Abstract
Growth mechanism for synthesizing PVD based Graphene using Amorphous Carbon, catalyzed by Copper is investigated in this work. Different experiments with respect to Amorphous Carbon film thickness, annealing time and temperature are performed for the investigation. Copper film stress and its effect on hydrogen diffusion through the film grain boundaries are found to be the key factors for the growth mechanism, and supported by our Finite Element Modeling. Low temperature growth of Graphene is achieved and the proposed growth mechanism is found to remain valid at low temperatures.
Similar content being viewed by others
Introduction
Graphene has been a material of great interest since its conception due to its extraordinary properties such as its single atomic thickness1, high current density tolerance2 of about 108 A/cm2, extremely high mobility3,4 of about 15,000 cm2/Vs, high thermal conductivity5 in the order of 5 × 103 W/mK, high optical transmittance of about 97% within the visible light wavelength-range of 400 to 700 nm6, high mechanical strength with breaking strength of 42 Nm−1 and second and third-order elastic stiffness of 340 Nm−1 and −690 Nm−1 respectively7.
Over a decade, Graphene synthesis methods have witnessed many pathways in order to produce high quality Graphene with a high degree of coverage area. Currently, the Graphene synthesis method used is Chemical vapor deposition (CVD) technique8,9,10,11 which produces uniform, large area, relatively low defect Graphene films and is known to be the cheapest for mass production and can meet the semiconductor industry requirement11.
Growth of Graphene on Copper using CVD has been demonstrated by Li et al.11,12 and others13,14. However, this method uses expensive carbon source which is also wasted considerably during chamber cleaning. This method also have no control over number of layers of synthesized Graphene when it comes to few‐layered and multi‐layered graphene growth.
On the other hand, a simpler and low cost technique to obtain non-transferable Graphene using amorphous carbon as solid source and Nickel or Cobalt thin film as catalyst annealed at elevated temperatures has been reported15,16,17. This technique can be instrumental in controlling the number of Graphene layers, eliminates the need for Graphene transfer and is economical as it uses amorphous carbon as the carbon source. Non-transferable Graphene synthesis is important especially for ULSI Interconnects which are used in BEOL (back-end-of-line) process and transfer of Graphene is quite infeasible. Moreover, the transfer of Graphene introduces many structural inhomogeneities which causes degradation of properties of graphene such as the mobility, electrical and thermal conductivity etc18,19,20. However, such Graphene synthesis method was reported to be not possible with Copper due to the very low solubility of Carbon in Copper16,17.
As the applications of Copper material is vast and important, such as printed circuit boards, transmission wires and cables, its application in ULSI interconnects, etc, the growth of Graphene on Copper (Cu) can overcome some limitations of Cu materials such as its ease of corrosion, ease of oxidation, limited Electromigration performance as interconnect, and push the electrical and thermal conductivity limits of Cu to higher values.
In view of the limitation of the reported methods, the use of amorphous carbon as solid source for Graphene synthesis on Cu was re-explored by Ji et al.21. However, their method involved Graphene deposition on a copper foil which is several microns thick and is not suitable for semiconductor industry as the Graphene so obtained needs to be transferred on to the required substrate which might introduce defects. Also, the Graphene is in direct contact with amorphous carbon in the method used by Ji et al.21, as the amorphous carbon layer atop Cu crystallizes to Graphene and this might result in crystal contaminations.
Recently, we successfully demonstrated the feasibility of crystallization of amorphous Carbon under sputtered Copper thin film which acts as a catalyst in order to obtain Graphene at the top surface of Cu experimentally22. However, its growth mechanism is yet to be explored.
In this work, various experiments are designed and performed in order to explore the growth mechanism of our Physical Vapor Deposition (PVD) Graphene growth method using amorphous carbon as the carbon-source. The proposed growth mechanism can also explain the claim of inability of Graphene growth on Copper thin film using this method as reported by others16,17. An attempt to achieve similar synthesis at comparatively lower temperature is presented and the growth mechanism is discussed for the same.
Methods
Sample Preparation
The experiments in this work involve deposition of amorphous Carbon (a-C) thin film of different thicknesses followed by Copper (Cu) (99.99%) film of 800 nm thickness on Si/SiO2 (300 nm) substrates using Physical Vapor Deposition (PVD) method. The samples are labelled as S1, S1.1 and S1.2 respectively as shown in Fig. 1a)22. (For sample preparation schematic and surface condition, please refer to Supplementary Figures S1 and S2 respectively in the Supplementary Information).
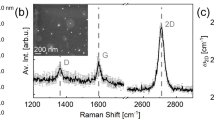
(a) Structure design for samples S1, S1.1 and S1.2 having 60 nm, 36 nm and 12 nm a-C layer sandwiched between 800 nm Cu and Si/SiO2(300 nm) substrate, (b) Schematic for annealing process, (c) Raman Spectrum of annealed sample S1, (d) Raman Spectrum of annealed sample S1.1, (e) Raman Spectrum of annealed sample S1.222.
