Abstract
Extracellular nucleotides are emerging as important regulators of inflammation, cell proliferation and differentiation in a variety of tissues, including the hematopoietic system. In this study, the role of ATP was investigated during murine hematopoiesis. ATP was able to reduce the percentage of hematopoietic stem cells (HSCs), common myeloid progenitors and granulocyte–macrophage progenitors (GMPs), whereas differentiation into megakaryocyte–erythroid progenitors was not affected. In addition, in vivo administration of ATP to mice reduced the number of GMPs, but increased the number of Gr-1+Mac-1+ myeloid cells. ATP also induced an increased proliferation rate and reduced Notch expression in HSCs and impaired HSC-mediated bone marrow reconstitution in sublethally irradiated mice. Moreover, the effects elicited by ATP were inhibited by suramin, a P2 receptor antagonist, and BAPTA, an intracellular Ca2+ chelator. We further investigated whether the presence of cytokines might modulate the observed ATP-induced differentiation. Treatment of cells with cytokines (stem cell factor, interleukin-3 and granulocyte–monocyte colony stimulator factor) before ATP stimulation led to reduced ATP-dependent differentiation in long-term bone marrow cultures, thereby restoring the ability of HSCs to reconstitute hematopoiesis. Thus, our data suggest that ATP induces the differentiation of murine HSCs into the myeloid lineage and that this effect can be modulated by cytokines.
Similar content being viewed by others
Main
Under steady-state conditions, hematopoietic bone marrow cells develop in intimate association with a highly organized, three-dimensional microenvironment. This structural scaffold is composed of many cell types, including osteoblasts and stromal cells, as well as their associated biosynthetic products: components of the extracellular matrix, adhesion molecules and hematopoietic growth factors.1 The specific bone marrow microenvironment in which hematopoietic stem cells (HSCs) reside is referred to as the stem cell niche. HSCs are a subset of bone marrow cells that are capable of self-renewal and of forming all types of blood cells.2 The hematopoietic niche has an essential role in regulating the self-renewal and differentiation of stem cells. In the bone marrow, two stem niches have been characterized: the osteoblastic niche, where osteoblasts primarily control the quiescence and asymmetric proliferation of HSCs,3 and the vascular niche, which is mainly associated with myeloid differentiation.4
The cells present in hematopoietic niches regulate hematopoiesis through the release of different cytokines, which then activate distinct cytokine receptors with intrinsic kinase activity or Janus kinase-dependent receptors. However, new families of G-protein-coupled receptors and ion channels have been recently associated with the regulation of hematopoiesis,5 such as the P2 receptor family, which is activated by extracellular nucleotides.6, 7 These extracellular nucleotides are released by endothelial and osteoblast cells,8, 9, 10 and may be associated with myeloid differentiation.6
The functions of ATP are mediated by receptors of the P2 receptor family, which are further divided into the P2X (ionotropic receptor) and P2Y (metabotropic receptor) subfamilies.11 P2 receptor activation triggers many physiological responses in various cell types that are mediated by a Ca2+ influx through the P2X receptor or via intracellular Ca2+ (Ca2+i) release by P2Y receptors that activate phospholipase C (PLC). PLC hydrolyzes phosphatidylinositol-4,5-bisphosphate and a subsequent increase in inositol 1,4,5-trisphosphate (InsP3) and diacylglycerol (DAG) occurs.12 In addition, changes in the level of adenylate cyclase activity have been shown to influence the concentration of cyclic adenosine monophosphate.13, 14 The activation of P2 receptors controls other intracellular pathways by changing the concentration levels of Ca2+i and cyclic adenosine monophosphate, modulating the mitogen-activated protein kinases and phosphoinositide 3-kinase, both related with proliferation and differentiation.15, 16
In hematopoietic cells, treatment with ATP leads to cellular activation, with an increase in proinflammatory activities, cell death,17, 18 platelet aggregation,14 and cellular proliferation and differentiation.6, 7 Studies in promyelocytic leukemia HL-60 cells have shown that the expression levels of P2 receptors change significantly during myeloid differentiation.19, 20 Nucleotides such as ATP and UTP enhance the response of HSCs to several cytokines.7 For example, UTP was able to significantly improve chemokine receptor 4-mediated (CxC4R) HSC migration.21 In addition, we have shown that large increases in ATP-induced Ca2+i promote transient proliferation and induce the differentiation of primitive hematopoietic cells in long-term bone marrow cultures (LTBMCs).6 Recently, the functional presence of P2X receptors was described in an HSC subset, which is probably related to proliferation.22
To understand the physiological role of P2 receptors in the hematopoietic system, the capacity of extracellular ATP to promote differentiation was investigated in the bone marrow. Furthermore, the ability of cytokines to inhibit ATP-dependent differentiation was explored. Our data show that extracellular ATP induces a reduction in the number of HSCs and myeloid progenitors (MPs), which leads to an increase of mature myeloid cells in the bone marrow, and that this effect can be modulated by the presence of cytokines.
Results
ATP induces changes in the percentage of primitive and mature bone marrow populations
Because ATP induces the differentiation of primitive cells in LTBMCs, we determined whether ATP stimulation could alter the percentages of the HSC and MP populations, including the common myeloid progenitors (CMPs), granulocyte–macrophage progenitors (GMPs) and megakaryocytic–erythroid progenitors (MEPs). An HSC phenotype was defined in this study as Lin−c-Kit+Sca-1+Thy1.1+Flk2−, a subset capable of extensive self-renewal and differentiation into all types of blood cells.23 Other myeloid progenitors were recognized as described in previous reports.2, 23 The identification of the populations used in this study is provided in the Supplementary information (Supplementary Figure 1). For this purpose, bone marrow cells were stimulated with ATP at different times and concentrations. Following stimulation, the samples were labeled to permit the identification of primitive hematopoietic populations by flow cytometry. A high concentration of ATP (1 mM) was required to promote different effects in the hematopoietic cells.7, 24 However, this concentration did not induce hematopoietic cell death6, 17 (Supplementary Figure 2). Concentrations lower than 1 mM did not induce significant changes in the percentage of HSCs (Supplementary Figure 3).
