Abstract
The physical properties of carbon materials can be altered by doping. For instance, the electronic properties of graphene can be modulated by controlling the substitutional doping of the carbon lattice with N. In addition, C–N bonding configurations with three ring types are recognized: pyridinic-N, pyrrolic-N, and graphitic-N. Controlling the type and relative density of various types of substitutional N is an important objective that requires an extremely high level of precision when the atomic lattice is constructed. This control can be accomplished only via bottom-up methods, such as chemical vapor deposition (CVD). The number of reports on N-doped graphene (NDG) grown via CVD has increased over the past decade, but a reliable wafer-scale production strategy that can realize the desired atomic-precision growth of NDG is still lacking. To identify the most promising strategies and analyze the consistency of the results published in the literature, we review the CVD growth and characterization of two-dimensional NDG and two of the most popular applications of NDG films: field-effect transistors and energy storage devices.
Similar content being viewed by others
Introduction
Graphene is a two-dimensional (2D) structure in which sp2-hybridized C atoms are densely packed into a benzene-ring structure. Graphene exhibits ultrahigh mobility (~200,000 cm2 V−1 s−1)1, broadband optical absorbance (2.3%) in the visible range, a large Young’s modulus of 1 TPa, high thermal conductivity at room temperature (~5000 W/mK)2, and large surface area (2630 m2/g)3. These extraordinary properties have sparked great interest in using graphene in applications, such as sensors4,5,6,7, conducting channels8,9, nanoelectronics10, flexible electronics11, electromagnetic interference shielding12, and energy storage13,14,15,16.
Nonetheless, the zero bandgap of graphene impedes the development of graphene-based nanoelectronic devices and battery applications17,18,19,20,21. Intrinsic graphene properties can be extended via bandgap tuning; thus, researchers have doped pure graphene with heteroatoms, molecules, nanoparticles, chemicals, adsorbates, and impurities22,23. Heteroatom dopants can be electron donors (n-type) or electron acceptors (p-type). Among the heteroatoms explored to date are the N (n-type)17,24,25,26,27, B (p-type), P (n-type)28, S (p- or n-type)29, I30, F31, Si (p-type), and Ge19,22,32,33,34,35,36,37,38. Elements with larger atomic radii than C, such as S, Si, and Ge, can replace one or two C atoms19,22,32,33,34,35,36,37,38. Moreover, halogen atoms (F, Cl, Br, and I) form ionic or covalent bonds with graphene, albeit with varying degrees of halogenation31,36,37,39,40. Both B and N, the neighboring elements of C on the periodic table, easily substitute C in the graphene lattice, resulting in p- and n-type doping, respectively22,24,27. As a dopant, N is more popular than B because it is more easily substituted into graphene24. In particular, N has five valence electrons in the 2s and 2p atomic orbitals, which could form strong bonds with C atoms24.
In addition, N-doped graphene (NDG) is widely applied in electronic devices26,41, sensors42,43,44, solar cells45, enhanced catalysis46,47,48,49,50,51,52, energy conversion, and storage49,53,54,55 (Fig. 1). However, most NDG-related reports consider the effect of the overall N-dopant concentration on device performance. Further, detailed studies focusing on the role of each type of C–N bonding configuration and N-dopant location are needed, as demonstrated by a few researchers (e.g., Wei et al. demonstrated NDG with graphitic-N configuration use for application in field-effect transistors (FETs)56). In NDG, the N-dopant bonding configurations are of three types: graphitic‑N (sp2 hybridization), pyridinic-N (sp2 hybridization), and pyrrolic-N (sp3 hybridization)26,32,33,34,35,56,57,58. Each type of N-doping configuration affects the device performance in a specific way49. Therefore, an appropriate synthesis approach that controls the N-doping configuration and N content for a specific device is in high demand.
The gray hexagon in the center displays a molecular model of the N-doping configurations in a graphene lattice (gray and blue denote C and N atoms, respectively). Surrounding the NDG model expresses the properties of NDG needed in applications. The outermost circles enclose typical NDG applications with representative images (e.g., the field-effect transistor, etc.).