Annealing
After samples S1, S1.1 and S1.2 are produced, they are annealed in an H2 environment with a flow rate of 50 sccm at a low pressure of 1 torr and different annealing temperatures and annealing times as shown in the annealing process schematic in Fig. 1b).
The graphene synthesis method used has been described in detail by Narula et al.22.
Characterization
Raman scattering spectra are collected using PTT RAMaker Micro Raman/PL/TR-PL Spectrometer with laser excitation wavelength of 473 nm and laser spot-size of about 0.5 μm.
Results
In order to understand the underlying mechanism of this graphene growth, experiments and characterization are performed to examine the effect of thickness of a-C, annealing time and annealing temperature for Graphene crystallization on Cu. In order to avoid any changes or modifications in the properties of synthesized Graphene, the experimental analysis is performed completely on the growth substrate. Therefore, all the reported data have been obtained with graphene still being on the Copper thin film.
Effect of a-C layer thickness on Graphene growth
Figure 1c,d and e show the Raman spectra of the annealed samples S1, S1.1 and S1.2 (each having different a-C thicknesses) respectively, and Table 1 summarizes their Raman characteristics22.
It is well known that the IG peak is associated with the doubly degenerate phonon mode at the Brillouin zone center which comes from a normal first order Raman scattering process in graphene and originates from the interplanar sp2-bonded C-C stretching vibrations23. The I2D and ID peaks originate from a second-order process, involving two iTO phonons near the K point for the 2D band and one iTO phonon and one defect in the case of the D band which can be often used to evaluate the number of graphene layers and the grain size of graphene respectively23.
From these information, we can see that in Fig. 1d, the I2D/IG peak ratio of sample S1.1 is much higher (close to 1) than that of sample S1 (Fig. 1c), and its ID/IG peak ratio is nearly the same as S1. As for S1.2 (Fig. 1e), its I2D/IG peak ratio is also higher (but less than 1) than sample S1, and its ID/IG peak ratio is much less as compared to S1. I2D/IG peak ratio equal or greater than 1 indicates the presence of few layers (i.e. layer number around 3–9) of Graphene while the peak ratio less than 1 indicates multi-layer Graphene (i.e. layer number more than 10)24. As all the three samples have I2D/IG peak ratio less than 1, their graphene have multi-layer, with sample S1 having the largest number of layer, and sample S1.1 having the least number of layer, despite that the a-C thickness of S1.1 is not the least. This will be explained later from our proposed growth mechanism.
Effect of annealing time on Graphene growth
Samples S1.1 and S1.2 are subjected to annealing with different annealing times, and Raman signatures with higher 2D peak are indeed observed. Figure 2 shows the plot of I2D/IG and ID/IG vs annealing time. Figures 3 and 4 shows the Raman Maps for samples S1.1 and S1.2 respectively for different annealing times. The means plotted with error bars in Fig. 2 are consistent with the Raman Mappings in Figs 3 and 4.
As I2D/IG ratio greater than 1 represents the presence of few layers of graphene, a sharp increase in the ratio is expected from zero (where no graphene is present) to a value much greater than 1, followed by a decrease in the ratio towards less than 1 as the number of graphene layer is increasing. Likewise, when the graphene layer is being etched away, a sharp change in the ratio from some values less than 1 to a value much greater than 1 and then down to zero should be expected. However, due to the discrete annealing time used in our experiments, such sharp change in the ratio is difficult to observe, and only dotted lines are shown in Fig. 2 for the period where these sharp changes are expected.
For sample S1.1, no Graphene is present for 10 minutes of annealing time and its surface layer is highly defective as indicated by its ID/IG ratio. Graphene formation can be found with 20 minutes of annealing time while the ID/IG ratio drops to a lower value. The largest I2D/IG ratio is observed with 30 minutes of annealing time which is a signature of few layers Graphene (since I2D/IG ratio is higher than 1) and an increase in ID/IG ratio can also be observed. This is followed by a drop in I2D/IG and ID/IG ratio with 40 minutes of annealing time and then a small rise in the I2D/IG for 50 minutes of annealing time while the ID/IG ratio also increases at this point.
On the other hand, the thin a-C layer in sample S1.2 undergoes early crystallization within 5 mins of annealing to produce few layers of Graphene which continues to form multi layered Graphene with a small increase in I2D/IG ratio during 20 to 30 minutes of annealing time. Beyond 30 minutes of annealing time, its I2D/IG and ID/IG ratios decrease continuously, in contrast to sample S1.1, and approaches zero for 60 minute of annealing time. The defects in sample S1.2 is much less than that in sample S1.1 for annealing time more than 40 minutes.
In the light of the above findings, we can understand the reason of the conclusion made by the reported works on the infeasibility of crystallization using Cu as catalyst because they were either having annealing time less than 10 minutes and/or the thickness of a-C film is too thick where the formation of graphene formation will begin only at longer annealing time17.