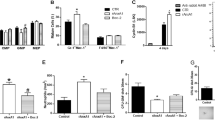
Our results showed a significant decrease in the percentage of the HSC (to 44% of untreated levels), CMP (to 28% of untreated levels) and GMP (to 27% of untreated levels) populations after stimulation with ATP for 4 h in vitro (Figure 1a), whereas the MEP population was not affected. Similar results were obtained by stimulation performed in short culture for 12 h (Supplementary Figure 4A).
ATP decreases the percentages of murine primitive hematopoietic cell populations. (a) Bone marrow suspensions were stimulated with 1 mM ATP for 4 h, and the hematopoietic populations present were quantified, n=6. Hematopoietic stem cell (HSC), common myeloid progenitor (CMP) and granulocyte–macrophage progenitor (GMP) populations were reduced by stimulation with ATP, whereas megakaryocytic–erythroid progenitors (MEPs) were not affected. (b and c) Mice were treated in vivo with ATP (47 mg/kg) for 4 days, n=6. (b) ATP significantly reduces the GMP population (c) and increases the number of mature myeloid cells (Gr-1+Mac-1+). (a–c) The effects induced by ATP were abolished in the presence of suramin, a P2 receptor antagonist. (a) In vitro experiment: suramin 100 μM. (b and c) In vivo experiment: suramin 10 mg/ml. (d) c-Kit+Lin− cells were plated in methylcellulose in the presence or absence of 1 mM ATP, n=4. ATP was able to partially reduce the number of CFU-GM colonies. (e) In addition, the same experiment shown in (a) was performed with an isolated c-Kit+Lin− population on the stroma cell line MS5. Similar results were obtained under these conditions, n=4. Data are expressed as the mean±S.E.M. *,#P<0.05. *Statistical analysis was performed against a control. #Statistical analysis was performed against samples stimulated with ATP. (a, b and c) ANOVA test. (d and e) t-test
To verify the ATP-induced differentiation, a dose of 0.47 g/kg of ATP was administered in vivo for 4 days. This dose injected intraperitoneally has been used previously in other studies to stimulate P2 receptors and did not induce adverse effects in the treated animals.25, 26 After treatment with ATP, bone marrow cells were collected for immunophenotyping. An evaluation of the hematopoietic cell populations showed a significant reduction in GMPs (40%). The HSC population was reduced in 35% (of untreated levels), although this did not constitute a significant decrease, whereas the CMP and MEP populations were not affected (Figure 1b). Furthermore, an evaluation of the mature myeloid population (Gr-1+Mac-1+) from experiments performed in vivo indicated a significant increase (43%) after 4 days of treatment (Figure 1c).
In additionally, suramin, a P2 antagonist were used to verify the participation of P2 receptors in the observed effects. Suramin was able to inhibit the decrease of the hematopoietic cell populations induced by ATP, both in vivo and in vitro (Figure 1a and b). Moreover, in the absence of ATP, suramin did not affect the percentage of hematopoietic populations (Supplementary Figures 4B and C). Furthermore, adenosine, which is produced by ATP degradation activating P1 receptors, and xanthine, an adenosine receptor antagonist, were used to evaluate P1 receptors' involvement. From our results, we observed that adenosine did not reduce the percentage of hematopoietic populations (Supplementary Figures 5A and B) and that xanthine did not abolish the effect of ATP in vivo and in vitro (Supplementary Figures 5C and D).
Furthermore, the number of colony-forming units of granulocytes and macrocytes (CFU-GM) and burst-forming units-erythroid (BFU-E) was quantified using methylcellulose assays. ATP induced a 42% reduction in the number of CFU-GM, but did not alter the number of BFU-E (Figure 1d). Concentrations lower than 1 mM did not induce significant changes in the percentage of CFU-GM colonies (Supplementary Figure 6). These data strongly suggest that ATP is involved in myeloid differentiation, reducing primitive MPs and increasing their mature forms.
Moreover, as the presence of macrophages in whole bone marrow could participate indirectly in the effects of differentiation induced by ATP, the same test was conducted with isolated c-Kit+ cells cultured on the stroma cell line MS5 for 24 h. Similar effects of differentiation in response to ATP were observed in the absence of macrophages (Figure 1e).
ATP reduces HSC potency
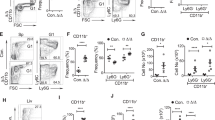
Subsequently, other tests were performed to verify the ability of ATP to promote myeloid differentiation, and important features of HSCs were evaluated, such as exhibiting a quiescent state, Notch receptor expression and the ability of HSCs to repopulate the bone marrow. We measure the proliferative state using an antibody against Ki-67 protein, which is specifically expressed during all active phases of the cell cycle, but is absent from resting cells. Stimulation of bone marrow cells with ATP induced an increase of the HSC-Ki-67+ population from 20±1 to 46±3%, showing that ATP induces HSC proliferation (Figure 2a). Notch receptors have been associated with the self-renewal, quiescence, maintenance and differentiation of HSC, and its expression is used as an indicator of the undifferentiated state of HSC.27, 28 Stimulation of bone marrow cells with 1 mM ATP for 2 or 4 h led to a marked reduction in the Notch receptor expression of the HSC and MP populations (Figure 2b). In addition, an evaluation of the competence of HSCs to repopulate the bone marrow microenvironment after stimulation with 1 mM ATP was performed using transgenic mice expressing the green fluorescent protein (GFP). Freshly isolated bone marrow cells from GFP animals were stimulated with ATP for 4 h and immediately injected by retro-orbital injection into sublethally irradiated (700 Gy) wild-type animals. After 8 weeks, the animals were killed, and the frequency of GFP+ cells within the different hematopoietic populations was determined. The hematopoietic populations from the ATP-stimulated group showed a clear reduction in the frequency of GFP+ cells compared with controls, indicating a decrease in the ability of ATP-treated HSCs to reconstitute the hematopoietic system (Figure 2c). Furthermore, the expression of CxCR4 and calcium-sensing receptors (CaSR), which are homing receptors of HSCs, were evaluated. Stimulation of bone marrow cells in vitro did not alter CxCR4 or CaSR expression (Supplementary Figures 7A and B).
