Abstract
Immunotherapy failures can result from the highly suppressive tumour microenvironment that characterizes aggressive forms of cancer such as recurrent glioblastoma (rGBM)1,2. Here we report the results of a first-in-human phase I trial in 41 patients with rGBM who were injected with CAN-3110—an oncolytic herpes virus (oHSV)3. In contrast to other clinical oHSVs, CAN-3110 retains the viral neurovirulence ICP34.5 gene transcribed by a nestin promoter; nestin is overexpressed in GBM and other invasive tumours, but not in the adult brain or healthy differentiated tissue4. These modifications confer CAN-3110 with preferential tumour replication. No dose-limiting toxicities were encountered. Positive HSV1 serology was significantly associated with both improved survival and clearance of CAN-3110 from injected tumours. Survival after treatment, particularly in individuals seropositive for HSV1, was significantly associated with (1) changes in tumour/PBMC T cell counts and clonal diversity, (2) peripheral expansion/contraction of specific T cell clonotypes; and (3) tumour transcriptomic signatures of immune activation. These results provide human validation that intralesional oHSV treatment enhances anticancer immune responses even in immunosuppressive tumour microenvironments, particularly in individuals with cognate serology to the injected virus. This provides a biological rationale for use of this oncolytic modality in cancers that are otherwise unresponsive to immunotherapy (ClinicalTrials.gov: NCT03152318).
Similar content being viewed by others
Main
High-grade gliomas (HGGs) are central nervous system tumours of glial origin with highly malignant morphologic and genetic features5,6. Among these, GBM is characterized by the worst outcome in terms of survival, with rapid recurrence after neurosurgical resection and chemoradiation7. Recurrent HGG (rHGG), including recurrent GBM (rGBM), is characterized by rapid neurological morbidity and survival of less than 10 months8. Although much is known of the genetics, cellular composition and evolution of HGG/GBM, this has not translated into successful therapies. Traditional immunotherapy has also been ineffective in rHGG/rGBM1. This is thought to be due to the scarcity of infiltrating antitumour lymphocytes caused by a highly immunosuppressive tumour microenvironment (TME), defining these tumours as ‘lymphocyte depleted’2. For rGBMs and several other highly immunosuppressive solid cancers, there is a need to find treatment modalities that can convert the TME into one that is more amenable to immunotherapy and immune activation.
Oncolytic viruses are a form of immunotherapy in which oncolytic-virus-induced oncolysis alters the TME, promoting proinflammatory pathways, activating resident and newly recruited immune cells through exposure of viral and possibly tumour antigens9,10,11,12,13. Several oncolytic viruses have been and continue to be tested in oncology, with one approved as a single-agent intralesional injection into melanoma14 and a second one approved for injection into rGBM in Japan15,16,17. Notably, several early-phase oncolytic-virus clinical trials for HGG have been published in recent high-profile literature17,18,19,20,21,22,23. Yet, immunological profiling of rGBMs treated with oncolytic viruses in numbers sufficient to correlate with a therapeutic outcome has been lacking.
Here we report safety data for a first-in-human phase I clinical trial in 41 patients with rHGG/rGBM who were treated with CAN-3110—an oncolytic virus derived from herpes simplex virus type 1 (oncolytic HSV (oHSV); ClinicalTrials.gov: NCT03152318). We found that patients whose survival response after CAN-3110 was the longest were characterized by positive HSV1 serology with CAN-3110 clearance from infected tumour, differences in T cell clonotype metrics, and tumour transcriptomic signatures associated with immune activation programs. These findings provide human immunological and biological evidence supporting intralesional oncolytic treatment modalities to change the immunosuppressive TME into one that is more favourable for immunotherapy, providing broad relevance for the therapy of many solid cancers that are otherwise impervious to immune rejection.
Safety of CAN-3110 in patients with rHGG/rGBM
Most clinical oHSVs to date have deleted or removed the viral gene encoding ICP34.5 (refs. 3,4); although ICP34.5 enables robust replication of HSV in infected cells24,25, it is also responsible for neurotoxicity in mice26. To take advantage of ICP34.5’s functions that enhance viral replication/persistence and minimize neurotoxicity, CAN-3110 (former designation, rQNestin34.5v.2) was engineered to express a copy of the viral ICP34.5 gene under transcriptional control of the promoter for nestin, restricting viral replication and virulence to HGG/GBM cells3,4. To further ensure safety for initial use in humans, a multi-cohort clinical trial design was implemented (Extended Data Fig. 1a). Moreover, to ensure that the injections occurred in tumour, intraoperative MRI guidance was used to visualize the injections (Extended Data Fig. 1b,c and Supplementary Methods). A total of 41 patients with rHGG/rGBM (42 interventions, see the note on participant 042/054 in the Supplementary Methods; Extended Data Tables 1 and 2) were recruited to the trial. The patients were enrolled at their first (n = 18), second (n = 9) or third (n = 3) recurrence for cohorts 1–9 and at the first (n = 5), second (n = 3), third (n = 1) or fourth (n = 3) recurrence for cohort 10 (Extended Data Table 3). Tumour genomic data were typical for a rHGG/rGBM population (Extended Data Fig. 2), including the presence of mutations in the CDKN2A/B (encoding p16) tumour suppressor pathway, previously shown to complement viral replication of oHSVs, such as CAN-3110, with defects in the viral ribonucleotide reductase function27. Serious adverse events, consisting of seizures requiring hospitalization and intervention, were observed in two patients, but there were no dose-limiting toxicities or clinical/pathological evidence of ICP34.5-induced HSV1 encephalitis/meningitis (Tables 1 and 2 and Extended Data Table 4). Thus, these data indicate the relative human safety of CAN-3110 at all tested doses despite the presence of the HSV1 ICP34.5 neurovirulence gene.
HSV1 serology predicts efficacy
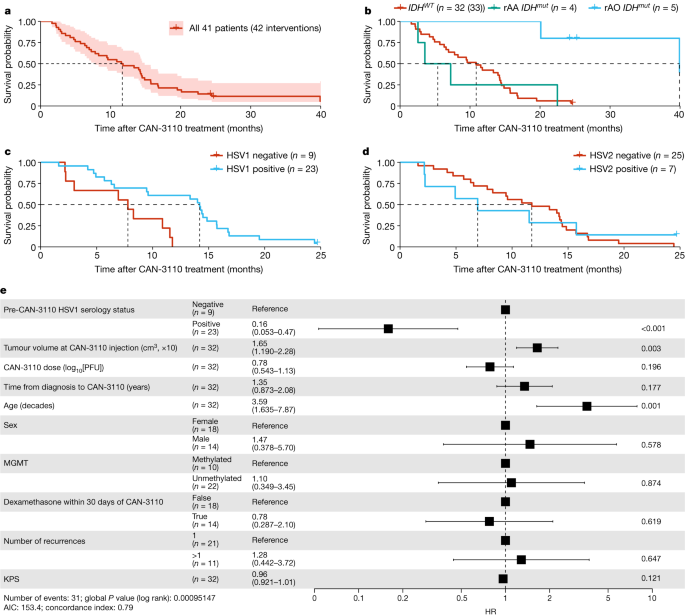
We tried to determine whether there were patients who benefited the most from treatment. Notably, 9 out of 41 patients (22%) had tumours associated with reduced survival28,29,30, such as depth (insular, thalamic), multifocality/multicentricity or bilateral laterality. In these latter cases, only one of the tumours or one hemispheric side of tumour was injected. Notably, patients like these are not routinely eligible for clinical trials, compounding the difficulty in comparing to historical clinical trial data. The estimated median overall survival (mOS) of the entire rHGG/rGBM group was 11.6 months (95% confidence interval (CI) = 7.8–14.9 months) (Fig. 1a). On the basis of the latest WHO classification5, we observed that, for the isocitrate dehydrogenase (IDH1/2) wild-type (WT) rGBM subgroup (n = 32 patients, 33 interventions), the mOS was 10.9 months (95% CI = 6.9–14.4 months), whereas, for the subgroup with recurrent IDHmutant (IDHmut) anaplastic astrocytoma (rAA; grade 3 or 4) (n = 4), the mOS was 5.4 months (95% CI = 2.6–∞ months) and, for the recurrent anaplastic oligodendroglioma (IDHmut; 1p/19q co-deleted), the mOS was 39.9 months (95% CI = 39.9–∞ months) (n = 5) (Fig. 1b). Progression-free survival times for the entire cohort and the cohort divided by the three rHGG diagnostic groups are shown in Extended Data Fig. 3a,b, respectively, and the clinical course of treated patients is shown in Extended Data Fig. 3c,d. Note that, in the swimmer plots, the timepoint of post-injection tumour resection is illustrated by a coloured triangle, with most additional antitumour therapies administered after resection. Full patient treatment histories have been included in Supplementary Table 1. Examples of significant clinical and radiographic responses are illustrated in Extended Data Fig. 4, including a response in a multifocal/multicentric rGBM.
a, Kaplan–Meier survival analysis of 41 patients with rHGG (42 interventions) after treatment with CAN-3110 (day 0). The shaded area shows the 95% CIs; the Kaplan–Meier estimate of survival probability is shown. Data maturity, October 2022. Median survival time (MST), 11.6 months (95% CI = 7.8–14.9 months). b, Kaplan–Meier survival analysis of patients with IDHWT rGBM (n = 32 patients, 33 interventions), IDHmutrAA (grades 3 and 4; n = 4 patients) and IDHmut rAO (grade 3; n = 5 patients). MST, 10.9 months (IDHWTrGBM; 95% CI = 6.9–14.4 months), 5.4 months (IDHmutrAA; 95% CI = 2.6–∞ months) and 39.9 months (IDHmut rAO; 95% CI = 39.9–∞ months). Hazard ratio (HR): IDHmutrAO, 0.07 (95% CI = 0.01–0.49, P = 0.0079, two-sided Cox proportional-hazard test); IDHmutrAA, 1.09 (95% CI = 0.38–3.16, P = 0.87, two-sided Cox proportional-hazard test). c, Kaplan–Meier survival analysis of 31 patients with IDHWT rGBM (32 interventions) by negative (n = 9) or positive (n = 22 patients, 23 interventions) HSV1 serological status after treatment with CAN-3110. MST, HSV1 positive, 14.2 months (95% CI = 9.5–15.7 months); and HSV1 negative, 7.8 months (95% CI = 3.0–∞ months). P = 0.007 (two-sided likelihood ratio test). d, Kaplan–Meier survival analysis of 31 patients with IDHWT rGBM (32 interventions) by negative (n = 24 patients, 25 interventions) or positive (n = 7) HSV2 serological status before treatment with CAN-3110. MST, HSV2 positive, 6.9 months (95% CI = 2.2–∞ months); and HSV2 negative, 11.8 months (95% CI = 8.3–14.5 months). P = 0.9 (two-sided likelihood ratio test). e, Cox proportional-hazard ratio multivariate analyses for independent predictors of survival in patients with IDHWT rGBM after treatment with CAN-3110. The error bars and values in parentheses show the 95% CIs. P values calculated using two-sided Cox proportional-hazard tests are shown on the right for each covariate. The unit of tumour volume is increments of 10 cm3. Partial MGMT promoter methylation was treated as unmethylated. For patients who were administered dexamethasone within the 30 days before or after CAN-3110 treatment, the median dose was 4 mg per day, and the median number of treated days during this time was 14.5 days. KPS, Karnofsky performance score. For c–e, participant 045 was excluded due to non-GBM mortality. PFU, plaque-forming units.