Effect of annealing temperature on Graphene growth
Samples S1.1 and S1.2 are annealed comparatively at lower temperature of 920 °C and 820 °C for 50 mins. Figure 5 shows plots of Raman spectrum for sample S1.1 and S1.2 annealed at 920 °C and 820 °C for 50 mins. It is evident from Fig. 5 that Graphene with higher 2D peak can be grown in low temperature regimes. However, for sample S1.1, Graphene is no longer synthesized at 820 °C, in contrast to for sample S1.2. No graphene is observed in both samples with further reduction in annealing temperature to 800 °C.
The distinct difference in the behavior of the graphene formation with annealing time and temperature for S1.1 and S1.2 is interesting since the difference in the two samples is only the thickness of the a-C layer. This kind of distinct behavior is believed to be due to the thermo-mechanical stress driven crystallization of a-C into Graphene, and we would like to verify it using finite element analysis.
Finite Element Analysis
Finite Element Analysis (FEA) is performed using ANSYS Workbench R16.2 platform. As thermo-mechanical stress25 is given as:

where Ef and vf are the Young’s modulus and Poisson’s ratio respectively, α is the coefficient of thermal expansion, and ΔT is the range of temperature excursion; the subscripts ‘s’ and ‘f’ refer to substrate and film, respectively, we can see that the stress is independent of the thickness of the a-C layer, and thus the thermo-mechanical stresses in each sample are the same. On the other hand, the thermo-mechanical stress causes the sample to warp, and the resultant Maximum Principal Stress is given by the Stoney’s equation26,27 given as:

where Δ(1/R) = (1/R−1/R0), and R0 and R are the radius of curvature of the samples before and after the thermo-mechanical stresses are developed respectively. ‘t’ is for thickness of the layer in the sample with subscripts ‘s’ and ‘f’ refer to substrate and film, respectively. Here Maximum Principal Stress is considered in the Stoney’s equation for a-C as a-C is known to be a brittle material28.
As Δ(1/R) is due to the warpage of the sample caused by thermo-mechanical stress which is independent of layer thickness in the sample, the value of Δ(1/R) can be assumed to be a constant for the three samples.
According to Equation (2), the Stoney’s equation indicates that thin film stress is inversely proportional to film thickness, with samples S1, S1.1 and S1.2 having a-C thicknesses of 60 nm, 36 nm and 12 nm respectively, the total stress in sample S1.2 should be the highest and that in sample S1 should be the lowest, as indeed shown in Fig. 6a–c 29 as computed by ANSYS. The thermo-mechanical properties of the materials used in the simulation are shown in Supplementary Table S1. Figure 6a–c 29 shows the comparison of the Maximum Principal Stress distributions in the three samples at 1020 °C, and the maximum stress values in a-C layers are decreasing from sample S1.2 to S1.1 and then to S1 as expected from the Stoney’s equation.
ANSYS simulation results of (a–c)29 Maximum Principal Stress distributions in the three samples at 1020 °C, zoning into the maximum stress areas; the area of maximum stress distribution is the largest in S1.2 and the least in S1, and the stresses are compressive, (d–f) Von-Mises stress in Cu (tensile) at the corner of each sample, showing the delamination at the a-C/substrate and Cu/a-C interfaces in these samples; maximum delamination can be observed in sample S1.2.
Figure 6d–f shows the delamination of Cu and a-C films from their respective substrates at the corners where stresses are the highest within each sample. Here we consider Equivalent Von-Mises Stress analysis for Cu because Cu is ductile in nature30. Again, we can see that the delamination is most severe for S1.2 due to the presence of highest stress in Cu and a-C layer. The von-Mises stresses in Cu film are tensile in nature and their values are believed to be high enough that can either cause cracking in Cu film and/or enhancement of H2 diffusion in Cu film through its grain boundaries31,32,33.
Furthermore, thermal strain analysis has also been performed on synthesized Graphene on Cu using ANSYS and is depicted in Fig. 7. The simulation results suggest that the Cu thin film possesses tensile strain (positive strain value) while the Graphene layer possesses compressive strain (negative strain value).
Based on our FEA simulation results, we revisit and explain our experimental results in the following discussion.
Discussions
Sample S1 has the most number of Graphene layers as observed and shown in Fig. 1c. In the same token, S1.2 should have the least number of Graphene layers as its a-C layer thickness is minimum, but Fig. 1e shows otherwise. This abnormality can be better understood by analyzing and comparing the trends of I2D/IG and ID/IG ratio, (as shown in Fig. 2) with the aid of the following governing chemical reactions for graphene growth as given by Vlassiouk et al.34. These reactions are shown in Fig. 8 and are modified for our experiments29 since methane is not used. Formation of hydrocarbons responsible for Graphene formation by the reaction of a-C and H2 at elevated temperatures as shown in Reaction (1) has been confirmed by Ji et al.21 as well.