ATP decreases the undifferentiated characteristics of hematopoietic stem cells (HSCs). (a) Stimulation of bone marrow cells with ATP for 4 h induces an increase of Ki-67 expression in HSCs. (b) A reduction in Notch expression was observed after ATP treatment. The graphs are representative of three independent experiments. Open histogram: unstimulated sample. Filled histogram: stimulated sample. (c) Bone marrow cells from green fluorescent protein (GFP) mice were stimulated with 1 mM ATP for 4 h and transferred to non-lethally irradiated mice. After 8 weeks, the animals that received GFP+ cells were killed, and the percentage of GFP+ cells was quantified in each population: HSCs, hematopoietic progenitors (HPs), myeloid progenitors (MPs) and the mature myeloid population (Gr-1+Mac-1+). The stimulation of hematopoietic cells with ATP led to a decrease in the capacity of HSCs to reconstitute bone marrow. (a and c) Data are expressed as the mean±S.E.M. *P<0.05, (a and c) t-test. n=5–7
Osteoblastic and endothelial niches regulate the quiescent state of HSCs. Therefore, if ATP induces HSC differentiation, then it will likely result in a high level of expression of ectonucleotidases in hematopoietic progenitors (HPs) and osteoblast cells. The expression of CD39 and CD73, which hydrolyze ATP into ADP and AMP into adenosine, respectively, was analyzed in freshly isolated bone marrow cells. HSC, HP and stromal cells express CD39 and CD73 (Supplementary Figures 7C–F). Moreover, activity of ectonucleotidases was compared among three populations: immature hematopoietic population (c-Kit+ cells), stromal cells (CD45−Ter-119−) and mature cells of bone marrow (whole bone marrow cells). We observed that immature hematopoietic cells exhibit a higher ectonucleotidase activity than mature or stromal cells (Supplementary Figure 8).
ATP promotes increased [Ca2+]i in hematopoietic cells
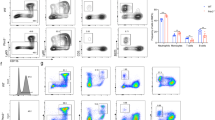
The observed ATP-induced differentiation in LTBMCs is related to a large increase in Ca2+i.6 To verify that the differentiation of primitive bone marrow hematopoietic cells is dependent on Ca2+i signaling, initial measurements of Ca2+i were carried out in freshly isolated HSC and c-Kit+Lin− populations (a population that contains HPs). After separation, the cells were loaded with the Ca2+ indicator Fluo-4-AM, and the emission of fluorescence was visualized by confocal microscopy. The Ca2+i concentration was represented as a temporal insensitive graph or as representative pseudocolored images. As shown in Figure 3a, ATP promoted a transient increase in the Ca2+i concentration in both primitive populations. Then, the Ca2+i chelator BAPTA-AM was used to verify the participation of Ca2+i in ATP-induced differentiation. BAPTA-AM was incubated with fresh bone marrow cells for 1 h before stimulus with ATP. The Ca2+i chelator was able to abolish the in vitro ATP-induced differentiation.
ATP promotes a decrease of primitive hematopoietic populations via intracellular Ca2+ (Ca2+i). (a) Hematopoietic cells were isolated and incubated with the Ca2+ fluorophore Fluo-4-AM (10 μM), and images were captured using confocal microscopy. Confocal images are shown with pseudocolor according to a fluorescence intensity scale (0=blue; 255=red). The records of fluorescence intensity as a function of time correspond to the images shown on the right. Each line represent the Ca2+i concentration of an individual cell. ATP increased Ca2+i in hematopoietic stem cells (HSCs) and Lin-c−Kit+ cells in a transient manner. Data are representative of at least three experiments. (b) Bone marrow suspensions were stimulated with 1 mM ATP for 4 h, and the hematopoietic populations present were quantified. BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid)-AM (10 μM) was incorporated into the cells for 1 h. The reduction in the number of HSCs, common myeloid progenitor (CMPs) and granulocyte–macrophage progenitors (GMPs) by ATP was abolished in the presence of the Ca2+i chelator BAPTA. *,#P<0.05. *Statistical analysis was performed against a control. #Statistical analysis was performed against samples stimulated with ATP. ANOVA test. n=4
Cytokines modulate the observed ATP-induced differentiation
Cytokines and ATP may be released by cells in hematopoietic stem cell niches. Thus, these molecules may regulate HSC activity together. We investigated whether the presence of cytokines could modulate the differentiation induced by ATP. For this purpose, known differentiation effects of ATP were induced to evaluate whether the previous stimulation of cytokines altered the response to ATP.
First, we evaluated whether the addition of cytokines could alter the ATP-dependent increase in the Ca2+i concentration because the effects of ATP-dependent differentiation occur mainly via increased Ca2+i (Figure 3). We observed that a previous 5 min stimulation with cytokines (granulocyte–monocyte colony stimulator factor (GM-CSF), interleukin-3 (IL-3) and stem cell factor (SCF) at 10 ng/ml) partially reduced the ATP-dependent increase in the Ca2+i concentration in LTBMCs. The Ca2+i concentration was represented as a graph of fluorescence intensity versus time or as pseudocolor images. Representative images are shown in Figure 4a and the temporal Ca2+i concentration values are shown in Figure 4b. The increase in Ca2+i was normalized using the Ca2+ ionophore A23187 (Response 100%). A23187 forms membrane channels that allow the passage of Ca2+ to the cytoplasm.
