Abstract
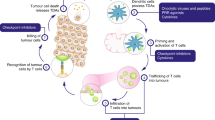
We present here the updated results after 9 years of the beginning of a trial on canine patients with malignant melanoma. This surgery adjuvant approach combined local suicide gene therapy with a subcutaneous vaccine composed by tumor cells extracts and xenogeneic cells producing human interleukin-2 and granulocyte-macrophage colony-stimulating factor. Toxicity was absent or minimal in all patients (0⩽VCOG-CTCAE grade⩽1). With respect to surgery-treated controls (ST), the complete surgery (CS) arm of this combined treatment (CT) significantly increased the fraction of local disease-free patients from 13 to 81% and distant metastases free from 32 to 84%. Even though less effective than the CS arm, the partial surgery (PS) arm of this CT was significantly better controlling the disease than only surgery (14% while PS-ST: 0%, P<0.01 and CS-ST: 5%, P<0.05). In addition, CT produced a significant sevenfold (CS) and threefold (PS) increase in overall survival. The CS-CT arm significantly improved both CS-ST metastasis-free- and melanoma overall survival from 99 days (respective ranges: 11–563 and 10–568) to >2848 days (81–2848 and 35–2848). Thus, more of 50% of our CT patients died of melanoma unrelated causes, transforming a lethal disease into a chronic one. Finally, surgery adjuvant CT delayed or prevented post-surgical recurrence and distant metastasis, significantly improved disease-free and overall survival maintaining the quality of life. Long-term safety and efficacy of this treatment are supported by the high number of CT patients (283) and extensive follow-up (>9 years). The successful clinical outcome encourages the further translation of similar approaches to human gene therapy trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hansen K, Khanna C . Spontaneous and genetically engineered animal models; use in preclinical cancer drug development. Eur J Cancer 2004; 40: 858–880.
Modiano JF, Breen M, Lana SL, Ehrhart N, Fosmire SP, Thomas R et al. Naturally occurring translational models for development of cancer gene therapy. Gene Ther Mol Biol 2006; 10: 31–40.
Ramos-Vara JA, Beissenherz ME, Miller MA, Johnson GC, Pace LW, Fard A et al. Retrospective study of 338 canine oral melanomas with clinical, histologic, and immunohistochemical review of 129 cases. Vet Pathol 2000; 37: 597–608.
Smith SH, Goldschmidt MH, McManus PM . A comparative review of melanocytic neoplasms. Vet Pathol 2002; 39: 651–678.
Finocchiaro LME, Glikin GC . Cancer gene therapy in large animals. In: Gustafsson WB, (ed) New Gene Therapy and Cancer Research. Nova Science Publishers: New York, 2008 pp 279–292.
Quintin-Colonna F, Devauchelle P, Fradelizi D, Mourot B, Faure T, Kourilsky P et al. Gene therapy of spontaneous canine melanoma and feline fibrosarcoma by intratumoral administration of histoincompatible cells expressing human interleukin-2. Gene Ther 1996; 3: 1104–1112.
Hogge GS, Burkholder JK, Culp J, Albertini MR, Dubielzig RR, Keller ET et al. Development of human granulocyte-macrophage colony-stimulating factor-transfected tumor cell vaccines for the treatment of spontaneous canine cancer. Hum Gene Ther 1998; 9: 1851–1861.
Alexander AN, Huelsmeyer MK, Mitzey A, Dubielzig RR, Kurzman ID, Macewen EG et al. Development of an allogeneic whole-cell tumor vaccine expressing xenogeneic gp100 and its implementation in a phase II clinical trial in canine patients with malignant melanoma. Cancer Immunol Immunother 2006; 55: 433–442.
Dow SW, Elmslie RE, Willson AP, Roche L, Gorman C, Potter TA . In vivo tumor transfection with superantigen plus cytokine genes induces tumor regression and prolongs survival in dogs with malignant melanoma. J Clin Invest 1998; 101: 2406–2414.
Bergman PJ, McKnight J, Novosad A, Charney S, Farrelly J, Craft D et al. Long-term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: a phase I trial. Clin Cancer Res 2003; 9: 1284–1290.
Manley CA, Leibman NF, Wolchok JD, Rivière IC, Bartido S, Craft DM et al. Xenogeneic murine tyrosinase DNA vaccine for malignant melanoma of the digit of dogs. J Vet Intern Med 2011; 25: 94–99.
Bianco SR, Sun J, Fosmire SP, Hance K, Padilla ML, Ritt MG et al. Enhancing antimelanoma immune responses through apoptosis. Cancer Gene Ther 2003; 10: 726–736.
Reed SD, Fulmer A, Buckholz J, Zhang B, Cutrera J, Shiomitsu K et al. Bleomycin/interleukin-12 electrochemogene therapy for treating naturally occurring spontaneous neoplasms in dogs. Cancer Gene Ther 2010; 17: 457–464.
von Euler H, Sadeghi A, Carlsson B, Rivera P, Loskog A, Segall T et al. Efficient adenovector CD40 ligand immunotherapy of canine malignant melanoma. J Immunother 2008; 31: 377–384.
Glikin GC, Finocchiaro LME . Gene therapy for canine cancer: clinical results. In: Andrade RL, Batista CI, (eds) Canine Behavior, Classification and Diseases. Nova Science Publishers: New York, 2012 pp 37–56.