Governing reactions29 for Graphene growth forming the basis for proposed mechanism of Graphene synthesis on Copper film; Here C represents Carbon atom, Gr represents Graphene, CH*/C* represent Hydrogen bonded-Carbon radical/Carbon radical, H* represent Hydrogen radical and ∆H represents heat.
For annealing time below 10 minutes, the Graphene obtained for sample S1.1 is totally defective as indicated by the nearly zero I2D/IG peak and very high ID/IG in Fig. 2. This is believed to be due to the lower tensile stress in Cu film, and hence longer annealing time is required for hydrogen to reach the underneath a-C for Graphene crystallization. Consequently, defective Graphene is observed below 10 minutes.
As annealing time increases to 20 minutes, the crystallization rate increases with time. The increase in crystallization rate is according to Reaction (2)29 in Fig. 8, which is an endothermic reaction32. As H2 gas has a dual role of carbon activator and Graphene etchant, etching of graphene by the H2 gas occur concurrently, and the number of Graphene layers is reduced. With the availability of the Hydrogen radicals, the exothermic etching reaction (Reaction (3)29 in Fig. 8) is shifting in the forward direction35. The combined effect of crystallization and etching of Graphene results in formation of few layers of Graphene, which can be observed by an increased value of I2D/IG ratio beginning from the 20 minutes of annealing. Due to discretization of annealing times used in our experiment, the exact extent of crystallization and etching of Graphene during 10 to 20 minutes of annealing times are unknown. For 20 minutes of annealing, the quality of Graphene film is improved as compared to that with 10 minutes of annealing and is less defective as reflected by a decreased value of ID/IG value. The production of carbon radicals is also accompanied by production of hydrogen radicals as per the Reaction (1)29 in Fig. 8.
As the annealing time increases to 30 minutes, the amount of hydrogen radicals increases to sufficient quantity to trigger Reaction (3). Also the heat of absorption due to Reactions (1) and (2) reduces the temperature of the Cu film surface. As a result, the crystallization rate is dominated by the etching rate of Graphene, and thus Graphene is continuously etched by H2, primarily at defective sites because these sites facilitates preferential etching of Graphene36. This results in an even lesser number of Graphene layers than the previous shorter annealing times with increased defective sites. Hence, a continuous increase in the I2D/IG and ID/IG ratios up to 30 minute of annealing time in Fig. 2 is observed.
When the annealing time goes beyond 30 minutes, the remaining graphene layer is so thin and the heat generated from Reaction (3) plus the increasing amount of carbon radicals as a by-product of Reaction (3) enhance the crystallization Reaction (2) in the forward direction. This facilitates further crystallization, and more number of Graphene layers is formed as indicated by a decrease in I2D/IG ratio, by the end of 40 minutes of annealing in Fig. 2. Also a decrease in ID/IG is observed because the newly formed layers fill the defective sites created due to etching in the earlier annealing process.
For annealing time beyond 40 minutes, the scenario is similar to what happened during 20 to 30 minutes of annealing time where the hydrogen radicals are also produced with carbon radicals. This increased number of hydrogen radicals and reduction of the Cu film surface temperature because of heat of absorption due to Reactions (1) and (2) triggers Reaction (3) and shifts it in the forward direction, resulting in reduction in the number of Graphene layers. Thus only few layers of Graphene are formed by the end of 50 minutes of annealing. Also the defects increase due to loss of carbon atoms from the Graphene crystal forming defective sites. This is indicated by an increase in I2D/IG and ID/IG ratios in Fig. 2.
Similar behavior depicted in Fig. 1e for sample S1.2 may be explained with the help of Fig. 2. One may attribute the phenomena to the higher tensile stress in Cu film that enhance hydrogen diffusion through Cu film and graphene synthesis with fairly low ID/IG ratio as can be observed even for annealing time as low as 5 minutes in Fig. 2. Due to higher tensile stress in S1.2, the carbon radical supply onto the Cu surface is high because of the enhanced hydrogen diffusion through the Cu film, renders Reaction (2) favorable and thus graphene growth continues. An increase in number of graphene layers is observed for annealing times between 8 to 20 minutes as indicated by the decrease in I2D/IG ratio in Fig. 2. The ID/IG ratio also decreases because the newly formed layers fill up the defective sites, thus reducing the effective defects in the Graphene crystal so formed. The high rate of Reaction (1) produces more H*, and the heat of absorption due to Reactions (1) and (2) reduces the temperature of the Cu film surface. These two factors enhance Reaction (3) in its forward direction and reduces the layer of graphene synthesized when the annealing time is prolonged to 30 minutes. Thus, the number of graphene layer is no longer increasing continuously with the annealing time, and a slow reduction in the number of layer takes place as Reaction (3) starts dominating slowly, and this is indicated by a small increase in I2D/IG while the ID/IG ratio also increases due to etching of graphene as explained before. Thereafter, the growth Reaction (2) becomes active swiftly for the annealing time beyond 30 minutes, and I2D/IG and ID/IG ratio keep on decreasing. This is because the Reaction (3) in the previous step produces carbon radicals as a by-product and Graphene growth Reaction (2) will be shifted to its forward reaction, giving hydrogen radicals lesser chance to etch away the graphene. When the annealing time is long enough (40 minutes and beyond), unlike the situation in sample S1.1, a different mechanism kick in as can be seen from the different rate of change of the I2D/IG ratio as seen in Fig. 2. This is believed to be due to two possible mechanisms.
