresh organ culture medium in a new well of a 6-well plate. 4) One milliliter of fresh culture medium is slowly added onto the membrane; the Transwell-COL membrane insert is then removed from the well, and extra medium on the membrane is aspirated using a pipette. 5) The Transwell-COL membrane insert is placed in a new well containing 1.3 mL fresh organ culture medium. 6) Seven hundred twenty microliters of organ culture medium is added onto the membrane.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1. Procedures for switching the medium of organ culture on day 5 or 11. (a) New 6-well plates are prepared with fresh organ culture medium with or without ICI on day 5 or 11, respectively. (b) Images show each step of the medium-switching procedure. (1) A cultured Transwell-COL membrane insert is removed from an old well using sterilized forceps. (2) Old medium on the Transwell-COL membrane insert is aspirated using a pipette. (3) The Transwell-COL membrane insert is placed into a well containing 2 ml of fresh organ culture medium in a new well of a 6-well plate. (4) One milliliter of fresh culture medium is slowly added onto the membrane; the Transwell-COL membrane insert is then removed from the well, and, using a pipette, extra medium on the membrane is aspirated. (5) The Transwell-COL membrane insert is placed into a new well containing 1.3 ml of fresh organ culture medium. (6) 720 μl of organ culture medium is added onto the membrane. (PDF 293 kb)
Separation of a genital ridge from the mesonephros.
A genital ridge is separated from the mesonephros using a curved tungsten needle in L-15 handling medium. (MOV 3370 kb)
Isolation of secondary follicles from an ovary cultured for 17 d.
Follicles are gouged out from the ovary using a straight tungsten needle. (MOV 17523 kb)
Removal of theca cells from follicles on day 20 of culture.
Using a glass capillary, follicles are pipetted up and down until theca cells are removed. (MOV 18157 kb)
Rights and permissions
About this article
Cite this article
Morohaku, K., Hirao, Y. & Obata, Y. Development of fertile mouse oocytes from mitotic germ cells in vitro. Nat Protoc 12, 1817–1829 (2017). https://doi.org/10.1038/nprot.2017.069
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.069
This article is cited by
-
In vitro oogenesis from murine premeiotic germ cells using a new three-dimensional culture system
Cell Death Discovery (2023)
-
Dissecting the initiation of female meiosis in the mouse at single-cell resolution
Cellular and Molecular Life Sciences (2021)
-
The antiandrogenic vinclozolin induces differentiation delay of germ cells and changes in energy metabolism in 3D cultures of fetal ovaries
Scientific Reports (2020)
-
Reconstitution of mouse oogenesis in a dish from pluripotent stem cells
Nature Protocols (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.