Clinical trials of oncolytic-virus therapy in cancer have not shown that viral serology predicts response19,31. We checked whether HSV1 serology or seroconversion predicted survival in our study. In total, 14 out of 41 patients were seronegative for HSV1 before CAN-3110 treatment, with 4 out of 14 patients seroconverting after (Extended Data Table 3). Given the impact of IDHmut on survival32 and the small number of IDHmutpatients in the study, we focused analyses on the patients with IDHWT rGBM. Notably, HSV1 seropositivity both before and after treatment was associated with significantly longer survival after treatment (P = 0.009 and P = 0.007, respectively) (Extended Data Fig. 5a). In a survival analysis, HSV1-seropositive patients lived a median of 14.2 months (95% CI = 9.5–15.7 months) versus only 7.8 months (95% CI = 3.0–∞ months) for seronegative patients (P = 0.007, likelihood ratio test; Fig. 1c). By contrast, HSV2 serology was not associated with survival (P = 0.9, likelihood ratio test; Fig. 1d). Similarly, the trend towards longer survival for HSV1-seropositive patients was observed in the small number of patients with IDHmutrAA (Extended Data Fig. 5b). Cox proportional hazard analyses in IDHWT rGBMs validated pre-CAN-3110 positive HSV1 serology as a highly significant independent predictor of survival (Fig. 1e). As previously reported, age and tumour volume were also independent survival predictors33,34. These results therefore suggest the importance of an immunological mechanism for the response of patients with IDHWT rGBM to CAN-3110 therapy.
CAN-3110 increases T cells in tumours
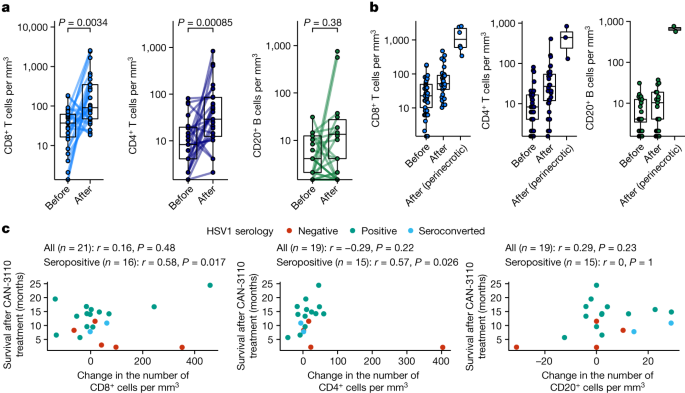
There has been understandable reluctance to routinely collect rHGGs/rGBMs after an experimental therapy as it requires a surgical procedure. Even post-mortem examinations are rarely performed. To determine whether CAN-3110 induced a significant increase in lymphocytes in this lymphocyte-depleted tumour2, we endeavoured to recover as many post-treatment tumours as feasible either by re-resections at suspected progression and/or by post-mortem. Paired tumours from before and various timepoints after CAN-3110 treatment were analysed for a majority of separate rHGGs/rGBMs from patients after CAN-3110 treatment (Supplementary Table 2a–c and Supplementary Methods). In total, all analysed (except one) tumour pairs retained immunohistochemical expression for nestin and nectin-1, one of the major HSV receptors in cells35, both before and after injection (one tumour pair had insufficient material for pre-injection immunohistochemistry analysis) (Extended Data Fig. 6a,b and Supplementary Table 2b). Histological and immunohistochemical analyses showed increases in CD8+ and CD4+ tumour-infiltrating lymphocytes (TILs) in most paired tumours after CAN-3110 treatment (Extended Data Fig. 6c and Supplementary Table 2b). TILs could be visualized in a perivascular distribution, as well as with diffusely scattered cells and occasional clusters throughout the tumour (Extended Data Fig. 6d) and surrounding large areas of tumour necrosis (Extended Data Fig. 6e). Quantitative analyses showed a significant increase in CD4+ (P = 0.00085) and in CD8+ (P = 0.0034) TILs in most analysed paired tumours after CAN-3110 treatment (Fig. 2a and Supplementary Table 2c). There was a non-significant trend in CD20+ B cell increases in almost half of post-treatment samples. The most significant increases in CD8+ and CD4+ T cells were adjacent to perinecrotic areas that were possibly due to CAN-3110 cytotoxicity (Fig. 2b). The observed post-treatment increases in CD8+ and CD4+ T cells were significantly correlated with post-treatment survival in IDHWT rGBMs, but only in HSV1-seropositive patients (r = 0.58, P = 0.017 (CD8+) and r = 0.57, P = 0.026 (CD4+); Fig. 2c). Importantly, the overall quantitative assessments of CD8+, CD4+ and CD20+ TILs used in this analysis were not significantly confounded by the time of tissue collection (Extended Data Fig. 7a–c). Furthermore, longitudinal analyses of patient immune counts over time showed a non-significant trend towards a time-dependent decrease in CD8+ T cell numbers (albeit, without much change in CD4+ or B cells) over several months in HSV1-seronegative patients (Kruskal–Wallis test, P = 0.16; Extended Data Fig. 7d,e) more so than in HSV1-seropositive patients (P = 0.45), suggesting that the immune response induced by CAN-3110 may be durable over long periods of time in the latter. Multiplex immunofluorescence analysis in two of the analysed patients also showed CD68+ macrophage populations (specifically CD68+CD163+ myeloid cells expressing PD-L1) after CAN-3110 treatment, particularly in perinecrotic tumour regions (Extended Data Fig. 7f–i). These results therefore indicate that CAN-3110 induced an increase in TILs that was associated with longer survival in HSV1-seropositive patients but not in HSV1-seronegative patients.
a, Quantification of CD4+ and CD8+ T cells and CD20+ B cells from patients with available paired pre-treatment biopsies and post-treatment tumour samples distal from and/or directly adjacent to the virus injection site. n = 26 patients and 27 interventions (CD8+), and 24 patients and 25 interventions (CD4+ and CD20+). P values were calculated using two-sided Wilcoxon matched-pairs signed-rank tests. b, Quantification of CD8+, CD4+ and CD20+ cells in pre-treatment and post-treatment samples in tumour areas far from the CAN-3110 injection site versus tumour areas near to necrotic foci associated with CAN-3110 injection. For pre-treatment, post-treatment and perinecrotic areas, respectively, n patients (interventions) = 39 (40), 29 (30) and 6 (6) (CD8+); 37 (38), 29 (30) and 3 (CD4+); 36 (37), 29 (30) and 2 (2) (CD20+). c, Correlations between changes in immune counts and post-treatment survival for CD8+ (left), CD4+ (middle) and CD20+ (right) cells in IDHWT rGBMs. Pearson’s correlation coefficient r and P values (two-sided, based on t-distribution) are provided above each plot calculated either using all patients or using only patients who were HSV1 seropositive before or after treatment. When counts were available for multiple post-treatment timepoints for a patient, the timepoint with the highest number of CD4+CD8+ cells was chosen. Importantly, TIL counts were not significantly confounded by the collection timepoint (Extended Data Fig. 7a–c). Patient 045 was excluded from the analyses in c due to early non-GBM mortality. The box plots show the median (centre line), 25th and 75th percentiles (box limits) and up to 1.5× the interquartile range or to the minimum/maximum values (if <1.5 × interquartile range distance from the box) (whiskers).
Persistence is linked to seronegativity
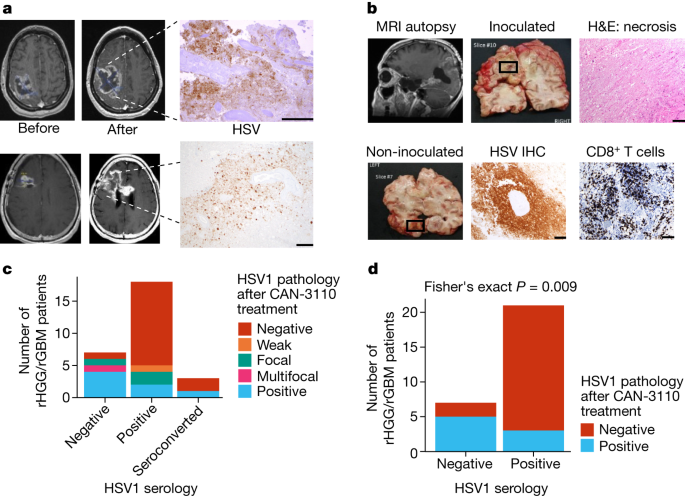
It has been rare to find oncolytic viruses in injected tumours and, even when observed, persistence is limited to a few weeks21. We examined whether the observed immune infiltrates were associated with oHSV persistence in injected tumours. In 12 out of 29 tumours, oHSV antigen was present even several months after CAN-3110 injection (with the longest at 801 days) (Fig. 3a and Supplementary Table 2c). Importantly, in one case of multicentric GBM, a non-injected temporal lesion analysed 8 months after CAN-3110 injection showed positivity for HSV antigen in the absence of antigen detection in the original injected lesion (Fig. 3b). PCR was used to confirm the presence of CAN-3110-specific viral DNA, indicating probable ongoing replication, and spread from the injected lesion to the non-injected tumour (Extended Data Fig. 8). Coupled with the previous findings, these results showed that there was prolonged persistence of CAN-3110 in some patients, with increased CD4+ and CD8+ T cells in injected rHGGs in most participants and evidence of ongoing replication even in a tumour that was not initially injected in a patient with multicentric rGBM.
a, oHSV-positive immunohistochemistry (IHC) images from two participants. Top, magnetic resonance imaging (MRI) images before and 41 days after CAN-3110 injection (106 PFU) from patient 005. oHSV-positive immunohistochemistry was visualized in the large area of tumour necrosis. The area was also positive for oHSV DNA as determined using PCR and positive for ICP22 oHSV transcripts as determined using quantitative PCR with reverse transcription (RT–qPCR; data not shown). Bottom, MRI images from patient 028 before and 253 days after CAN-3110 injection (109 PFU). oHSV-positive immunohistochemistry images were visualized in the area of resected tumour necrosis; the positive status of ICP22 oHSV transcript was determined using RT–qPCR (data not shown). b, Participant 014 had multifocal GBMs in the left temporal and left occipital lobes. The left occipital lobe lesion was injected with 107 PFU of CAN-3110. Post-mortem analyses were performed 252 days after injection. Top left, MRI scan before post-mortem brain collection, with the necrotic injected occipital lesion, shown in the grossly necrotic lesion (top middle), confirmed by histological haematoxylin and eosin (H&E) staining (top right). The CAN-3110 non-injected temporal-lobe post-mortem gross section (bottom left) exhibited oHSV positivity (bottom middle) and dense infiltrates of CD8+ T cells (bottom right). Extended Data Fig. 8 shows that this oHSV-positive focus was CAN-3110 and not reactivated latent wild-type HSV1 from this patient who was otherwise seronegative for HSV throughout the trial. c, HSV1 pathology staining in tumour tissue from patients with rGBM/rHGG (n = 28 interventions, 27 patients) after CAN-3110 treatment relative to HSV1 serological status. d, The same data as in c, but with patients who were initially seropositive grouped with patients who seroconverted after treatment with CAN-3110. Focal/weak pathology staining was grouped with negative staining; and multifocal staining was grouped with positive staining. P values were calculated using two-sided Fisher’s exact tests. For a and b, scale bars, 100 µm.