Cytokines partially inhibit the ATP-induced intracellular Ca2+ concentration ([Ca2+]i) increase in long-term bone marrow cultures (LTBMCs). LTBMCs were incubated with the Ca2+ fluorophore Fluo-4-AM (10 μM), and images were captured using confocal microscopy. LTBMCs were stimulated with 1 mM ATP in the presence or absence of cytokines (granulocyte–monocyte colony stimulator factor (GM-CSF), stem cell factor (SCF) and interleukin-3 (IL-3): 10 ng/ml). (a) Confocal images are shown with pseudocolor according to a fluorescence intensity scale (0=black; 255=white). Representative images of basal [Ca2+]i intensity and the intensity following ATP stimulation and administration of the Ca2+ ionophore A23187 are shown. Control corresponds to the response without previous stimulation with cytokines. The records of fluorescence intensity as a function of time correspond to the images shown on the right. The trace is a mean of 10–20 cells. (b) The average increase in the Ca2+i concentration elicited by ATP. The increase in [Ca2+]i was normalized using the Ca2+ ionophore 10−6 M A23187 (Response 100%). Data are expressed as the mean±S.E.M. *P<0.05. ANOVA test. n=4–5
Second, we determined whether cytokines could prevent ATP-dependent differentiation in LTBMCs, as reported previously.6 LTBMCs were incubated with GM-CSF, SCF and IL-3 for 5 min and subsequently stimulated with ATP (1 mM). The cells were collected after 3 days of stimulation, and the percentages of the different populations were analyzed by flow cytometry. Our results showed that the presence of cytokines (10 ng/ml) abolished the increase in the mature myeloid population (Gr-1+Mac-1+) elicited by 1 mM ATP (Figure 5a). The presence of cytokines also inhibited the ATP-dependent decrease in primitive cells (Gr-1−Mac-1−c-Kit+) in LTBMCs (Figure 5b). In addition, Giemsa/May–Grünwald staining was performed to corroborate the flow cytometry results. The counts of different cell types in the LTBMCs indicated a reduction in the number of the immature and blast forms induced by ATP in LTBMCs, and this effect was inhibited in the presence of cytokines (Table 1).
Cytokines inhibit ATP-induced differentiation in long-term bone marrow cultures (LTBMCs). LTBMCs were incubated for 24 h in Iscove's modified Dulbecco's medium (IMDM) medium supplemented with 0.5% fetal bovine serum (FBS) before stimulation with ATP in the presence or absence of cytokines (granulocyte–monocyte colony stimulator factor (GM-CSF), stem cell factor (SCF) and interleukin-3 (IL-3): 10 ng/ml). The percentages represented in each histogram were obtained 3 days after stimulation with or without cytokines. Cytokines were added 5 min before stimulation with ATP. (a) Analysis of LTBMC populations showed that cytokines inhibit the increase of the mature Gr-1+Mac-1+ population. (b) The ATP-induced decrease in primitive hematopoietic populations (Gr-1−Mac-1−c-Kit+) was abolished in the presence of cytokines in LTBMCs. Data are expressed as the mean±S.E.M. *P<0.05. ANOVA test. n=6–8
Finally, we determined whether previous stimulation with cytokines would prevent the ATP-dependent decrease in the HSC reconstitution capacity. Thus, bone marrow cells from GFP+ animals were incubated for 5 min with GM-CSF (10 ng/ml) and IL-3 (10 ng/ml) before stimulation with ATP for 4 h. These cells were then injected into sublethally irradiated animals. After 8 weeks, the bone marrow cells were extracted, and the percentages of GFP+ cells within different hematopoietic populations were calculated. As shown in Figure 6, pretreatment with cytokines followed by 4 h of stimulation with ATP led to a recovery in the reconstitution ability of HSCs. A significant increase in GFP+ cells was observed within the immature population (HSC, HP and MP) and the mature myeloid population (Gr-1+Mac-1+) compared with the group treated with ATP alone.
Cytokines recover the reconstitution capacity of hematopoietic stem cells (HSCs). Bone marrow cells extracted from green fluorescent protein (GFP) mice were stimulated with 1 mM ATP for 4 h and transferred to non-lethally irradiated mice. After 8 weeks, the animals were killed, and the percentage of GFP+ cells were quantified in each hematopoietic population: (a) HSCs, (b) hematopoietic progenitors (HPs), (c) myeloid progenitors (MPs) and (d) the mature myeloid population (Gr-1+Mac-1+). Pre-incubation with cytokines restores HSC potency after ATP stimulation. Data are expressed as the mean±S.E.M. *,#P<0.05. *Statistical analysis was performed against a control. #Statistical analysis was performed against samples stimulated with ATP. ANOVA test. n=5–7
Discussion
Under physiological conditions, HSCs exhibit a quiescent state and asymmetric proliferation following an interaction with particular osteoblasts in the trabecula bone area. In contrast, myeloid differentiation occurs adjacent to blood vessels.3, 4 In addition, some types of unidentified osteoblast and endothelial cells can release ATP in response to certain stimuli or physiological conditions.8, 9, 10
ATP released by the endothelium or by osteoblasts could participate in myeloid differentiation. In a previous report, we showed that ATP and its analogs could induce the transient proliferation and myeloid differentiation of primitive HPs present in LTBMCs through the activation of P2 receptors.6 In light of these findings, this study was undertaken to investigate the ability of extracellular ATP to induce direct differentiation of HSC and MPs in vivo and in vitro assays.
Initially, we determined the percentage of HSCs and myeloid precursor cells (CMP, GMP and MEP) after stimulation with ATP in freshly isolated bone marrow cells. The percentages of the HSC, CMP and GMP populations present in the bone marrow were reduced by stimulation with ATP, whereas the MEP population remained unchanged (Figure 1a). Subsequently, in vivo experiments showed that treatment with ATP for 4 days led to a reduction in the number of MPs (Figure 1b) and a corresponding increase in the mature myeloid population (Figure 1c). To determine whether the observed changes in blood cell populations were associated with differentiation, we evaluated different HSC characteristics. The presence of a quiescent state, which is a characteristic of HSCs,2 was evaluated. ATP was able to reduce the number of HSCs in quiescent state (Figure 2a). In addition, we investigated whether ATP could alter the expression the receptors Notch, CxCR4 and CaSR. Notch receptors have been associated with the self-renewal, quiescence, maintenance and differentiation of HSCs, and their expression is used as an indicator of the undifferentiated state of HSCs,27, 28, 29 whereas CxCR4 and CaSR are homing receptors required for the colonization of bone marrow by HSCs during the development of hematopoiesis.30, 31 ATP reduced the expression of Notch receptors (Figure 2b). However, there was no observed alteration in the expression of CxCR4 or CaSR (Supplementary Figures 7A and B). Moreover, ATP reduced the ability of HSCs to reconstitute important characteristics of HSC potency in bone marrow (Figure 2c). Therefore, we have showed that ATP reduces the HSC and primitive macrophage/granulocytic progenitor populations, leading to an increase of the mature myeloid population.