One mechanism is stress induced migration37 or stress induced voiding38,39 in Cu that enhance the movement of vacancies in Cu film. These voids move outwards toward the edge of the Cu film and during this movement, they combine and form bigger voids, enhancing the diffusion of hydrogen and carbon radical supply. The rationale behind this postulation is derived from the computed tensile stresses in Cu film on SiO2 substrate and on a-C film with SiO2 substrate. Our finite element calculations using Equations (1) and (2) show that the tensile stress in Cu film for the former is 1190 MPa, and that for the latter ranges from 460 MPa to 690 MPa depending upon the a-C layer thickness as depicted in Fig. 9. With a-C film thickness of 12 nm at the beginning for S1.2 sample, and after 40 minutes of annealing, the a-C film thickness has become very small, and hence the tensile stress in Cu film could be sufficiently high for stress induced voiding to occur, especially under elevated temperature during annealing. This rationale is further strengthened by the ANSYS simulation for the deformation in the samples shown in Fig. 10. It shows that the maximum deformation which is a direct measure of area under high stress, is distributed at the corners for sample S1 and S1.1 while the same is densely distributed at the center for sample S1.2. This is because initially the corners are comparatively free to deform so the high values of deformation concentrate there. With the decrease in a-C layer, the stress at a-C/Cu interface starts to increase slowly causing delamination at the corners as shown in Fig. 6d–f. Hence, the corners become stress free and the deformation to migrate from the corners to the center area resulting in higher stress distribution at the center. Therefore, the carbon radicals move out slowly from the corners in S1.1 and with the decrease in a-C layer thickness, the movement of the radicals become higher from the center. In other word, the movement of carbon is large at the center of S1.2 in the beginning, and the distribution area is wide which keeps on widening.
Another mechanism is the sharp increase in the a-C film compressive stress as computed using finite element as shown in Fig. 6. When the annealing time is longer than 40 minutes, the remaining thickness of a-C layer could be below 4 nm for S1.2 since its initial thickness is only 12 nm, and the stress in a-C layer becomes very high as depicted in Fig. 9. This large compressive stress in a-C layer will “push” the carbon atoms in perpendicular directions in order to release the stress, enhancing both the hydrogen and carbon diffusion29.
As a result of the above two mechanisms, the rate of crystallization of Graphene is much faster than the rate of etching, and the number of layer of the synthesized Graphene increases at a higher rate in S1.2, resulting in multi-layered graphene formation. With such a higher rate of diffusion of carbon radicals and formation of graphene, and the thin layer of a-C in S1.2 at the start, when annealing time go up to 60 minutes and beyond, the remaining a-C is quickly depleting. As a result, the Reaction (2) shifts in the backward direction and Graphene etching reaction becomes dominant and hence all the grown Graphene is etched away by H2 gas. The points at which the growth of Graphene layers stops and etching reaction takes over is unknown due to discretization of annealing times in our experiments.
On the other hand, the above-mentioned two mechanisms are unlikely to happen in S1.1. First of all, its a-C thickness is 36 nm to start with, and after 40 minutes of annealing, its thickness is still well above 4 nm, and thus the increase in the compressive stress of a-C is slow as seen in Fig. 9. Also, with thick a-C film still underneath, the tensile stress in Cu film is unlikely to increase significantly to trigger the stress induced voiding.
When the annealing temperature is lowered, the value of the von-Mises stress in Cu film and the area of maximum stress are reduced. Figure 11a–c shows the ANSYS simulation results of the von-Mises at different annealing temperatures for S1, and one can see a reduction of the maximum stress area, and the values of the maximum stress decreases from 9.61 GPa to 7.65 GPa as the temperature decreases from 1020 °C to 820 °C. Thus, the enhanced hydrogen diffusion through Cu film decreases significantly which slow down the reaction between H2 and a-C. Consequently, carbon radicals’ supply is reduced but Graphene etching by H2 gas still persists. Thus, only formation of nearly single layer or very few layers Graphene are possible.