We examined whether the prolonged persistence of CAN-3110 in injected tumours was associated with HSV1 serological status. Indeed, oHSV persistence was significantly correlated with the absence of HSV1 seropositivity either before or after CAN-3110 treatment (Fig. 3c,d). These findings suggested that oHSV persistence in injected rHGGs/rGBMs may have been due to absence of a robust anti-HSV1 immune response. Coupled with the extended survival for patients with positive HSV1 serology (Fig. 1c), this suggests that tumour clearance of CAN-3110 characterized patients with an improved survival response to CAN-3110.
T cell metrics are linked to survival
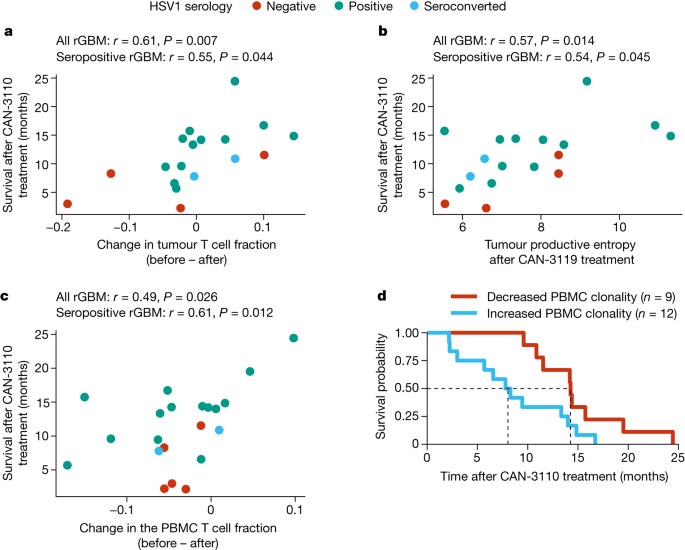
The previous data (Fig. 2c) showed that CAN-3110 elicited an increased number of TILs in post-treatment samples that correlated with patient survival in the HSV1-seropositive patients. To further validate this finding, we examined whether survival was also correlated with changes in T cell clonotype metrics in tumour and/or peripheral blood mononuclear cells (PBMCs). Again, we focused the analyses on the IDHWT rGBM population: out of the 29 paired rHGGs/rGBMs, 21 were IDHWT rGBMs (corresponding to 20 patients). T cell receptor β chain (TCRβ) DNA sequencing (DNA-seq) was performed on tumours and corresponding PBMCs collected at various timepoints after injection (range, 7–349 days). These data were used to calculate changes in the T cell fraction and metrics of TCRβ diversity (productive entropy and productive Simpson clonality; Supplementary Methods). Again, these metrics were not significantly confounded by the collection timepoint (Extended Data Fig. 9a). We found that changes in the tumour T cell fraction (a measure of T cell frequency) after CAN-3110 treatment were positively correlated with prolonged post-treatment survival both in tumours of all of the patients and in tumours of the patients who were HSV1 seropositive (Fig. 4a and Extended Data Fig. 9b). Increased tumour TCRβ diversity (increased entropy/decreased clonality) was associated with prolonged post-treatment survival both in tumours of all of the patients and in tumours of patients who were HSV1 seropositive (Fig. 4b and Extended Data Fig. 9c,d). The same findings were observed for PBMCs (Fig. 4c,d and Extended Data Fig. 9e), suggesting that evolution of a polyclonal T cell response was correlated with survival. Notably, the association between HSV1 serology status and survival was maintained in the subset of patients with IDHWT rGBM for which TCRβ sequencing data were available (Extended Data Fig. 9f). Tumours from patients positive for HSV1 had nominally higher productive entropy (that is, higher TCRβ rearrangement diversity) compared with those from patients negative for HSV1 after (P = 0.070) but not before (P = 0.65) CAN-3110 treatment (Extended Data Fig. 9g), suggesting that TCRβ diversity after CAN-3110 treatment was influenced by positive HSV1 serological status.
a, The correlation between the change in tumour T cell fraction (after versus before CAN-3110 treatment) and survival after CAN-3110 treatment. The T cell fraction is the fraction of nucleated cells that are T cells on the basis of TCRβ DNA-seq analysis (see the ‘Definition of TCR based metrics’ section in the Supplementary Methods). b, The correlation between post-CAN-3110 tumour TCR productive entropy (Supplementary Methods) and survival. A higher entropy indicates a greater diversity of TCRβ rearrangements. n = 18 interventions and 17 patients. For a and b, three participants were excluded (two who survived longer than 1 year, and one who survived less than 1 year) with <200 ng of gDNA. n = 18 interventions and 17 patients. Extended Data Fig. 9b,c shows analyses with all patients, regardless of the amount of gDNA collected. c, The correlation between the change in PBMC TCR clonotype fraction (after versus before CAN-3110 treatment) and survival after CAN-3110 treatment. n = 21 interventions and 20 patients. For a–c, Pearson’s r correlation coefficients and P values (two-sided, based on t-distribution) are shown above the plots. d, Kaplan–Meier survival analysis based on an increase (change > 0) or decrease (change < 0) in PBMC productive Simpson’s clonality (Supplementary Methods) after CAN-3110 treatment. HRincreased = 2.79 (95% CI = 1.08–7.21), P = 0.034 (two-sided Cox proportional-hazard test). Higher clonality indicates a lower diversity of TCRβ rearrangements.
We also performed bulk RNA-seq analysis of a subset of IDHWT rGBMs for which tumours were frozen (to obtain good-quality RNA) and identified transcripts that possessed a V(D)J junction (indicating a T or B cell receptor transcript). The total number of pre-treatment V(D)J transcripts was significantly correlated with post-treatment survival, with a trend towards significance with total post-treatment V(D)J transcript counts (Extended Data Fig. 9h,i), whereas the numbers of unique V(D)J transcripts both before and after treatment were significantly correlated with survival (Extended Data Fig. 9j,k), further validating the association between TCR abundance/diversity and post-treatment survival.
Specific public T cells are linked to survival
We next examined whether there were specific T cell clonotypes that were associated with participant response to therapy. To do this, we focused on public T cell clonotypes36, shared among the 21 IDHWT rGBMs for which we had TCRβ sequencing data. As expected, public TCRβs between patients were relatively rare in PBMCs and even more so in tumours (Extended Data Fig. 10a–f and Supplementary Methods). We found 55 public TCRβ sequences in 21 paired PBMC samples that we could analyse. There were highly significant changes in the frequency of two public PBMC T cell clones that were significantly associated with survival after treatment with CAN-3110: CASSLGGNTEAFF37,38 (Extended Data Fig. 10g; false-discovery rate (FDR) = 0.0035) and CASSSSTDTQYF39 ((Extended Data Fig. 10h; FDR = 0.018). Taken in conjunction, these findings show that survivorship after CAN-3110 treatment in the studied patients was significantly correlated with overall changes in T cell clonotype metrics and changes in the frequency of at least two specific public T cell clonotypes in PBMCs.
Changes in T cell repertoire
Given the little overlap (very few public TCRs) in TIL-specific TCR clonotypes between patients (Extended Data Fig. 10), the relationship between survival after CAN-3110 treatment and TCR clonotype frequency changes could not be meaningfully analysed in TILs. There has been recent interest in analyses of tumour/PBMC T cell clonal repertoire changes as a function of oncologic immunotherapy40. Similarly, we sought to determine whether the tumour/PBMC T cell clonal repertoire changed after treatment with CAN-3110. We found 63 TCRs that were significantly (FDR ≤ 0.05) expanded or depleted in TILs of 11 of the analysed patients with IDHWT rGBM (Supplementary Table 3). If we looked at TCRs that concordantly changed in TILs and PBMCs, four TCRs significantly (FDR ≤ 0.05) expanded and five TCRs were significantly depleted in both TILs and PBMCs (Extended Data Fig. 11a). Of the four expanded TCRs common between TILs and PBMCs, three were from a single patient—patient 021—who was an exceptional responder after CAN-3110 treatment and remained radiologically tumour free for more than 2 years after CAN-3110 treatment before dying due to a non-GBM-related event (Extended Data Fig. 4b and Supplementary Video 1). Notably, all TCRs that concordantly expanded/depleted in both TILs and PBMCs were in longer-surviving patients (Extended Data Fig. 11b), suggesting that defined and concordant PMBC/TIL T cell clonal repertoire changes denoted responses after CAN-3110 treatment. In one participant (previously discussed in Fig. 3b and Extended Data Fig. 8) who remained HSV1 seronegative throughout the trial and was therefore unlikely to have T cell reactivity against HSV1, there were four expanding emergent T cell clonotypes (Extended Data Fig. 11c). This suggested that these were unlikely to be reactive against CAN-3110. When assessing V(D)J gene usage, we also identified a correlation between post-treatment TCRBV09-01*01 (refs. 41,42) usage and survival in HSV1-seropositive patients (Extended Data Fig. 11d; Pearson’s r = 0.00019, FDR = 0.0095). Taken in conjunction, the analyses of T cell clonotypes in tumours revealed that longer-term survivors showed concordance between TIL and PBMC expansion, suggesting that there were alterations in the T cell repertoire after CAN-3110 treatment in the patients who survived for longer. In at least one participant, there was suggestive evidence that tumour TCR expansion was unlikely to be against CAN-3110.