Our results showed that the observed ATP-induced differentiation occurred by P2 receptor activation. Participation of the P2 receptor was shown using suramin, a well-known P2 antagonist, in vitro and in vivo experiments (Figure 1). On the other hand, the participation of P1 receptors, activated by adenosine, was excluded by the use of adenosine and xanthine, a P1 receptor inhibitor (Supplementary Figure 5). However, the identification of the P2 receptor subtypes deserves further investigation.
In addition, the maintenance of HSCs in a quiescent state in the osteoblast niche can also be related to a high level of ectonucleotidase expression, including that of CD39 and CD73 (Supplementary Figures 7C–F). Interestingly, primitive hematopoietic cells, characterized as c-Kit+ cells, showed higher ectonucleotidase activity than stromal cells and mature hematopoietic cells (whole bone marrow) (Supplementary Figure 8). These results could explain the lower differentiation effect caused by ATP in vivo assay (Figure 1b). It is possible that the high expression and activity of HSC and stromal cells reduced the differentiation induced by ATP in the hematopoietic niche. Hence, the expression and activity of ectonucleotidases arises like a new important feature to undifferentiated state. However, further investigation on the role of ectonucleotidases in hematopoietic differentiation is needed.
On the other hand, the signaling pathways involved in the differentiation by the activation of P2 receptors have not been completely elucidated. In fact, Ca2+i signaling is the main intracellular pathway triggered by P2 receptors and we have shown its participation in the differentiation induced by ATP. Ca2+ is a ubiquitous messenger that controls many physiological cellular processes in different tissues, including the cells of the bone marrow microenvironment.6, 32 Our results show that ATP induces an increase in Ca2+i in HSC and c-Kit+Lin− populations (Figure 3a) and that this differentiation is Ca2+i dependent (Figure 3b). The involvement of Ca2+ in cytokine-mediated signaling was recently reported by our group.33 However, the molecular mechanisms stimulated by Ca2+i to carry out the response to ATP and cytokines remain unknown. The Ca2+i increase elicited by ATP is large and transiently induced in the cells studied here (Figure 3a). In contrast, the change in Ca2+i elicited by cytokines exhibits a short and oscillatory signal.6, 33 Ca2+ signaling stimulated by signaling molecules can influence proliferation, differentiation and cell death, and the response to Ca2+ signaling is determined by proteins that are sensitive to the intensity and duration of the Ca2+ signal.32 It is known that activation of PLCγ, an enzyme that hydrolyzes phosphatidylinositol 4,5-bisphosphate to generate the two second messengers, InsP3 and DAG, and cytosolic Ca2+ modulate several important transcription factor pathways required for the development or differentiation of B cells.34 In myeloid differentiation, the participation of PLCγ2 by the activation of cytokines receptors was described recently.33 Moreover, an unidentified PLCβ is activated by ATP in HSC, which produces InsP3 and DAG similarly to PLCγ. These two PLC isoforms can modulate hematopoiesis depending on the amount of Ca2+i released. Therefore, the increase in Ca2+i levels elicited by ATP or cytokines leads to different cellular effects because distinct Ca2+-sensitive proteins could be activated.
The presence of ATP in hematopoietic niches can be an important factor in regulating HSC activity together with other signaling molecules that regulate hematopoiesis, such as cytokines. Here, we show that the presence of cytokines can modulate ATP-induced differentiation. Previous stimulation of LTBMCs with cytokines reduced the effects of ATP-dependent Ca2+i increases (Figure 4) and hematopoietic differentiation (Figure 5). Finally, previous incubation with cytokines also restored the ability of HSCs to repopulate bone marrow after non-lethal irradiation (Figure 6). Therefore, the intracellular pathways induced by ATP led to differentiation, whereas at the same time, cytokines and other signals activate specific pathways that regulate the maintenance of HPs.
Cytokines activate several intracellular mechanisms that could prevent some overload responses. GM-CSF was able to reduce cell death by activation of chimeric receptor GM-CSF-Bcl-XL protein.35 In addition, moderate elevation of Ca2+i concentration also induces cytokine-independent survival and proliferation of human myelo-erythroid CD34+ leukemia cell line.36. The low increase of Ca2+i concentration by cytokines probably activates the mechanism related with survival decreasing the ATP-induced differentiation.
The results of this study show that extracellular ATP decreases HSC and MPs populations, promoting myeloid differentiation. Moreover, we showed that cytokines are able to modulate ATP-induced differentiation. These findings are important for understanding the HSC niche and point out the importance of the ATP and the P2 receptors in the hematopoiesis.
Materials and Methods
Animals
The wild-type mice (C57BL/6, 8–12 weeks old) used in this study were supplied by the INFAR/UNIFESP Animal Facility (São Paulo, Brazil), and the GFP mice (C57BL/6-Tg(act-EGFP)C14-Y01-FM131Osb) were maintained at CEDEME/UNIFESP. All experiments were approved by the Animal Care and Ethics Committee of the Federal University of São Paulo (0254/08).
In vivo assays
Solutions of nucleotides in saline were prepared as described by Rapaport.26 Briefly, 0.47 g/kg of ATP was diluted in saline (sterile saline, pH 6.2) and intraperitoneally injected daily for 4 days. A control group received an equivalent volume of the vehicle daily (sterile saline) by intraperitoneally injection.25, 26 In some experiments, 10 mg/kg suramin37 and 3 μg/kg xanthine38 were applied 1 h before to ATP injection.