Finocchiaro LME, Glikin GC . Cytokine-enhanced vaccine and suicide gene therapy as surgery adjuvant treatments for spontaneous canine melanoma. Gene Ther 2008; 15: 267–276.
Grosenbaugh DA, Leard AT, Bergman PJ, Klein MK, Meleo K, Susaneck S et al. Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. Am J Vet Res 2011; 72: 1631–1638.
Finocchiaro LME, Villaverde MS, Gil Cardeza ML, Riveros MD, Glikin GC . Cytokine-enhanced vaccine and interferon-β plus suicide gene as combined therapy for spontaneous canine sarcomas. Res Vet Sci 2011; 91: 230–234.
Finocchiaro LME, Spector A, Rossi UA, Gil-Cardeza ML, Suárez JL, Riveros MD et al. The potential of suicide plus immune gene therapy for treating osteosarcoma: the experience on canine veterinary patients. In: Butler EJ, (ed) Sarcoma: Symptoms, Causes and Treatments. Nova Science Publishers: New York, 2012 pp 107–122.
Finocchiaro LME, Fiszman GL, Karara AL, Glikin GC . Suicide gene and cytokines combined non viral gene therapy for spontaneous canine melanoma. Cancer Gene Ther 2008; 15: 165–172.
San H, Yang ZY, Pompili VJ, Jaffe ML, Plautz GE, Xu L et al. Safety and short-term toxicity of a novel cationic lipid formulation for human gene therapy. Hum Gene Ther 1993; 4: 781–788.
Gao X, Huang L . Cationic liposome-mediated gene transfer. Gene Ther 1995; 2: 710–722.
Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340: c869.
VCOG. Veterinary Co-operative Oncology Group—Common Terminology Criteria for Adverse Events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Vet Comp Oncol 2004; 2: 195–213.
Mesnil M, Yamasaki H . Bystander effect in herpes simplex virus-thymidine kinase/ganciclovir cancer gene therapy: role of gap-junctional intercellular communication. Cancer Res 2000; 60: 3989–3999.
Li S, Wilkinson M, Xia X, David M, Xu L, Purkel-Sutton A et al. Induction of IFN-regulated factors and antitumoral surveillance by transfected placebo plasmid DNA. Mol Ther 2005; 11: 112–119.
Roman M, Martin-Orozco E, Goodman JS, Nguyen MD, Sato Y, Ronaghy A et al. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med 1997; 3: 849–854.
Rosenberg SA . Progress in human tumour immunology and immunotherapy. Nature 2001; 411: 380–384.
Rochlitz C, Dreno B, Jantscheff P, Cavalli F, Squiban P, Acres B et al. Immunotherapy of metastatic melanoma by intratumoral injections of Vero cells producing human IL-2: phase II randomized study comparing two dose levels. Cancer Gene Ther 2002; 9: 289–295.
Dranoff G . GM-CSF-based cancer vaccines. Immunol Rev 2002; 188: 147–154.
Soiffer R, Lynch T, Mihm M, Jung K, Rhuda C, Schmollinger JC et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci USA 1998; 95: 13141–13146.
Pan PY, Li Y, Li Q, Gu P, Martinet O, Thung S et al. In situ recruitment of antigen-presenting cells by intratumoral GM-CSF gene delivery. Cancer Immunol Immunother 2004; 53: 17–25.
van Putten EH, Dirven CM, van den Bent MJ, Lamfers ML . Sitimagene ceradenovec: a gene-based drug for the treatment of operable high-grade glioma. Future Oncol 2010; 6: 1691–1710.
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364: 2507–2516.
Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364: 2517–2526.
Acknowledgements
We have properly acknowledged all the help received for the period 2003–2006 in the previous publication.16 For the period 2007–2011, we are grateful to our earlier and new patients and their owners for their cooperation and participation in this study. We thank Dr Marcela S Villaverde and Dr María L Gil-Cardeza for psCMVtk, MSc. María D Riveros for cytokine-producing cells and vaccine preparation, VMD Marie Maminska and VMD Fernando Calcagno for patients’ treatment and care, and the Centro de Especialidades Médicas Veterinarias (CEMV, Buenos Aires) for kindly lending its facilities for patients’ treatment. This work was supported by grants from ANPCYT/FONCYT (PICT2002-12084 and PICT2007-00539) and CONICET (PIP 112 200801 02920/2009-2012). LMEF and GCG are investigators of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Finocchiaro, L., Glikin, G. Cytokine-enhanced vaccine and suicide gene therapy as surgery adjuvant treatments for spontaneous canine melanoma: 9 years of follow-up. Cancer Gene Ther 19, 852–861 (2012). https://doi.org/10.1038/cgt.2012.72
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2012.72
Keywords
This article is cited by
-
Particulate mediators of the bystander effect linked to suicide and interferon-β transgene expression in melanoma cells
Gene Therapy (2021)
-
Combination of cytokine-enhanced vaccine and chemo-gene therapy as surgery adjuvant treatments for spontaneous canine melanoma
Gene Therapy (2019)
-
The importance of comparative oncology in translational medicine
Cancer Immunology, Immunotherapy (2015)