Tumour immune signatures are linked to survival
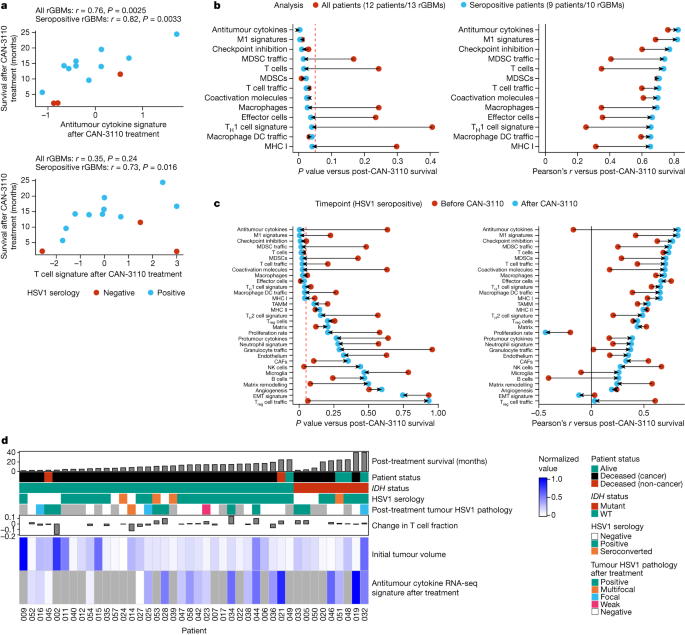
We next queried RNA transcriptomic signatures in paired pre- and post-treatment frozen tumours (to maximize isolation of high-quality RNA) from 14 IDHWTrGBMs (13 patients, 14 interventions). Notably, associations between post-treatment immune signatures and survival were stronger when analysing samples from only HSV1-seropositive patients compared with when analysing samples from all patients (Fig. 5a–c and Extended Data Figs. 12 and 13). In fact, analysis in HSV1-seropositive patients showed 13 post-treatment immune signatures associated with survival (Fig. 5b,c and Extended Data Fig. 13b), whereas, when analyses were conducted with all patients (HSV1 seronegative and seropositive), there were only 7 post-treatment signatures associated with survival (Fig. 5b and Extended Data Fig. 12b). Notably, most of the immune signatures in HSV1-seropositive patients became associated with survival only after treatment with CAN-3110 (Fig. 5c). The time to tumour collection after treatment did not influence the post-treatment signature analyses (Extended Data Fig. 12c). When considered together with other data from this study (Fig. 5d), these results demonstrate that CAN-3110 instigates a highly inflammatory and immunologically activated tumour microenvironment in HSV1 serologically positive patients that persists beyond detectable HSV1 antigen and is significantly correlated with post-treatment survival in a way that is not true of the pretreatment tumour immune state.
A total of 13 paired IDHWTrGBMs with good-quality RNA was analysed by bulk RNA transcriptomics. Transcriptomic signatures for different biological programs were estimated for each sample, and these signatures were assessed for correlation with survival after CAN-3110 treatment either in all patients or only in patients who were HSV1 seropositive before or after CAN-3110 treatment. a, Example of two immune signatures (antitumour cytokine and T cell signatures) that are strongly correlated with survival after CAN-3110 treatment when analysed in HSV1 seropositive patients. Pearson’s r correlation coefficients and P values (two-sided, based on t-distribution) are shown above the plots. Importantly, these signatures did not appear to be significantly confounded by the tissue collection timepoint (Extended Data Fig. 12c). b, The change in Pearson’s correlation P (left) (two-sided, based on t-distribution) and r (right) values when correlations between post-treatment immune signatures and survival were performed in all patients (red points) or in only HSV1-seropositive patients (teal points). Only gene signatures that reached P ≤ 0.05 (dashed red line) in either analysis were plotted. DCs, dendritic cells; MDSCs, myeloid-derived suppressor cells; TH1, T helper 1. c, The change in Pearson’s correlation P (left) (two-sided, based on t-distribution) and r (right) values for pre-treatment (red points) and post-treatment (teal points) samples from HSV1-seropositive patients. This panel includes all of the analysed RNA-seq gene signatures. The dashed red line indicates P = 0.05. CAFs, cancer-associated fibroblasts; EMT, epithelial–mesenchymal transition; NK cells, natural killer cells; TAMM, tumour-associated monocyte/macrophage; Treg cells, regulatory T cells. d, Combined data for all of the patients in the study, including survival after CAN-3110 treatment, HSV1 serology, HSV1 tumour pathology, T cell fraction changes based on TCRβ DNA-seq, initial tumour volumes and bulk RNA-seq-based antitumour cytokine signature scores. The grey boxes indicate missing data. For b and c, HSV1 serology remained unchanged after CAN-3110 treatment for all of the patients, and one patient (045) was omitted from the analysis due to early non-GBM mortality.
Discussion
In this first-in-human clinical trial of CAN-3110, HSV meningitis or encephalitis was not seen, despite ongoing CAN-3110 persistence/replication for several months and maintenance of the ICP34.5 neurovirulence gene. All inflammatory responses remained confined to injected tumours and were not detected in the surrounding brain tissue. This was true in HSV-seropositive and HSV-seronegative patients. Overall, CAN-3110 was well tolerated without dose-limiting toxicities.
A major challenge faced by solid tumour immunotherapy is to create a microenvironment that is favourable for an efficient immune response against cancer cells43. CD8+ cytotoxic and CD4+ helper T cells are important by expressing effector programs against tumour antigens. More recently, public (for example, the same TCR sequence is shared between different individuals) T cell clones, some of which recognize shared viral antigens, have also been shown to traffic into tumours, and their function in cancer immunity is a subject of debate36. In this trial, we analysed a large majority of paired pre- and post-CAN-3110 rHGG/rGBM tumours, with corresponding longitudinal PBMCs to show that (1) pre-existing HSV1-positive serology correlated with individuals who survived the longest after treatment with CAN-3110; (2) CAN-3110 persisted in injected tumours, with almost half of assayed rHGGs still positive even months after a single timepoint injection, but persistence was significantly associated with negative HSV1 serology; and (3) CAN-3110 led to quantitative increases in TILs in a large majority of assayed tumours. Furthermore, we showed for the subpopulation with IDHWT rGBM, for whom there were available paired tumour samples, (4) improved patient survival was correlated with changes in T cell clonotype metrics (elevated T cell clone frequency, increased TCRβ rearrangement diversity, decreased clonality in post-injection versus pre-injection tumours, and transcripts associated with immunological effector programs, particularly in the individuals seropositive for HSV1); and (5) there were changes in specific public peripheral TCR clonotypes significantly associated with survival after CAN-3110 treatment. Taken together, positive HSV1 serology with the observed changes in T cell clonotypes, including public ones, results in a more efficacious immune response, characterizing individuals whose immune system is more ‘fit’ and who can mount a more effective antiviral and possibly antitumour immune response. Note that two of the longest survivors were treated with immune-checkpoint inhibition after their injected tumours were resected (see the swimmer plots of participant 019 and 021 in Extended Data Fig. 3c,d), based on the post-injection finding of extensive TILs. We speculate that CAN-3110 inflamed the TME, possibly improving the efficacy of immune-checkpoint inhibition therapy.
The finding that positive HSV1 serology before or after CAN-3110 treatment was a highly significant independent predictor of response was unexpected based on previously reported trials of other oHSVs16,17,19,31. A recent study showed no correlation between HSV1 serology in humans with GBM and survival44. We speculate that this finding may be specific to oncolytic viruses, based on the capacity of each oncolytic virus to replicate, persist and stimulate an innate and adaptive immune response. It may also be a factor related to sample size, at least for the brain tumour trials, as our trial had more participants. Note that the 22 participants (23 interventions) with IDHWT rGBM who were serologically positive for HSV1 before treatment with CAN-3110 had a mOS of 14.2 months (95% CI = 9.5–15.7 months; Fig. 1c), which is higher than the historical mOS of 6–9 months. Further prospective validation of this discovery in the next phase of planned trials will determine whether HSV1 serology can be used as a selection criterion for the likelihood of response.
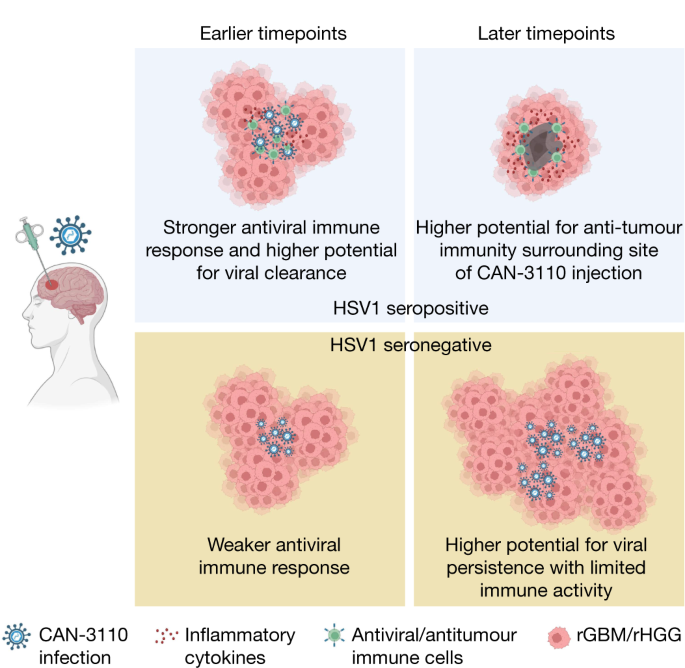
The observation that CAN-3110 was immunohistochemically detected in almost half of the injected tumours several months (and even years in some patients) and even in one uninjected tumour suggests ongoing replication of the agent. Other oncolytic viruses, such as ICP34.5-defective oHSV, have rarely been found in injected human tumours, particularly after several weeks17,19,20,21,31,45,46,47, suggesting that CAN-3110 expression of ICP34.5 may enable persistence. We speculate that this persistence may increase infiltration of virus-specific TCR clones that could initially function in antitumour immunity in a bystander manner36, but could also begin to stimulate T cell responses against tumour antigen. Mouse brain tumour models do show that tumour infiltration of T cells against both tumour and viral antigens correlate with survival48. The significant association of HSV1 seropositivity with the absence of CAN-3110 antigen and transcripts in tumours after injection suggests that an initial humoral and probably adaptive antiviral immune response led to an improved antitumour response based on the survival data and on the finding that there were still increased CD8+ and CD4+ T cells and increased immunological transcriptional programs in tumours despite absent CAN-3110 in the longer-surviving patients (Fig. 6). Identification of the expansion of emergent TCRs, such as those in patient 014 who was seronegative for HSV1 before and after CAN-3110 treatment, possibly suggest that oHSV therapy indeed promotes epitope spreading49, enabling expansion of T cell clones against tumour antigens. Future extensive studies determining whether the TCRs that we discovered in injected tumours react to viral versus tumour antigens are underway (data not shown).
In patients who are seropositive for HSV1, CAN-3110 elicits an initial augmented anti-HSV1 innate and T cell-mediated response (presumably by expansion and differentiation of memory into effector anti-HSV1 T cells) to clear the injected oHSV from tumours. This bystander T cell effect possibly mediates an effective antitumour effect by direct inflammation in the tumour and/or by stimulating ‘antigen spreading’ to also elicit T cell recognition of tumour antigens. In patients who are seronegative for HSV1, the absence of a rapid anti-HSV1 innate and T cell response leads to CAN-3110 replicative persistence with tumour growth overcoming viral-induced cytotoxicity and delayed immune activity against tumour antigens. The figure was generated using BioRender.com.