Several NDG synthesis methods have been developed to date59. These methods produce NDG with varied configurations, such as a different number of graphene layers and the type/distribution of N atoms. Moreover, they have distinct advantages and disadvantages regarding precursor costs, the overall process, thickness control, shape, defect size, reproducibility, and production scale. Some NDG synthesis methods use graphene flakes or graphene derivatives (graphene oxide, GO, or reduced GO) for scalable NDG production (Table 1). Although defects in graphene derivatives facilitate the doping process18, the end product is primarily synthesized in powdered form15. Controlling the defect sites (and hence the doping) in batch-to-batch samples of graphene derivatives is a challenging task60. Direct thermal annealing of graphene derivatives during N doping causes irreversible stacking of the graphene sheets due to strong π–π interactions. To circumvent these problems, NDG can be grown using the chemical vapor deposition (CVD) process. To balance the cost-quality tradeoff of 2D NDG synthesis, the CVD method is more suitable than annealing16, physical vapor deposition61, pyrolysis62,63, arc discharge27,64,65, hydrothermal61,66,67, and solvothermal21,68,69 syntheses (Table 1).
In FETs or battery applications, NDG is usually synthesized as a 3D network of 2D materials functionalized by additional moieties, such as metal nanoparticles70, or post-processing using solvents, plasma, heat, or another physical process. A few excellent reviews on NDG have been published in the last decade20,32,33,57,59,71,72. However, to our knowledge, a comprehensive review of CVD-grown 2D NDG is currently lacking. Recently, Ullah et al. reviewed the process and applications of 3D graphene modified with several dopants via CVD72. Focusing on graphene synthesis processes, Ikram et al. compared the uses of various carbon nanomaterials (3D networks) in battery applications20. Wang et al. summarized various doping methods and applications of the resulting products33. Still, the many challenges associated with controlling the N location and concentration in the graphene lattice, necessary for specific applications, have been overlooked.
We review the growth processes and characterization of CVD-based NDG, along with the quality consistency of the as-produced 2D material. The significance of controlling the N-doping configuration and using a straightforward CVD method is emphasized. Finally, we survey the current experimental and theoretical insight into FETs and energy storage applications based on as-grown NDG.
Chemical vapor deposition of N-doped graphene films
Although CVD promises a reproducible and controlled high-quality synthesis of graphite/graphene thin films, at a large scale and low cost4,11,37,73,74,75, fabricating reproducible wafer-scale 2D NDG with controlled properties remains challenging (Table 2)18. In addition, NDG synthesis via the CVD approach (Fig. 2) can employ a wide range of C- or N-containing precursors, either during graphene growth (in the same system, i.e., in situ) or afterward (i.e., ex situ treatment)34,35,53. The N-dopant content of NDG, synthesized in or ex situ, ranges from 0.2 to 16 at.%32,33,34,35,46,56. The ex situ synthesis properly controls the C–N bonding configuration76 and is sometimes preferred due to its lower cost, higher reliability, and increased throughput17.
The CVD approach for N doping can occur via surface-charge transfer or, more commonly, via substitutional doping18,28,37. In surface-charge transfer doping, metal atoms and gas molecules are adsorbed to the substrate;4,17,18,75 however, the electronic properties of the resulting materials are unstable in the long term37. Substitutional doping, which incorporates covalently bonded heteroatoms into the graphene lattice, stabilizes the material but degrades the extraordinary properties of graphene34,35. Ideally, the performance enhancement should not disturb the intrinsic properties of graphene.
To study and control N doping in graphene, 2D NDG grown via CVD is eminently suitable, along with zero-dimensional77 and 3D-NDG forms64,78 which are commonly produced using other synthesis methods. The CVD routes include thermal CVD25,26,56, plasma-enhanced CVD34,79,80, radio-frequency (RF) or microwave (MW) plasma CVD81, and electron cyclotron resonance CVD. Direct growth via plasma CVD on a dielectric substrate (e.g., glass or SiO2/Si) has been reported, but the as-produced NDG crystals are small with a high density of defects (N–O and C–O bonds) and low electron mobilities34,79,80,81,82. Thermal CVD produces NDG with significantly fewer defects and high electron mobilities25,26. Recently, NDG with few defects and high charge carrier mobility has been obtained via the combination of plasma and thermal CVD83. Table 2 compares the NDG growth methods developed in the most recent decade. Overall, the CVD NDG growth can be classified into two broad categories (see Fig. 2)14,26,32,33,46,81,84,85.
-
1.
In situ growth: N-containing gas/plasma treatment during the graphene growth or immediately after.