Quantification of hematopoietic populations
Bone marrow cells were extracted from femurs and stimulated with ATP at 37°C for 4 h. All experiments up to 4 h were performed in a physiological solution (137 mM NaCl, 2.68 mM KCl, 1.36 mM CaCl2, 0.49 mM MgCl2, 12 mM NaHCO3, 0.36 mM NaH2PO4, and 5.5 mM D-glucose). For experiments more than 12 h long, Iscove's modified Dulbecco's medium (IMDM) supplemented with 2% fetal bovine serum (FBS) was used. Cells were stimulated, and the percentages of each hematopoietic population were determined by flow cytometry and compared with the initial percentage. HP cells were identified as described previously.2, 23 Mature cells were excluded using a lineage marker cocktail (Lin: PE-conjugated B220, CD3, Ter-119, CD11b and Gr-1). In addition, several antibodies (FLK-2-PE, IL-7R-PE, CD90-FITC, Sca-1-Cy7/PE and c-Kit-APC) were used to recognize a subset rich in HSCs (Lin−FLK-2−Sca-1+c-Kit+Thy1.1+ or Lin−FLK-2−Sca-1+c-Kit+); HP (Lin−Sca-1−c-Kit+), a population that contained myeloid and lymphoid progenitors; and MP (Lin−Sca-1−IL-7c-Kit+). Moreover, CMP (Lin−IL-7R−c-Kit+Sca-1−CD34+FcγRlow), GMP (Lin−IL-7R−c-Kit+Sca-1−CD34+FcγRhigh) and MEP (Lin−IL-7R−c-Kit+Sca-1−CD34−FcγRlow) populations were identified using IL-7R-PE, Sca-1-PE, c-Kit-Cy7/PE, CD34-FITC and FCγR-APC antibodies. To quantify the percentage of hematopoietic populations, 3–5 × 106 events were acquired. All antibodies were purchased from Becton Dickinson (Franklin Lakes, NJ, USA) or eBioscience (San Diego, CA, USA). The mature bone marrow-resident myeloid populations were evaluated with antibodies that recognize granulocytic and monocytic populations (Gr-1-PE and CD11b-Cy7/PE, respectively). We captured 3 000 000 events to evaluate this mature population. Data analyses were performed on a FACSCalibur (Becton Dickinson) flow cytometer using the CellQuest software. The gating strategy for murine hematopoietic populations is shown in Supplementary Figure 1.
Murine Lin−c-Kit+ and CFU-GM assay
Separation of the murine Lin−c-Kit+ population was performed by gradient centrifugation methods using Ficoll Histopaque (1.077 g/cm3). After mononuclear isolation, the cells were labeled with specific antibodies. For cell isolation, a biotin-conjugated Lin antibody cocktail was used, followed by incubation with anti-biotin-conjugated microbeads (Miltenyi Biotec, Columbus, OH, USA). After labeling, the cells were placed in a magnetic column, and the negative population was extracted. The Lin− cells were labeled with anti-c-Kit-conjugated microbeads (Miltenyi Biotec), and the Lin−c-Kit+ population was isolated in a magnetic column.
CFU assays were performed by plating 5 × 103 murine (Lin−c-Kit+) bone marrow cells in methylcellulose-based medium with recombinant cytokines and EPO (Methocult M3434; Stem Cell Technologies, Vancouver, Canada) in 35-mm diameter dishes. The cells were cultured in a fully humidified air incubator under 5% CO2 at 37°C for 7 or 14 days for murine and human cells, respectively. At the end of the incubation period, colonies of more than 50 cells were counted using a dark-field microscope.
Notch and Ki-67 expression
Bone marrow cells were stimulated with ATP at 37°C for 2 or 4 h in a physiological solution. Subsequently, cells were fixed with 2% paraformaldehyde for 30 min, washed with 0.1 M glycine and permeabilized with 0.01% saponin for 15 min. The cells were incubated for 2 h with rabbit purified anti-Notch-1 or anti-Ki-67-Alexa Fluor-488 conjugated (Becton Dickinson). Anti-rabbit IgG-Alexa Fluor 488 conjugates (Molecular Probes/Invitrogen, Eugene, OR, USA) was used as secondary antibody against anti-Notch-1 and incubated for 40 min. Notch and Ki-67 expression were evaluated in hematopoietic populations using the antibodies described above. Data analyses were performed on a FACSCalibur flow cytometer using the CellQuest software.
Competitive repopulation assay
Whole bone marrow cells of GFP transgenic mice were stimulated with 1 mM ATP in the presence or absence of GM-CSF (10 ng/ml) and IL-3 (10 ng/ml) in physiological solution at 37°C for 4 h and then injected (106 cells per animal) into wild-type sublethally irradiated mice (700 Gy). The frequency of GFP+ bone marrow cells in each hematopoietic population (HSC, HP, MP and Gr-1+Mac-1+) was quantified at 8 weeks post-transfer using a FACSCalibur flow cytometer.
Dexter-type cultures
Dexter-type cultures or LTBMCs were prepared following the method of Dexter et al.39 with some modifications. Briefly, total bone marrow cells were plated onto 12-well plates and fed weekly with IMDM supplemented with 5% FBS (Cultilab, Rio Grande do Sul, Brazil), 20% horse serum (Stemcell Technologies) and 10−6 M hydrocortisone (Sigma-Aldrich, St Louis, MO, USA). Cultures were maintained at 37°C in a humid chamber with 5% CO2. After the establishment of a confluent stroma layer, the remaining non-adherent cells were removed, and 106 cells per well was added to precultured stroma cultures, along with fresh media. After 1 week of co-culture, the cells were further cultured in IMDM with 0.5% FBS for 24 h. Subsequently, LTMBCs were stimulated with ATP in the presence or absence of IL-3, GM-CSF and SCF. After 3 days, the cells were collected for immunophenotyping using the antibodies Gr-1-FITC, Mac-1-PE and c-Kit-APC. Data analyses were performed on a FACSCalibur flow cytometer (Becton Dickinson). In addition, co-cultured cells were stained with May–Grünwald and Giemsa solution (Sigma) and identified by morphological characteristics.