In summary, single-timepoint intralesional injection of rHGG/rGBM with CAN-3110 enriches the tumour microenvironment with TILs, inducing defined changes in peripheral and tumour T cell repertoires and tumour transcriptomic signatures. These changes are particularly evident in patients who are seropositive for HSV1 and are associated with improved survival in this otherwise therapy-refractory cancer. These findings therefore provide human immunological and biological evidence supporting intralesional oncolytic modalities to convert the immunosuppressive TME characteristic of many solid cancers into a TME that is more favourable to immunologic rejection of the tumour. We are now set to determine whether multiple-timepoint injections lead to further improvements in this therapy (ClinicalTrials.gov: NCT03152318).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Patient responses, demographic information and safety outcomes, as well IHC quantifications and RNA-seq gene signature scores are available within the Article and its Supplementary Information. Raw RNA-seq and TCRβ DNA-seq files have been deposited in a controlled-access repository in the Database of Genotypes and Phenotypes (http://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs003378.v1.p1). Source data are provided with this paper.
Code availability
Custom code used to perform analyses in this study have been deposited at OSF (https://doi.org/10.17605/OSF.IO/YBCG7).
Change history
03 November 2023
An amendment to the underlying article code was made to enable an author name to appear correctly in PubMed. In addition, a hyphen was missing in the data availability link, which has now been updated.
References
Chuntova, P. et al. Unique challenges for glioblastoma immunotherapy-discussions across neuro-oncology and non-neuro-oncology experts in cancer immunology. Meeting report from the 2019 SNO Immuno-Oncology Think Tank. Neuro Oncol. 23, 356–375 (2021).
Thorsson, V. et al. The immune landscape of cancer. Immunity 48, 812–830 (2018).
Chiocca, E. A., Nakashima, H., Kasai, K., Fernandez, S. A. & Oglesbee, M. Preclinical toxicology of rQNestin34.5v.2: an oncolytic herpes virus with transcriptional regulation of the ICP34.5 neurovirulence gene. Mol. Ther. Methods Clin. Dev. 17, 871–893 (2020).
Kambara, H., Okano, H., Chiocca, E. A. & Saeki, Y. An oncolytic HSV-1 mutant expressing ICP34.5 under control of a nestin promoter increases survival of animals even when symptomatic from a brain tumor. Cancer Res. 65, 2832–2839 (2005).
Louis, D. N. et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 23, 1231–1251 (2021).
Wen, P. Y. & Packer, R. J. The 2021 WHO classification of tumors of the central nervous system: clinical implications. Neuro Oncol. 23, 1215–1217 (2021).
Vargas Lopez, A. J. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 23, 502–503 (2021).
Taslimi, S., Ye, V. C., Wen, P. Y. & Zadeh, G. Lessons learned from contemporary glioblastoma randomized clinical trials through systematic review and network meta-analysis: part 2 recurrent glioblastoma. Neurooncol. Adv. 3, vdab029 (2021).
Macedo, N., Miller, D. M., Haq, R. & Kaufman, H. L. Clinical landscape of oncolytic virus research in 2020. J. Immunother. Cancer 8, e001486 (2020).
Martin, N. T. & Bell, J. C. Oncolytic virus combination therapy: killing one bird with two stones. Mol. Ther. 26, 1414–1422 (2018).
Peruzzi, P. & Chiocca, E. A. Viruses in cancer therapy—from benchwarmers to quarterbacks. Nat. Rev. Clin. Oncol. 15, 657–658 (2018).
Russell, L. & Peng, K. W. The emerging role of oncolytic virus therapy against cancer. Chin. Clin. Oncol. 7, 16 (2018).
Totsch, S. K. et al. Oncolytic herpes simplex virus immunotherapy for brain tumors: current pitfalls and emerging strategies to overcome therapeutic resistance. Oncogene 38, 6159–6171 (2019).
Harrington, K. J. et al. Clinical development of talimogene laherparepvec (T-VEC): a modified herpes simplex virus type-1-derived oncolytic immunotherapy. Expert Rev. Anticancer Ther. 15, 1389–1403 (2015).
Christie, J. D. & Chiocca, E. A. Treat and repeat: oncolytic virus therapy for brain cancer. Nat. Med. 28, 1540–1542 (2022).
Todo, T., Ino, Y., Ohtsu, H., Shibahara, J. & Tanaka, M. A phase I/II study of triple-mutated oncolytic herpes virus G47 in patients with progressive glioblastoma. Nat. Commun. 13, 4119 (2022).
Todo, T. et al. Intratumoral oncolytic herpes virus G47 for residual or recurrent glioblastoma: a phase 2 trial. Nat. Med. 28, 1630–1639 (2022).
Desjardins, A. et al. Recurrent glioblastoma treated with recombinant poliovirus. N. Engl. J. Med. 379, 150–161 (2018).
Gallego Perez-Larraya, J. et al. Oncolytic DNX-2401 virus for pediatric diffuse intrinsic pontine glioma. N. Engl. J. Med. 386, 2471–2481 (2022).
Friedman, G. K. et al. Oncolytic HSV-1 G207 immunovirotherapy for pediatric high-grade gliomas. N. Engl. J. Med. 384, 1613–1622 (2021).
Lang, F. F. et al. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J. Clin. Oncol. 36, 1419–1427 (2018).
Fares, J. et al. Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: a first-in-human, phase 1, dose-escalation trial. Lancet Oncol. 22, 1103–1114 (2021).
van Putten, E. H. P. et al. Convection enhanced delivery of the oncolytic adenovirus Delta24-RGD in patients with recurrent GBM: a phase I clinical trial including correlative studies. Clin. Cancer Res. 28, 1572–1585 (2022).
Kanai, R. et al. Effect of γ34.5 deletions on oncolytic herpes simplex virus activity in brain tumors. J. Virol. 86, 4420–4431 (2012).
Peters, C. et al. Restriction of replication of oncolytic herpes simplex virus with a deletion of γ34.5 in glioblastoma stem-like cells. J. Virol. https://doi.org/10.1128/JVI.00246-18 (2018).
Orvedahl, A. et al. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe 1, 23–35 (2007).
Aghi, M., Visted, T., Depinho, R. A. & Chiocca, E. A. Oncolytic herpes virus with defective ICP6 specifically replicates in quiescent cells with homozygous genetic mutations in p16. Oncogene 27, 4249–4254 (2008).
Fyllingen, E. H. et al. Survival of glioblastoma in relation to tumor location: a statistical tumor atlas of a population-based cohort. Acta Neurochir. 163, 1895–1905 (2021).
Lasocki, A., Gaillard, F., Tacey, M., Drummond, K. & Stuckey, S. Multifocal and multicentric glioblastoma: Improved characterisation with FLAIR imaging and prognostic implications. J. Clin. Neurosci. 31, 92–98 (2016).
Li, Y. et al. A systematic review of multifocal and multicentric glioblastoma. J. Clin. Neurosci. 83, 71–76 (2021).
Andtbacka, R. H. et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 33, 2780–2788 (2015).
Yan, H. et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 360, 765–773 (2009).
Ohgaki, H. & Kleihues, P. Epidemiology and etiology of gliomas. Acta Neuropathol. 109, 93–108 (2005).
Keles, G. E., Lamborn, K. R., Chang, S. M., Prados, M. D. & Berger, M. S. Volume of residual disease as a predictor of outcome in adult patients with recurrent supratentorial glioblastomas multiforme who are undergoing chemotherapy. J. Neurosurg. 100, 41–46 (2004).
Huang, Y. et al. Receptors and ligands for herpes simplex viruses: novel insights for drug targeting. Drug Discov. Today 27, 185–195 (2022).
Meier, S. L., Satpathy, A. T. & Wells, D. K. Bystander T cells in cancer immunology and therapy. Nat. Cancer 3, 143–155 (2022).
Massa, C. et al. Identification of patient-specific and tumor-shared T cell receptor sequences in renal cell carcinoma patients. Oncotarget 8, 21212–21228 (2017).
Werner, L. et al. Alterations in T and B cell receptor repertoires patterns in patients with IL10 signaling defects and history of infantile-onset IBD. Front. Immunol. 11, 109 (2020).
Tickotsky, N., Sagiv, T., Prilusky, J., Shifrut, E. & Friedman, N. McPAS-TCR: a manually curated catalogue of pathology-associated T cell receptor sequences. Bioinformatics 33, 2924–2929 (2017).
Luoma, A. M. et al. Tissue-resident memory and circulating T cells are early responders to pre-surgical cancer immunotherapy. Cell 185, 2918–2935 (2022).
Held, K. et al. αβ T-cell receptors from multiple sclerosis brain lesions show MAIT cell-related features. Neurol. Neuroimmunol. Neuroinflamm. 2, e107 (2015).
Savola, P. et al. Somatic mutations in clonally expanded cytotoxic T lymphocytes in patients with newly diagnosed rheumatoid arthritis. Nat. Commun. 8, 15869 (2017).
Tomaszewski, W., Sanchez-Perez, L., Gajewski, T. F. & Sampson, J. H. Brain tumor microenvironment and host state: implications for immunotherapy. Clin. Cancer Res. 25, 4202–4210 (2019).
Guerra, G. et al. Antibodies to varicella-zoster virus and three other herpesviruses and survival in adults with glioma. Neuro Oncol. https://doi.org/10.1093/neuonc/noac283 (2023).
Harrow, S. et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 11, 1648–1658 (2004).
Markert, J. M. et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol. Ther. 17, 199–207 (2009).
Ribas, A. et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 170, 1109–1119 (2017).
Alayo, Q. A. et al. Glioblastoma infiltration of both tumor- and virus-antigen specific cytotoxic T cells correlates with experimental virotherapy responses. Sci. Rep. 10, 5095 (2020).
Hu, Z. et al. Personal neoantigen vaccines induce persistent memory T cell responses and epitope spreading in patients with melanoma. Nat. Med. 27, 515–525 (2021).
Acknowledgements
We thank all of the patients and families who participated in the trial, particularly participant 021 and her family. We acknowledge assistance from S. Ventz, G. Park, L. M. Seften, K. Hoe Chow, A. Shetty, W. Pisano, E. Lapinskas and F. Watkinson; D. Hamm and B. Banbury for assistance in TCR analyses; and N. Caffo, J. Dwyer, D. Lane and H. White for assistance.