When the temperature is further lowered, the diffusion of H2 through the Cu film will be so slow that Reaction (1)29 (mentioned in Fig. 8) will proceed very slowly, resulting in much slower Reaction (2), and the Graphene so formed will be etched away immediately by the H2 gas on top (as H2 gas diffusion through Cu film is also slow down, and thus its surface concentration will be high for etching). Thus no formation of graphene layers is observed below a certain temperature, where we term it as threshold temperature. This threshold temperature is lower for S1.2 since it has thinner a-C film and thus the stress in Cu film is higher than S1.1 for a given temperature as shown earlier, and this is consistent with our experimental results. ANSYS simulation for total deformation as shown in Fig. 11d validates the fact the stress in Cu for sample S1.2 is the highest followed by sample S1.1 and S1 even at lower temperatures.
Moreover, the growth mechanism discussed for annealing temperature value of 1020 °C stands true for lower temperature regimes as well because the trends in stress variation are similar to the high temperature regimes. (For more details, please refer to Supplementary Figures S3,S4 and S5 in Supplementary Information).
The thermal strain results presented in Fig. 7 are verified using Raman characteristics as shown in Fig. 12. Figure 12 represents the plot of Raman frequencies of G (ωG) vs 2D (ω2D) modes. Dashed line in purple color with a slope (Δω2D/ΔωG) of 2.2 represents Unstrained Graphene40 and point ‘O’ represents intrinsic frequencies of the two modes which are not affected by strain or excess charges40,41. The chances of doping in our samples are minimum as the experiments are conducted in vacuum. The Raman frequencies of G and 2D modes for samples S1.1 and S1.2 (represented by red-dotted linearly fitted lines) are nearly parallel to the Unstrained-Graphene line and the belong to Compressive Strain region40.
A worth-noting point observed in our samples is that the ID peak intensity, which is also connected with defects, is on a higher side but these defects are advantageous with respect to contact resistance on metals42,43, and thus will be beneficial for ULSI Interconnects’ applications.
Proposed Mechanism
From the above investigations, we can see that graphene synthesis on Cu is having a different mechanism as compared to that in the conventional CVD and gaseous carbon-source based graphene synthesis. Based on the results obtained in this work, we propose the plausible mechanism for the Graphene synthesis using amorphous carbon as the solid carbon source in presence of copper catalyst as depicted in Fig. 8 which consists of four steps.
Step I- The process begins with native copper oxide reduction by H2 gas at high temperatures ranging from 820–1020 °C, releasing water molecules in the gas phase as by-product and stress induced grain growth44,45.
Step II- The high annealing temperature causes thermo-mechanical stress to develop in the films of the sample, and its subsequent warping, rendering high tensile stress in the Cu film.
Step III- The high tensile stress due to thermo-mechanical stress in Cu film then enhances hydrogen gas diffusion through the Cu film and reach out to the underlying a-C layer for graphene crystallization.
Step IV- H2 gas in the meanwhile reacts with the a-C to form carbon radicals in the presence of Cu catalyst and moves out through the grain boundaries and crystallizes into Graphene. The Graphene formation and etching are simultaneous in the presence of the H2 gas based on discussed reactions. For a given annealing time and annealing temperature, the supply of carbon radical depends on the a-C layer thickness and different stress levels, and hence different quality of Graphene is obtained for different samples.
Conclusion
Various experiments are successfully conducted in this work in order to explore the growth mechanisms of Graphene synthesis using a-C as the carbon source and Cu as the catalyst. Diffusion of H2 gas through copper grain boundaries to interact with a-C underneath and thermo-mechanical stress in copper and a-C films are proposed to be the mechanisms for the Graphene synthesis. Therefore, even though samples S1.1 and S1.2 are similar in configuration, their dependence of the graphene growth rate on the annealing time and temperature are different due to their difference in thickness of a-C, which result in different film stresses in the two samples. The proposed growth mechanisms are found to valid for in the temperature range of 1020 to 820 °C. With this mechanism, the infeasibility of Graphene formation on copper film as reported by previous works can also be explained.
Additional Information
How to cite this article: Narula, U. et al. Growth Mechanism for Low Temperature PVD Graphene Synthesis on Copper Using Amorphous Carbon. Sci. Rep. 7, 44112; doi: 10.1038/srep44112 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science. 306, 666, doi: 10.1126/science.1102896 (2004).
Moser, J., Barreiro, A. & Bachtold, A. Current-induced cleaning of graphene. Appl. Phys. Lett. 91, 163513, doi: 10.1063/1.2789673 (2007).
Geim, A. K. & Novoselov, K. S. The rise of graphene. Nature Materials. 6, 183, doi: 10.1038/nmat1849 (2007).
Bolotin, K. I. et al. Ultrahigh electron mobility in suspended graphene. Solid State Comm. 146, 351, doi: 10.1016/j.ssc.2008.02.024 (2008).
Balandin, A. A. et al. Superior thermal conductivity of single-layer graphene. Nano Lett. 8, 902, doi: 10.1021/nl0731872 (2008).
Nair, R. R. et al. Fine structure constant defines visual transparency of graphene. Science. 320, 1308, doi: 10.1126/science.1156965 (2008).