Calcium measurements by confocal microscopy
Lin−c-Kit+ and HSC populations were previously isolated to measure Ca2+i. The Lin− cells were isolated as described above. HSC isolation was performed by cell sorting in a FACSCalibur flow cytometer.
After the separation of populations, the cells were cytocentrifuged onto glass coverslips (25 mm). For intracellular Ca2+ concentration ([Ca2+]i) measurements, the isolated cells or LTBMCs were incubated for 40 min at room temperature with 10 μM Fluo-4/AM (Molecular Probes/Invitrogen) and 0.01% pluronic acid (Molecular Probes/Invitrogen) and washed with physiological solution. Images were captured with a microscope (Zeiss, Axiovert 100M, Oberkochen, Germany) equipped with a laser scanner (Zeiss, LSM 510 META) and a 63 × objective (Plan-Neofluor, 1.4 numerical aperture) under oil immersion. The Fluo-4 probe was excited with an argon laser (λEx=488 nm), and light emission was detected using a Zeiss META detector (λEm=500–550 nm). The pinhole device was not used for Ca2+i measurements. Images were collected at approximately 4- to 6-s intervals. Fluorescence intensity was normalized with reference to basal fluorescence using the Examiner 3.2 (Zeiss) and Spectralyzer (NJ, USA) software. Representative pseudocolored images are shown with reference to the basal intensity on a fluorescence intensity scale ranging from 0 (black or blue) to 255 (white or red).
Statistical analysis
Ca2+ mobilization in response to ATP was normalized with reference to the response to 10−6 M A23187 (100%), and results were presented as representative pseudocolored images according to a fluorescence intensity scale. Data were expressed as means±S.E.M. Statistical analyses were performed using Student's t-test for comparisons between two groups, and analysis of variance and Dunnett's post hoc test were used for multiple comparisons among groups. Values of P<0.05 were considered statistically significant.
Abbreviations
- BFU-E:
-
burst-forming units-erythroid
- CaSR:
-
calcium-sensing receptors
- CFU-GM:
-
colony-forming units of granulocytes and macrocytes
- CMP:
-
common myeloid progenitors
- GMPs:
-
granulocyte–macrophage progenitors
- CxCR4:
-
CxC chemokine receptor 4
- DAG:
-
diacylglycerol
- GM-CSF:
-
granulocyte–monocyte colony stimulator factor
- HP:
-
hematopoietic progenitor
- HSCs:
-
hematopoietic stem cells
- InsP3:
-
inositol 1,4,5-trisphosphate
- IL-3:
-
interleukin-3
- Ca2+i:
-
intracellular Ca2+
- Lin:
-
lineage marker cocktail
- LTBMCs:
-
long-term bone marrow cultures
- MEPs:
-
megakaryocytic–erythroid progenitors
- MP:
-
myeloid progenitor
- PLC:
-
phospholipase C
- SCF:
-
stem cell factor
References
Nilsson SK, Johnston HM, Coverdale JA . Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood 2001; 97: 2293–2299.
Passegue E, Wagers AJ, Giuriato S, Anderson WC, Weissman IL . Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J Exp Med 2005; 202: 1599–1611.
Zhang J, Niu C, Ye L, Huang H, He X, Tong WG et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003; 425: 836–841.
Yin T, Li L . The stem cell niches in bone. J Clin Invest 2006; 116: 1195–1201.
North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 2007; 447: 1007–1011.
Paredes-Gamero EJ, Leon CM, Borojevic R, Oshiro ME, Ferreira AT . Changes in intracellular Ca2+ levels induced by cytokines and p2 agonists differentially modulate proliferation or commitment with macrophage differentiation in murine hematopoietic cells. J Biol Chem 2008; 283: 31909–31919.
Lemoli RM, Ferrari D, Fogli M, Rossi L, Pizzirani C, Forchap S et al. Extracellular nucleotides are potent stimulators of human hematopoietic stem cells in vitro and in vivo. Blood 2004; 104: 1662–1670.
Kawai Y, Yokoyama Y, Kaidoh M, Ohhashi T . Shear stress-induced ATP-mediated endothelial constitutive nitric oxide synthase expression in human lymphatic endothelial cells. Am J Physiol Cell Physiol 2010; 298: C647–C655.
Alvarenga EC, Rodrigues R, Caricati-Neto A, Silva FC, Paredes-Gamero EJ, Ferreira AT . Low-intensity pulsed ultrasound-dependent osteoblast proliferation occurs by via activation of the P2Y receptor: role of the P2Y(1) receptor. Bone 2010; 46: 355–362.
Hayton MJ, Dillon JP, Glynn D, Curran JM, Gallagher JA, Buckley KA . Involvement of adenosine 5′-triphosphate in ultrasound-induced fracture repair. Ultrasound Med Biol 2005; 31: 1131–1138.
Abbracchio MP, Burnstock G . Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther 1994; 64: 445–475.
Nicholas RA, Lazarowski ER, Watt WC, Li Q, Boyer J, Harden TK . Pharmacological and second messenger signalling selectivities of cloned P2Y receptors. J Auton Pharmacol 1996; 16: 319–323.
Suh BC, Kim TD, Lee IS, Kim KT . Differential regulation of P2Y(11) receptor-mediated signalling to phospholipase C and adenylyl cyclase by protein kinase C in HL-60 promyelocytes. Br J Pharmacol 2000; 131: 489–497.
Takasaki J, Kamohara M, Saito T, Matsumoto M, Matsumoto S, Ohishi T et al. Molecular cloning of the platelet P2T(AC) ADP receptor: pharmacological comparison with another ADP receptor, the P2Y(1) receptor. Mol Pharmacol 2001; 60: 432–439.
Giltaire S, Lambert S, Poumay Y . HB-EGF synthesis and release induced by cholesterol depletion of human epidermal keratinocytes is controlled by extracellular ATP and involves both p38 and ERK1/2 signaling pathways. J Cell Physiol 2011; 226: 1651–1659.