Author information
Authors and Affiliations
Contributions
Conceptualization: E.A.C. Methodology: A.L.L., I.H.S., A.M.L., H.N., J.K.W., A. Santos, N. Masud, G.F., X.M., A.S.Y., J.G., A.Z., J.D.B., E. Torio, H.I., J.L., N. Shono, M.O.N., P.H., R.P., H.S., B.S., W.L.B., P.P., E.C., S.W.M., M.C.P., Y.Z., A.C., S.J.R., K.K., E. Tikhonova, N. Miheecheva, D. Tabakov, N. Shin, A.G., A. Shumskiy, F.F., E.A.-C., L.K.A., J.W., D. Krisky, A.M., C.M., P.P.T., F.B., D. Kovarsky, I.T., M.L.S., K.W.W., K.L., D.A.R. and E.A.C. Investigation: A.L.L., I.H.S., A.M.L., H.N., J.K.W., A. Santos, N. Masud, G.F., X.M., A.S.Y., J.G., A.Z., J.D.B., E. Torio, H.I., J.L., N. Shono, M.O.N., D. Triggs, P.H., R.P., H.S., B.S., W.L.B., P.P., S.W.M., M.C.P., Y.Z., A.C., S.J.R., P.Y.W., E.Q.L., L.N., U.C., L.N.G.C., S.D.D., T.B., K.K., E. Tikhonova, N. Miheecheva, D. Tabakov, N. Shin, A.G., A. Shumskiy, F.F., E.A.-C., L.K.A., D. Krisky, J.W., A.M., C.M., P.P.T., F.B., D. Kovarsky, I.T., M.L.S., K.W.W., K.L., D.A.R. and E.A.C. Visualization: A.L.L., I.H.S., A.M.L., H.N., A. Santos, G.F., X.M., N. Shono, S.W.M., M.C.P., E. Tikhonova, N. Miheecheva, D. Tabakov, N. Shin, A.G., A. Shumskiy, F.F., F.B., K.L. and E.A.C. Funding acquisition: A.L.L., J.K.W., N. Shono, E.A.-C., P.P.T., M.L.S., K.W.W., K.L., D.A.R. and E.A.C. Project administration: H.N., S.J.R., P.Y.W., T.B., N. Shin, E.A.-C., P.P.T., F.B., I.T., M.L.S., K.W.W., K.L., D.A.R. and E.A.C. Supervision: I.H.S., H.N., G.F., X.M., S.J.R., P.Y.W., T.B., N. Shin, P.P.T., F.B., E.A.-C., L.K.A., M.L.S., K.W.W., I.T., K.L., D.A.R. and E.A.C. Writing—original draft: A.L.L. and E.A.C. Writing—review and editing: A.L.L., I.H.S., A.M.L., H.N., J.K.W., G.F., X.M., J.D.B., M.C.P., P.Y.W., L.N.G.C., N. Shin, E.A.-C., L.K.A., P.P.T., F.B., D. Kovarsky, M.L.S., K.W.W., D.A.R. and E.A.C.
Corresponding author
Ethics declarations
Competing interests
J.D.B. has an equity position in Treovir, an oHSV clinical stage company and is a member of the POCKiT Diagnostics board of scientific advisors; and has a provisional patent (application number 63/273,577) entitled ‘Methods and formulations related to the intrathecal delivery of oncolytic viruses’. W.L.B. consulted for Stryker. P.P. is cofounder and member of the board of directors of Ternalys Therapeutics; he is also named as an inventor on patents related to non-coding RNA technology. P.Y.W. received research support from AstraZeneca/Medimmune, Beigene, Celgene, Chimerix, Eli Lily, Genentech/Roche, Kazia, MediciNova, Merck, Novartis, Nuvation Bio, Puma, Servier, Vascular Biogenics and VBI Vaccines and served on advisory boards for AstraZeneca, Bayer, Black Diamond, Boehringer Ingelheim, Boston Pharmaceuticals, Celularity, Chimerix, Genenta, GlaxoSmithKline, Karyopharm, Merck, Mundipharma, Novartis, Novocure, Nuvation Bio, Prelude Therapeutics, Sapience, Servier, Sagimet, Vascular Biogenics and VBI Vaccines, and on data safety monitoring committees for Day One Bio and Novocure. L.N. serves as a consultant for Ono and Brave Bio. L.N.G.C. received research support from Merck (to the Dana-Farber Cancer Institute); he also has received research support from the NIH, the American Society of Clinical Oncology and the Robert Wood-Johnson Foundation. E.Q.L. receives royalties from Wolter Kluwer (Up to Date) and consulting fees from GCAR. T.B. receives clinical trial support from ONO Pharmaceuticals, publishing royalties from UpToDate and Oxford University Press. S.J.R. receives research support from Bristol Myers-Squibb and KITE/Gilead; and is on the scientific advisory board for Immunitas Therapeutics. K.K. is employed by and owns equity in Clearpoint. E. Tikhonova, N. Miheecheva, D. Tabakov, N. Shin, A.G., A. Shumskiy and F.F. are employed by BostonGene and have equity options in BostonGene. E.A.-C. is a founder, board member of and holds equity in Candel Therapeutics. L.K.A. is co-founder and holds equity in Candel Therapeutics. D. Krisky, J.W., A.M., C.M., P.P.T. and F.B. are employees of and hold equity in Candel Therapeutics. I.T. is an advisory board member of Immunitas Therapeutics. M.L.S. and K.W.W. are equity holders, scientific co-founders and advisory board members of Immunitas Therapeutics. D.A.R. is an advisor to Agios, AnHeart Therapeutics, Avita Biomedical, Blue Rock Therapeutics, Bristol Myers Squibb, Boston Biomedical, CureVac, Del Mar Pharma, DNAtrix, Hoffman-LaRoche, Imvax, Janssen, Kiyatec, Medicenna Therapeutics, Neuvogen, Novartis, Novocure, Pyramid, Sumitomo Dainippon Pharma, Vivacitas Oncology and Y-mabs Therapeutics. E.A.C. is an advisor to Amacathera, Bionaut Labs, Genenta, Insightec, DNAtrix, Seneca Therapeutics and Theravir; he has equity options in Bionaut Laboratories, DNAtrix, Immunomic Therapeutics, Seneca Therapeutics and Ternalys Therapeutics; he is co-founder and on the board of directors of Ternalys Therapeutics. Patents related to oHSV and CAN-3110 are under the possession of Brigham and Women’s Hospital with E.A.C. and H.N. named as co-inventors. These patents have been licensed to Candel Therapeutics. Present and future milestone license fees and future royalty fees are distributed to Brigham and Women’s Hospital from Candel. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 (related to Sub-heading, Safety of CAN-3110 in patients with rHGG/rGBM) Clinical trial design and treatment strategy.
(a) Dose-escalation schema- Subjects with a previous diagnosis of rHGG (Glioblastoma, Grade IV or III astrocytoma or anaplastic astrocytoma, grade III anaplastic oligodendroglioma, including molecular grading with or without a mutation in IDH and with or without hypermethylation of the MGMT promoter) were eligible for the trial. At the time of stereotactic biopsy, the neuropathologist had to confirm that there was histologic evidence consistent with glioma to exclude inclusion of subjects with radiation necrosis and/or infection. The first 9 cohorts of subjects underwent one stereotactic inoculation of CAN-3110 at a tumour site selected to be different from the antecedent biopsy site (to avoid blood contamination of the injectate) in a 3 + 3 dose-escalation design, starting from 106 pfus up to 1010 pfus in half-log increments. The biopsy and injections for each subject were carried out in an intraoperative MRI to visualize injections in gadolinium-enhancing tumour. The volume of injectate was 1 ml delivered over 5 min using the SmartFlow cannula (ClearPoint Neuro, Inc.) that minimizes reflux. When all 30 subjects in the first 9 cohorts were treated (September 2017- February 2020) without a dose limiting toxicity, the protocol was amended to include a tenth cohort of 12 subjects, where up to 5 regions of tumour were injected with a dose of 109 pfus divided into 1 to 5 mls based on tumour diameter (e.g., for each mm of tumour diameter, 1 ml of CAN-3110 was injected). No DLTs were encountered. Subjects in cohort 10 were accrued from June, 2020 until January, 2021. (b-c) Representative intraoperative MRIs during (b) and after (c) CAN-3110 injection. b: Subject 021 was positioned prone in the intraoperative MRI. The SmartFlow cannula is shown in the occipital area penetrating the skull through a drilled burr hole (white arrow). The T2 dark area shows the needle trajectory through the occipital and temporal lobe to reach the area of rGBM where the tip of the needle (yellow arrowhead) is placed for injection. The gadolinium-enhanced tumour was manually overlaid with purple colour. c: Representative intraoperative MRI (subject 002) showing the view from the intraoperative console after injection of CAN-3110 (106 pfus/1 ml). The T1 dark injectate is indicated by the blue and yellow cross-hatch, with the red dot showing where the tip of injection needle was after injection and needle removal, showing persistence of the injectate at site of injection with minimal reflux. The rGBM consisted of a bifrontal mass and the needle was inserted from the frontal vertex to reach an area of gadolinium-enhancement located in the inferior frontal lobe. The 3 images shown in the console are from the same brain section in coronal, sagittal and axial planes.
Extended Data Fig. 2 (related to Sub-heading, Safety of CAN-3110 in patients with rHGG/rGBM) Genomic alterations in 34/41 subjects (42 separate interventions) in the CAN-3110 clinical trial.
34 rHGG/rGBM specimens underwent exome sequencing for the 18 genes shown on the left of the panel (PTEN, EGFR, etc). On the right side of the panel, the percentage of tumours expressing each genetic mutation is listed together with colour coded relative frequencies of specific types of genomic alteration (amplification, gain, etc). The top of the map displays a bar graph representation of tumour mutational burden (limited to these 18 genes), as well as indications of age, trial cohort, diagnosis at time of injection, and MGMT promoter methylation status for each patient included. To the far right of the figure, colour coding legends indicate designations for different types of genomic alteration, trial cohort, diagnosis, and MGMT promoter methylation status.
Extended Data Fig. 3 (related to Sub-Heading, HSV1 serology predicts efficacy). Trial Survival Outcomes.
(a,b) Progression-free survival for the entire rHGG/rGBM group (a) and the 3 rHGG sub-groups divided by rGBM IDHwt, rAA IDHmut, and rAO IDH mutant (b), based on the 2021 WHO classification of central nervous system tumours5. (a) Kaplan Meier progression free survival (PFS) for all 41 subjects (42 interventions). Shaded region = 95% confidence interval of KM estimate of survival probability. The data is mature as of October 2022. The median PFS was 1.9 months (95% CI: 1.6 – 4.5). (b) Kaplan Meier PFS curves for 41 subjects, divided into rGBM IDH wild type (n = 32 subjects, 33 interventions), rAA IDH mutant (n = 4), and rAO IDH mutant (n = 5). The median PFS were 1.9 (95% CI: 1.6 – 4.6), 0.9 (95% CI: 0.5 - Inf) and 9.0 months (95% CI: 4.5 - Inf), respectively. The PFS HR for rAO IDH mut was 0.30 (95% CI, 0.10 – 0.86, p = 0.026 (CoxPH, 2-sided)) and PFS HR for rAA IDHmut was 2.63 (95% CI: 0.91 – 7.65, CoxPH p = 0.075 (CoxPH, 2-sided)). (c, d) Swimmer plots for Cohorts 1-9 (i.e., I-IX) (c) and Cohort 10 (i.e., X) (d). All 41 subjects’ (42 interventions, with subject 042/054 treated twice, 6 months apart) clinical course since day 0 CAN-3110 injection time is shown. Cohort number and CAN-3110 dose are indicated on the far left, with next column showing each subject clinical trial ID. Months after CAN-3110 injection is shown below. After CAN-3110 injection, subjects were followed and when there was MRI evidence of progression or pseudoprogression with or without clinical deterioration, additional treatments were instituted including craniotomy and/or biopsy. All instituted treatments (bevacizumab, immune checkpoint inhibitors, carboplatin, temozolomide, reirradiation, lomustine, LITT, targeted inhibitors) have shown no benefit in this setting in advanced trials and were used for palliative purposes. Post-CAN-3110 treatments are shown in colour coding on the far right and time/duration of treatment is overlaid on the swimmer plot for each subject. Below each bar, colour coding for dexamethasone dosing and duration is shown for each subject. As of September 2022, there are 4 surviving subjects (032, 048, 049, 051), 3 of which are IDH mutant anaplastic oligodendroglioma (1p/19q co-deleted) and one is IDH wt GBM (049).