Lee, C., Wei, X., Kysar, J. W. & Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science. 321, 385, doi: 10.1126/science.1157996 (2008).
Reina, A. et al. Growth of large-area single- and bi-layer graphene by controlled carbon precipitation on polycrystalline Ni surfaces. Nano Res. 2, 509, doi: 10.1007/s12274-009-9059-y (2009).
Thiele, S. et al. Engineering polycrystalline Ni films to improve thickness uniformity of the chemical-vapor-deposition-grown graphene films. Nanotechnology. 21, 015601, doi: 10.1088/0957-4484/21/1/015601 (2010).
Reina, A. et al. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 9, 30, doi: 10.1021/nl801827v (2009).
Li, X. et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science. 324, 1312, doi: 10.1126/science.1171245 (2009).
Li, X. et al. Transfer of large-area graphene films for high-performance transparent conductive electrodes. Nano Lett., 9, 4359, doi: 10.1021/nl902623y (2009).
Levendorf, M. P., Ruiz-Vargas, C. S., Garg, S. & Park, J. Transfer-free batch fabrication of single layer graphene transistors. Nano Lett. 9, 4479, doi: 10.1021/nl902790r (2009).
Lee et al. Wafer-scale synthesis and transfer of graphene films. Nano Lett. 10, 490, doi: 10.1021/nl903272n (2010).
Pan, G. et al. Transfer-free growth of graphene on SiO2 insulator substrate from sputtered carbon and nickel films. Carbon. 65, 349, doi: 10.1016/j.carbon.2013.08.036 (2013).
Zheng, M. et al. Metal-catalyzed crystallization of amorphous carbon to graphene. Appl Phys Lett. 96, 063110, doi: 10.1063/1.3318263 (2010).
Orofeo, C. M., Ago, H., Hu, B. & Tsuji, M. Synthesis of large area, homogeneous, single layer graphene films by annealing amorphous carbon on Co and Ni. Nano Res. 4, 531, doi: 10.1007/s12274-011-0109-x (2011).
Park, B. J. et al. Realization of large-area wrinkle-free monolayer graphene films transferred to functional substrates. Sci. Rep. 5, 9610, doi: 10.1038/srep09610 (2015).
Pirkle, A. et al. The effect of chemical residues on the physical and electrical properties of chemical vapor deposited graphene transferred to SiO2 . Appl. Phys. Lett. 99, 122108, doi: 10.1063/1.3643444 (2011).
Calado, V. E., Schneider, G. F., Theulings, A. M. M. G., Dekker, C. & Vandersypen, L. M. K. Formation and control of wrinkles in graphene by the wedging transfer method. Appl. Phys. Lett. 101, 103116, doi: 10.1063/1.4751982 (2012).
Ji, H. et al. Graphene growth using a solid carbon feedstock and hydrogen. ACS Nano. 5, 7656, doi: 10.1021/nn202802x (2011).
Narula, U., Tan, C. M. & Lai, C. S. Copper induced synthesis of graphene using amorphous carbon. Microelectron Reliab. 61, 87, doi: 10.1016/j.microrel.2016.01.005 (2016).
Malard, L. M., Pimenta, M. A., Dresselhaus, G. & Dresselhaus, M. S. Raman spectroscopy in graphene. Physics Reports. 473, 51, doi: 10.1016/j.physrep.2009.02.003 (2009).
Ferrari, A. C. Raman spectroscopy of graphene and graphite: disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 143, 47, doi: 10.1016/j.ssc.2007.03.052 (2007).
Hoffman, R. W. Physics of Non-Metallic Thin Films Vol. 14 (eds Dupuy, C. H. S. & Cachard, A. ) Part III, Ch. 12, 273–354 (Plenum Press, 1976).
Stoney, G. G. The tension of metallic films deposited by electrolysis. Proc. R. Soc. London. A82, 172, doi: 10.1098/rspa.1909.0021 (1909).
Xu, S. et al. Properties of carbon ion deposited tetrahedral amorphous carbon films as a function of ion energy. J. Appl. Phys. 79, 7234, doi: 10.1063/1.361440 (1996).
Ichim, I., Kuzmanovic, D. V. & Love, R. M. A finite element analysis of ferrule design on restoration resistance and distribution of stress within a root. International Endodontic Journal. 39, 443, doi: 10.1111/j.1365-2591.2006.01085.x (2006).
Narula, U. & Tan, C. M., Determining the parameters of importance of a graphene synthesis process using design-of-experiments method. Appl. Sci. 6, 204, doi: 10.3390/app6070204 (2016).
De-Groot, R., Peters, M. C., De-Haan, Y. M., Dop, G. J. & Plasschaert, A. J. Failure stress criteria for composite resin. J Dent Res. 66, 1748, doi: 10.1177/00220345870660121001 (1987).