Ornelas IM, Ventura AL . Involvement of the PI3K/AKT pathway in ATP-induced proliferation of developing retinal cells in culture. Int J Dev Neurosci 2010; 28: 503–511.
Paredes-Gamero EJ, Dreyfuss JL, Nader HB, Miyamoto Oshiro ME, Ferreira AT . P2X7-induced apoptosis decreases by aging in mice myeloblasts. Exp Gerontol 2007; 42: 320–326.
Costa-Junior HM, Hamaty FC, da Silva Farias R, Einicker-Lamas M, da Silva MH, Persechini PM . Apoptosis-inducing factor of a cytotoxic T cell line: involvement of a secretory phospholipase A2. Cell Tissue Res 2006; 324: 255–266.
Communi D, Janssens R, Robaye B, Zeelis N, Boeynaems JM . Rapid up-regulation of P2Y messengers during granulocytic differentiation of HL-60 cells. FEBS Lett 2000; 475: 39–42.
Adrian K, Bernhard MK, Breitinger HG, Ogilvie A . Expression of purinergic receptors (ionotropic P2X1-7 and metabotropic P2Y1-11) during myeloid differentiation of HL60 cells. Biochim Biophys Acta 2000; 1492: 127–138.
Rossi L, Manfredini R, Bertolini F, Ferrari D, Fogli M, Zini R et al. The extracellular nucleotide UTP is a potent inducer of hematopoietic stem cell migration. Blood 2007; 109: 533–542.
Casati A, Frascoli M, Traggiai E, Proietti M, Schenk U, Grassi F . Cell-autonomous regulation of hematopoietic stem cell cycling activity by ATP. Cell Death Differ 2011; 18: 396–404.
Christensen JL, Weissman IL . Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci USA 2001; 98: 14541–14546.
Paredes-Gamero EJ, Craveiro RB, Pesquero JB, Franca JP, Oshiro ME, Ferreira AT . Activation of P2Y1 receptor triggers two calcium signaling pathways in bone marrow erythroblasts. Eur J Pharmacol 2006; 534: 30–38.
Shabbir M, Thompson C, Jarmulowiczc M, Mikhailidis D, Burnstock G . Effect of extracellular ATP on the growth of hormone-refractory prostate cancer in vivo. BJU Int 2008; 102: 108–112.
Rapaport E . Experimental cancer therapy in mice by adenine nucleotides. Eur J Cancer Clin Oncol 1988; 24: 1491–1497.
Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol 2005; 6: 314–322.
Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 2003; 423: 409–414.
Maillard I, He Y, Pear WS . From the yolk sac to the spleen: new roles for Notch in regulating hematopoiesis. Immunity 2003; 18: 587–589.
Kiel MJ, Morrison SJ . Maintaining hematopoietic stem cells in the vascular niche. Immunity 2006; 25: 862–864.
Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 2006; 439: 599–603.
Cook SJ, Lockyer PJ . Recent advances in Ca(2+)-dependent Ras regulation and cell proliferation. Cell Calcium 2006; 39: 101–112.
Leon CM, Barbosa CM, Justo GZ, Borelli P, Resende JDJ, Oliveira JS et al. Requirement for PLCgamma2 in IL-3 and GM-CSF-stimulated MEK/ERK phosphorylation in murine and human hematopoietic stem/progenitor cells. J Cell Physiol 2011; 226: 1780–1792.
Scharenberg AM, Humphries LA, Rawlings DJ . Calcium signalling and cell-fate choice in B cells. Nat Rev Immunol 2007; 7: 778–789.
Antignani A, Youle RJ . The cytokine, granulocyte-macrophage colony-stimulating factor (GM-CSF), can deliver Bcl-XL as an extracellular fusion protein to protect cells from apoptosis and retain differentiation induction. J Biol Chem 2007; 282: 11246–11254.
Apati A, Janossy J, Brozik A, Bauer PI, Magocsi M . Calcium induces cell survival and proliferation through the activation of the MAPK pathway in a human hormone-dependent leukemia cell line, TF-1. J Biol Chem 2003; 278: 9235–9243.
Yang DM, Teng HC, Chen KH, Tsai ML, Lee TK, Chou YC et al. Clodronate-induced cell apoptosis in human thyroid carcinoma is mediated via the P2 receptor signaling pathway. J Pharmacol Exp Ther 2009; 330: 613–623.
Mino RP, Spoerri PE, Caballero S, Player D, Belardinelli L, Biaggioni I et al. Adenosine receptor antagonists and retinal neovascularization in vivo. Invest Ophthalmol Vis Sci 2001; 42: 3320–3324.
Dexter TM, Allen TD, Lajtha LG . Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol 1977; 91: 335–344.
Acknowledgements
This work was supported by grants from the ‘Fundação de Amparo à Pesquisa do Estado de São Paulo’ (FAPESP). CMVB was supported by a doctoral fellowship from FAPESP (2007/58589-9).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by A Stephanou
Supplementary Information accompanies the paper on Cell Death and Disease website
Supplementary information
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Barbosa, C., Leon, C., Nogueira-Pedro, A. et al. Differentiation of hematopoietic stem cell and myeloid populations by ATP is modulated by cytokines. Cell Death Dis 2, e165 (2011). https://doi.org/10.1038/cddis.2011.49
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2011.49
Keywords
This article is cited by
-
Purinergic system in cancer stem cells
Purinergic Signalling (2023)
-
P2X7Rs: new therapeutic targets for osteoporosis
Purinergic Signalling (2023)
-
p53-dependent induction of P2X7 on hematopoietic stem and progenitor cells regulates hematopoietic response to genotoxic stress
Cell Death & Disease (2021)
-
Involvement of P2 receptors in hematopoiesis and hematopoietic disorders, and as pharmacological targets
Purinergic Signalling (2020)
-
Extracellular annexin-A1 promotes myeloid/granulocytic differentiation of hematopoietic stem/progenitor cells via the Ca2+/MAPK signalling transduction pathway
Cell Death Discovery (2019)