Extended Data Fig. 4 (related to Sub-Heading, HSV1 serology predicts efficacy) MRI imaging responses to CAN 3110.
(a) Complete response in a multifocal GBM subject. Subject 007 (56 year old caucasian man, IDHwt GBM) had an initial right frontal GBM resected. After completion of standard of care radiochemotherapy, the right frontal lesion grew back and a second new lesion posterior and periventricular also appeared (Pre-operative MRI). The subject underwent injection of CAN-3110 (106 pfus in 1 ml) solely into the second new lesion (indicated by yellow arrow in MRI-guided CAN-3110 injection label). Serial MRIs on day 56, 111, 168, 220, 224 and 282 are shown. No other treatments and no dexamethasone were administered during this time, during which the patient experienced full time employment, travel and enjoyment from significant family events. At the 349 day mark, a new separate biopsy-proven recurrence in the right basal ganglia leading to a progressive hemiparesis and hemiplegia prompted the subject to seek hospice care and eventual demise. (b) Durable response in a right temporal GBM subject. Subject 021 (61 year old caucasian female, IDHwt GBM) had an initial GBM diagnosed 262 days (-d262) before CAN-3110 injection. After craniotomy and tumour resection (-d259), she underwent standard chemoradiation and then treatment with temozolomide for IDHwt GBM with methylated MGMT promoter. The tumour recurred (-d47) and she underwent a second subtotal resection (-d30), but because of visible rapid progression (-d14), she was enrolled in the CAN-3110 trial. On d0, she received single injection of 108 pfus (the MRI-compatible injection needle is indicated by the yellow arrow). On d91, MRI appeared to show progression and she was brought back to surgery for resection of the mass with postoperative MRI showing a gross total resection (d96). Histology and immunohistochemical staining showed a mixture of CD8+, CD4+, CD20+ lymphocytes and tumour (see Extended Data Fig. 6d, panels labelled with #21). The subject then remained tumour free for the next 630 days (d630), which was the time of her last MRI. Unfortunately, she passed as the passenger of a motor vehicle accident on d717. The subject’s personal story is shared in the supplementary video 1 with consent of her family.
Extended Data Fig. 5 (related to Sub-Heading, HSV1 serology predicts efficacy).
(a) Comparative analyses of differential survival based on HSV1 pre-operative (left panel) and post-operative (right panel) serology for the 31 rGBM IDHwt subjects (32 interventions). P value from two-tailed Student’s t-test. (b) Kaplan-Meier survival curves for IDH mutant patients as divided by pre-treatment HSV1 serological status. Median OS months with 95% confidence intervals = rAA HSV1−: 3.5 [2.6 - Inf]; rAA HSV1+: 22.5 [-Inf - Inf]; rAO HSV1−: not reached; rAO HSV1+: 39.9 [20.1 - Inf]. Boxplot centre = median, box bounds = 25th and 75th percentiles, whisker length = up to 1.5x inter-quartile range (IQR) or to minima/maxima (if <1.5x IQR distance from box).
Extended Data Fig. 6 (related to Sub-heading, CAN-3110 increases T cells in tumours.) Representative immunohistochemistry (IHC) Images.
(a) upper panel: Nestin IHC was carried out in a rGBM (IDHwt) resected 279 days after CAN-3110 injection (Subject 044, 1010 pfus in 1 ml); lower panel: Nectin-1/CD111 was carried out in a rGBM (IDHwt) resected 253 days after CAN-3110 (Subject 028, 109 pfu in 1 ml). (b) Time course of Nestin and Nectin-1 IHC. Subject 046 (IDHmut astrocytoma) was injected on day 0. Because of an SAE consisting of multiple seizures 2 days after injection (Table 1b), he was treated with antivirals with resolution of the event. He was then brought back for re-resection twice showing both times persistence of both nestin and nectin-1 expression with tumour progression. Non tumour brain shows nestin expression peri-vascularly with no nectin-1 expression. (c) Representative example of CD8+ T cell immunohistochemistry (IHC) from Pre- and Post-CAN-3110 injected tumour. Subject 016 was injected with 3 × 107 PFUs of CAN-3110 and post-injection tumour was resected 24 days after injection due to MRI evidence of continued progression. Areas shown were relatively far from the area of injection. (d) Representative examples of perivascular CD3+ T cell accumulation from 3 subjects (016, 019, 021) after CAN-3110 injection. Dose and time of post-injection tumour harvest are indicated for each. (e) Representative examples of perinecrotic accumulation of CD20+ B and CD8+ and CD4+ T cells from subject 034. Zoomed images of regions outlined in red are shown in bottom right corner of respective plots. Scale bars are 25 µm (panel c), 100 µm (panels a, b, and d), and 500 µm (panel e).
Extended Data Fig. 7 (related to Sub-heading, CAN-3110 increases T cells in tumours). Quantitative IHC.
IHC was performed by computer aided quantification of IHC stains (e.g., CD4, CD8, CD20) using slides scanned at 40X magnification using the Hamamatsu Nanozoomer S210. Using the Halo Image Analysis Sofware (PerkinElmer), 3 square regions of interest (approximately 160,000 mm2 each) are averaged for each case in in areas of tumour and in uninvolved/reactive brain tissue if present, and quantities are normalized by tissue area (mm2). (a-c) Pathological assessments of CD8+, CD4+, and CD20+ TILs are not systematically confounded by collection timepoint. Post-treatment IHC based counts of CD8+ (a), CD4+ (b), and CD20+ (c) TILs plotted versus the time of post-treatment tissue collection for the same IDHwt rGBM patients plotted in Fig. 2c (note that patient 045 was excluded due to early non-GBM mortality). Pearson’s correlation coefficient r and p values (2-sided, based on t-distribution) are provided above each plot calculated either using all patients or using only patients which were HSV1 seropositive before or after treatment. When counts were available for multiple post-treatment timepoints for a patient, the timepoint with the highest number of CD4+ or CD8+ or CD20+ cells were chosen. (d,e) Quantitative IHC for CD8+, CD4+ T cells and CD20+ B cells for each patient as a function of time. For each subject, the number of CD8+, CD4+ T and CD20+ B cells/mm2 are plotted as a function of time after CAN-3110 (i.e., when tumours underwent re-resection(s) and/or postmortem analyses after CAN-3110). Note that perinecrotic counts are not included here as they were only available for a few patients. n patients = 41. Kruskal-Wallis p = 0.33 (all patients), p = 0.16 (HSV1 seronegative patients), p = 0.45 (HSV1 seropositive/seroconverted patients) for CD8+. In (e), the same data is shown as in panel d but restricted to patients that have >1 post-treatment sample available (i.e., underwent more than 1 resection or had a resection and then also a postmortem analysis). n patients = 8. Kruskal-Wallis p = 0.39 (all patients), p = 0.30 (HSV1 seronegative patients), p = 0.44 (HSV1 seropositive/seroconverted patients) for CD8+. (f-i) Multiplex fluorescent imaging (mIF) for myeloid cell populations in pre- and post-CAN-3110 rGBM IDHwt. Each 20x region of interest (ROI) is plotted as a black dot. The overlaying bar graph is the mean of the ROIs and the error bars represent the standard deviation. For panels g and i, the values represent the percentage of the macrophage populations (CD68+ for panel g and CD68+ CD163+ for panel i) that are positive for PD-L1 expression. For panel h, the values are the cell density, or number of positive cells per mm2 of CD68+ CD163+ double positive cells. (f) Representative mIF images of post-treatment sample with quantified ROIs for comparison of solid tumour area and perinecrotic viral antigen positive tumour areas. Two subjects with pre-post-treatment pairs were examined (subjects 044 and 028). Scale bar = 50 μm. (g) Quantification of PD-L1 expression on total macrophage/microglial population in tumour near necrotic positive CAN-3110 region. (044: n = 3 ROIs (pre), 3 ROIs (Tumour), 6 ROIs (Necrotic); 028: n = 6 ROIs (pre), 3 ROIs (Tumour), 5 ROIs (Necrotic)) (h) post-treatment samples with CD163+ myeloid populations in both tumour and tumour-necrotic interface regions. Pre-treatment values are also shown. (044: n = 3 ROIs (pre), 3 ROIs (Tumour), 5 ROIs (Necrotic); 028: n = 6 ROIs (pre), 3 ROIs (Tumour), 5 ROIs (Necrotic)) (i) PD-L1 expression in CD163+ populations in perinecrotic interface regions. Pre-treatment values are also shown. DAPI blue nuclei enumeration, SOX2 white tumour nuclei enumeration, CD68 yellow pan-macrophage/microglia, CD163 orange macrophage/microglia. (044: n = 3 ROIs (pre), 3 ROIs (Tumour), 5 ROIs (Necrotic); 028: n = 6 ROIs (pre), 3 ROIs (Tumour), 5 ROIs (Necrotic)).
Extended Data Fig. 8 (Related to Sub-heading, Persistence is linked to seronegativity).Presence of CAN-3110 DNA in un-injected temporal lobe lesion of subject in Fig. 3b (8 months from injection in occipital lesion).
(a) Schematic of CAN-3110 viral genomic DNA showing the location of primers for Nestin-Hsp68 relative to other transcriptional cassette elements. (b) Gel electrophoresis of the PCR products from genomic DNA extracted from FFPE of postmortem brain from subject 014. Primers for Nestin-Hsp68 amplified a PCR product of ~112 bps within the promoter/enhancer transcriptional cassette. WT HSV represents a negative control with viral genomic DNA from HSV1 strain 17+. CAN-3110 represents a positive control with CAN-3110 viral genomic DNA amplifying a strong band of ~112 bp in the Nestin-Hsp68 promoter PCR reaction. BA-19-036 is the temporal lobe tumour that was not injected with CAN-3110 but was IHC-positive for HSV antigen (see Fig. 3b). The uncropped version of this gel is shown in Supplementary Fig. 1. Each PCR reaction was run in triplicate. (c) Sequencing reactions of CAN-3110 control and BA-19-036 PCR products show sequence homology with the original CAN-3110 sequence map.
Extended Data Fig. 9 (Related to Sub-Heading, T cell metrics are linked to survival). TCR clonotype analyses for IDHwt rGBM patients.