Birnbaum, H. K. Mechanisms of hydrogen related fracture of metals, Technical Report A-208–210, 1–18 (Defense Technical Information Center, Office of Naval Research: University of Illinois, USA, 1989).
Buehler, M. J., Hartmaier, A. & Gao, H. Constrained grain boundary diffusion in thin copper films. In MRS Proceedings: Symposium P – Nanoscale Materials & Modeling-Relations Among Processing, Microstructure and Mechanical Properties. 821–P1.2 (Materials Research Society: Boston, Massachusetts, Nov. 28–Dec. 2, 2004).
Yao, J. & Cahoon, J. R. Experimental studies of grain boundary diffusion of hydrogen in metals. Acta Metallurgica et Materialia. 39, 119, doi: 10.1016/0956-7151(91)90333-V (1991).
Vlassiouk, I. et al. Role of hydrogen in chemical vapor deposition growth of large single-crystal graphene. ACS Nano. 5, 6069, doi: 10.1021/nn201978y (2011).
Zhang, Y., Li, Z., Kim, P., Zhang, L. & Zhou, C. Anisotropic hydrogen etching of chemical vapor deposited graphene. ACS Nano. 6(1), 126, doi: 10.1021/nn202996r (2012).
Choubak, S., Biron, M., Levesque, P. L., Martel, R. & Desjardins, P. No graphene etching in purified hydrogen. J. Phys. Chem. Lett. 4, 1100, doi: 10.1021/jz400400u (2013).
Saito, N., Murata, N., Tamakawa, K., Suzuki, K. & Miura, H. Stress-induced migration of electroplated copper thin films used for 3D integration. In 5thInternational Microsystems Packaging Assembly and Circuits Technology Conference. 1–4 (IEEE: Taipei, Oct. 20–22, 2010).
Hou, Y. & Tan, C. M. Stress-induced voiding study in integrated circuit interconnects. Semiconductor Science and Technology. 23, 075023, doi: 10.1088/0268-1242/23/7/075023 (2008).
Hou, Y. & Tan, C. M. Comparison of stress-induced voiding phenomena in copper line–via structures with different dielectric materials. Semiconductor Science and Technology. 24, 085014, doi: 10.1088/0268-1242/24/8/085014 (2009).
Lee, J. E., Ahn, G., Shim, J., Lee, Y. S. & Ryu, S. Optical separation of mechanical strain from charge doping in graphene. Nat. Commun. 3, 1024, doi: 10.1038/ncomms2022 (2012).
Ding, F. et al. Stretchable graphene: a close look at fundamental parameters through biaxial straining. Nano Lett. 10, 3453, doi: 10.1021/nl101533x (2010).
Bharadwaj, B. K., Nath, D., Pratap, R. & Raghavan, S. Making consistent contacts to graphene: effect of architecture and growth induced defects, Nanotechnology. 27, 205705, doi: 10.1088/0957-4484/27/20/205705 (2016).
Smith, J. T., Franklin, A. D., Farmer, D. B. & Dimitrakopoulos, C. D. Reducing contact resistance in graphene devices through contact area patterning, ACS Nano. 7, 3661, doi: 10.1021/nn400671z (2013).
Zielinski, M., Vinci R. P. & Bravman, J. C. Effects of barrier layer and annealing on abnormal grain growth in copper thin films. J. Appl. Phys. 76, 4516, doi: 10.1063/1.357283 (1994).
Moriyama, M., Matsunaga, K. & Morita, T. The effect of strain distribution on abnormal grain growth in cu thin films. Materials Transactions. 45, 3033, doi: 10.1007/s11664-003-0219-7 (2004).
Acknowledgements
We acknowledge the support of Ministry of Science and Technology (Taiwan) grant (MOST-105-2221-E-182-060) and Semiconductor Laboratory in Department of Electronic Engineering of Chang Gung University, Taiwan. The samples are prepared and annealed at Semiconductor Laboratory in Department of Electronic Engineering of Chang Gung University. Raman datum are taken at Biosensor Laboratory in Chang Gung University. Finite Element Modeling was performed at the Centre of Reliability Sciences and Technologies in Chang Gung University.
Author information
Authors and Affiliations
Contributions
Experiments Conceived by: C.S. Lai and C.M. Tan, Experiments’Design, Simulation and Measurements performed by: U. Narula, Data Analyzed by: C.M. Tan and U. Narula, Manuscript compiled by: U. Narula and C.M. Tan.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Narula, U., Tan, C. & Lai, C. Growth Mechanism for Low Temperature PVD Graphene Synthesis on Copper Using Amorphous Carbon. Sci Rep 7, 44112 (2017). https://doi.org/10.1038/srep44112
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep44112
This article is cited by
-
The utilization of waste cooking palm oil as a green carbon source for the growth of multilayer graphene
Journal of the Australian Ceramic Society (2021)
-
Spectral and structural investigation of layered growth of copper and graphene deposited by sputtering and annealing
Applied Physics A (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.