(a) For the TCRβ DNAseq analysis of IDHwt patients (n = 21 interventions, 20 patients), Tumour T-cell fraction (left), productive T-cell entropy (middle), and productive T-cell Simpson Clonality (right) are not correlated with time from CAN-3110 treatment to tissue collection. (b) The change in tumour T cell fraction (Post minus Pre-CAN-3110) from all 21 interventions was analysed as a function of subject survival after CAN-3110 in all subjects and in the HSV seropositive ones. Unlike Fig. 4a, no gDNA filter was applied. (c) The post-CAN-3110 tumour productive entropy for the same 21 interventions is analysed as a function of subject survival after CAN-3110. Unlike Fig. 4b, no gDNA filter was applied. (d) Differences in Tumour Productive Simpson Clonality Indices in T cell TCRs from paired rGBM (n = 21 interventions, 20 patients) in the LS (> 1-year post-treatment survival) vs. SS (< 1-year post-treatment survival) patients as a function of CAN-3110 treatment. P value calculated using a Wilcoxon matched pairs signed rank test. (e) The change in productive entropy in TCRs from PBMCs for the same 21 tumour pairs is analysed as a function of subject survival after CAN-3110. Note that all panels omit patient 045 who had an early non-GBM mortality. (f) Comparison of post CAN-3110 survival time between pre- (left panel) or post- (right panel) CAN-3110 HSV1 serology positive (PRE: n = 23 interventions, 22 patients, POST: n = 26 interventions, 25 patients) and negative (PRE: n = 9 patients, POST: n = 6 patients) rGBM IDHwt patients for whom paired samples were available (e.g., for samples analysed in other panels in this figure). P values shown from 2-tailed Student’s t-test. (g) Boxplot comparisons of tumour productive entropy values as grouped by timepoint (PRE or POST CAN-3110 treatment) and pre-CAN-3110 HSV1 serology status (negative or positive). P values calculated using two-tailed Student’s t-test. Sample sizes for each group are as follows: 1) HSV1- POST = 7 patients; 2) HSV1 + POST = 14 interventions, 13 patients; 3) HSV1- PRE = 7 patients; 4) HSV1 + PRE = 14 interventions, 13 patients. Note that all panels omit patient 045 due to early non-GBM mortality. (h-k) Bulk RNAseq of tumours injected with CAN-3110. Both total (h,i) and unique (j,k) number of transcripts containing VDJ chain sequences were analysed before (n = 12 interventions, 11 patients) (h, j) or after (n = 15 interventions, 14 patients) (i, k) CAN-3110 and plotted against subject survival after CAN-3110. (a-c and e) Pearson’s correlation coefficient r and p values (two-sided, based on t-distribution) are provided above each plot. (h-k) Spearman’s correlation coefficient rho and p values (two-sided, based on t-distribution) are provided above each plot.
Extended Data Fig. 10 (Related to Specific public T cells are linked to survival.) Public and Private TCR clonotypes in rGBM IDHwt (a, b), PBMCs (c, d) and combined (e, f).
Jaccard Indices heatmaps (a, c, e) and Pearson’s correlation coefficient maps (b, d, f) are shown for amino acid-based shared (public) or unshared (private) TCRs between samples. For Jaccard maps (a, c, e), colour in box provides a gradient with white indicating no shared TCRs and increasing shades of blue indicating more shared TCRs between tumours. For (a-f) the top row denotes survival for each subject with the red bars denoting the SS (short survivors defined as survival <1-year post CAN-3110) and green bars the LS (long survival defined as survival >1-year post CAN-3110) subjects and the small y axis showing survival days. The right Y and X axes denote each subject with paired Pre- and Post-CAN-3110 rGBM IDHwt, ordered based on respective overall survival time. The number of private TCRs is shown in the bar graphs to the right of the heatmaps. For (b, d, f), each box represents a neutral (white), negative (red spectrum) or positive (blue spectrum) Pearson correlation coefficient between pre, post-CAN 3110 Tumour (a, b), PBMCs (c, d), or all combined (e, f). In TILs, there were 5 TCRs publicly shared amongst 4 patients, 45 amongst 3 patients, and 792 between 2 patients. The remaining 41,756 tumour TCRs were private (i.e., not shared between patients). Grey boxes denote no shared TCRs between samples. Asterisks inside boxes denote significant Pearson’s correlations p < 0.05, ** p < 0.01, *** p < 0.001 (2-sided, based on t-distribution). Note that 042 and 054 are the same individual treated at two different timepoints with CAN-3110. (g, h) PBMC TCRs for which post-CAN-3110 change in productive frequency associates with post-CAN-3110 survival (FDR ≤ 0.05 based on Pearson’s correlation p values calculated 2-sided using t-distribution) in rGBM IDHwt with available TCRβ sequences (n = 21 interventions/20 patients). Patient 045 excluded due to early non-GBM related mortality. TCRβ sequences were included if present in PBMCs from 21 interventions with a median read count of at least 2 pre- or post-CAN-3110. Pearson’s r, p (2-sided, based on t-distribution), and FDR are included in each plot. (g) CASSLGGNTEAFF37,38 was detected in the TIL TCRs of two patients post CAN-3110. (h) CASSSSTDTQYF39 was detected in the TIL TCRs of one patient pre CAN-3110.
Extended Data Fig. 11 (Related to Changes in T cell repertoire).
(a) Table of TIL TCRs which were either statistically enriched post-CAN-3110 in both tumour and PBMCs or statistically depleted post-CAN-3110 in both TILs and PBMCs from the same patient for rGBM IDHwt patients with available TCRβ sequencing data. Statistical enrichment/depletion was determined via Fisher’s Exact test with FDR correction on a per-patient basis. For TILs, FDR correction was applied across all detected TCRs. For PBMCs, FDR correction was applied only across TCRs that were statistically enriched/depleted in TILs and detected in PBMCs for that patient. The final column indicates whether the given TCR was reported in TCRdb (http://bioinfo.life.hust.edu.cn/TCRdb/#/) as of 11/04/2022. Further details of these TCR alterations are included in Supplementary Table 3. (b) Changes in Tumour and PBMC TCR repertoires were detected in long-survivors (post-CAN-3110 survival ≥ one-year post-CAN-3110) but not short-survivors (post-CAN-3110 survival <one-year post-CAN-3110) for rGBM IDHwt patients after CAN-3110. (c) TCR frequencies pre and post CAN-3110 for statistically expanded TIL TCRs (FDR ≤ 0.05) from patient 014, who was HSV1 seronegative both before and after CAN-3110 treatment (see also Fig. 3b and Extended Data Fig. 8). FDR values are calculated as in panel a. (d) Correlation between post-treatment TCRBV09-01*01 usage and post-treatment survival in IDHwt rGBM patients (n = 21 interventions, 20 patients) (excluding 045 due to early non-GBM mortality). Pearson’s correlation coefficients r and p values (2-sided, based on t-distribution) are shown above the plot when calculated using all patients or using only HSV1 seropositive + seroconverted patients. FDR correction was performed using all V genes with a median usage > 0 in both pre- and post-treatment samples from these patients. GLM = Generalized Linear Model, FDR = False Discovery Rate.
Extended Data Fig. 12 (Related to Sub-heading Tumour immune signatures are linked to survival). Few post-treatment RNAseq immune signatures are associated with survival when analysed using all available patients.
Bulk RNAseq immune signatures from paired pre- and post-treatment IDHwt rGBMs (n = 12 patients, 13 rGBMs) versus post CAN-3110 survival irrespective of HSV1 serology. (a) Pre-treatment Signatures which were significantly correlated (p ≤ 0.05, 2-sided, based on t-distribution) with post-treatment survival via Pearson’s correlation when analysed using all available patients irrespective of HSV1 serology. (b) Post-treatment Signatures which were significantly correlated (p ≤ 0.05, 2-sided, based on t-distribution) with post-treatment survival via Pearson’s correlation when analysed using all available patients irrespective of HSV1 serology. Note that one patient (045) was excluded from these analyses due to early non-GBM mortality. (c) Post-treatment Signatures were not significantly correlated (based on Pearson’s r with 2-sided p values calculated via t-distribution) with time from CAN-3110 injection to post-treatment tissue collection.
Extended Data Fig. 13 (Related to Sub-heading Tumour immune signatures are linked to survival). Many more post-treatment RNAseq immune signatures are associated with survival when analysed using only HSV1 seropositive patients than when analysed using all patients.
Bulk RNAseq immune signatures from paired pre- and post-treatment IDHwt rGBMs (n = 9 patients, 10 rGBMs) versus post CAN-3110 survival when analysed using only HSV1 seropositive patients. (a) Pre-treatment Signatures which were significantly correlated (p ≤ 0.05, 2-sided, based on t-distribution) with post-treatment survival via Pearson’s correlation when analysed using all available patients irrespective of HSV1 serology. (b) Post-treatment Signatures which were significantly correlated (p ≤ 0.05, 2-sided, based on t-distribution) with post-treatment survival via Pearson’s correlation when analysed using all available patients irrespective of HSV1 serology. Note that one patient (045) was excluded from these analyses due to early non-GBM mortality.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Notes and Supplementary Fig. 1 (the uncropped gels).
Supplementary Table 1
Patient treatment histories (related to the ‘HSV1 serology predicts efficacy’ section).
Supplementary Table 2
Patient tissue summary and pathology quantifications (related to the ‘CAN-3110 increases T cells in tumours’ and ‘Persistence is linked to seronegativity’ sections).
Supplementary Table 3
TCR sequences that were statistically changed in TILs after treatment with CAN-3110 (related to the ‘Changes in T cell repertoire’ section).
Supplementary Video 1
The experience of participant 021 with CAN-3110. Participant 021 underwent standard of care surgery and chemoradiation for her GBM, but then recurred. After a second surgery, she recurred rapidly. She then underwent intraoperative MRI-guided stereotactic injection of CAN-3110. An MRI 90 days later appeared to show recurrence again and she underwent a third resection. This resection showed significant new infiltration with CD8+ T cells. After this, she remained tumour-free by MRI for almost 2 years with adjuvant pembrolizumab infusions. She succumbed to a motor vehicle accident in which she was the passenger. The video features pictures and videos of Susan as well as interviews with her daughter.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ling, A.L., Solomon, I.H., Landivar, A.M. et al. Clinical trial links oncolytic immunoactivation to survival in glioblastoma. Nature 623, 157–166 (2023). https://doi.org/10.1038/s41586-023-06623-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06623-2
This article is cited by
-
Mechanistic insights and the clinical prospects of targeted therapies for glioblastoma: a comprehensive review
Experimental Hematology & Oncology (2024)
-
Optimizing oncolytic virus design: a “Swiss army knife” approach to create a systemically delivered therapeutic
Signal Transduction and Targeted Therapy (2024)
-
Personalizing Oncolytic Immunovirotherapy Approaches
Molecular Diagnosis & Therapy (2024)
-
Glioblastoma tackled with oncolytic virus
Nature Reviews Neurology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.