-
2.
Ex situ growth: Post-growth N-doping treatment of as-made graphene.
In the in situ CVD approach, the NDG is usually grown on a transition metal catalyst (e.g., Cu or Ni). Similar to CVD graphene growth4,75, a reactive mixture containing the C and N precursors is introduced at the growth temperature (~700–1000 °C)26,35,44,53,56,86. These precursors dissociate on the surface of the catalyst, and NDG forms via the organization/precipitation of the C and N atoms. When the precursors are gases (e.g., CH4 or C2H4 as the C source; NH3 or N2 as the N source), the amount of N dopant is varied by adjusting the flow rate, the ratio of the C and N precursor gases, and processing time46,56,80,85. The N content is usually low (0.2–6 at.%) but a few reports have claimed otherwise (9–16 at.% sourced from NH3; see Table 2).
NDG has also been grown using solid/liquid organic precursors, such as pyridine26,46,58,87, acetonitrile53, CH3CN88, dimethylformamide35, melamine, and monoethanolamine89. However, in most of these reports, the quantity of liquid precursor was not mentioned or is vaguely stated. Therefore, their reproducibility is uncertain. In addition, solid/liquid N precursors35 yield a high O content in the form of C=O and C–O or N–O bonds (as detected via x-ray photoelectron spectroscopy [XPS])88, which lowers the quality of the resulting NDG material. Among the solid/liquid precursors, the sublimation point of melamine is too high (343 °C), and acrylonitrile (containing C−C, C=C, and C≡N bonds) cannot form graphitic-N-type when heated to a temperature of >500 °C57. In contrast, pyridine has bond energies similar to graphitic-N-type, inducing the formation of NDG with dominant graphitic-N-type bonds that do not significantly degrade the graphene lattice26. However, pyridine is highly flammable (with a flashpoint of −21 °C) and toxic. Therefore, its use requires extreme care and the CVD growth must proceed in well-regulated conditions.
Thus far, catalytic Cu foils (rather than Ni) are preferred to obtain controlled 2D NDG, a trend that follows the CVD graphene processes26. As the solubility of C in Ni is high90 (C solubility in Cu is 0.03 at.% at 1000 °C91, while in Ni is 0.29 at.% at 700 °C and 0.9 at.% at 900 °C92,93), C atoms diffuse in the Ni substrate during the growth step. Knowingly, these C atoms out-diffuse and form multilayer graphene during the cooling step94,95. Similarly, if N is implanted in the Ni lattice (the diffusion coefficient of N in Ni is 4.46 × 10−13 m2/s)96, the N atoms out-diffuse to the Ni surface and N atoms can become trapped in the graphene lattice, forming NDG during the cooling step. This hypothesis was experimentally confirmed in the ultrahigh-vacuum growth of NDG on a Ni (111) single-crystal substrate, which extended over a few square nanometers76. Interestingly, these authors pretreated the substrate under a porphyrin flux prior to graphene growth, achieving precise control of the C and N quantities. Similarly, Zhang et al. performed vacuum annealing on a Ni/B/SiO2/Si substrate with trace elements (C, N, etc.) present in the reactor. Upon cooling, an analog of NDG was formed (N = 0.6–2.3 at.%). The C atoms were trapped in Ni while N was trapped in the B films, thus segregating the C and N97. It would be pertinent to test these experiments at wafer-scale and under thermal CVD conditions (low-to-high pressure and elevated temperatures).
Ex situ CVD processes include the post-treatment of previously grown graphene layers14 or flakes17,79,81,98. The ex situ treatments are similar to those of thermal annealing (Table 1). Note that, in the literature, thermal annealing often refers to the processing of powdered graphene derivatives (3D networks)59; these are outside the scope of this review. The ex situ growth of NDG is uniquely advantageous for simultaneous device production17,79.
We noticed a considerable variation among the N-doping environment of CVD processes (Table 2). The variable parameters include activation source (heat, plasma, or both), treatment time (few seconds to a few hours), chamber pressure (from ultrahigh-vacuum to atmospheric pressure), substrate temperature (450 to 1040 °C), and catalyst material thickness (a few tens to a few hundred micrometers). Such scattering offers little value as each of these parameters can control the quality of the as-produced NDG material. In fact, few teams have claimed dominant N-doping configuration (graphitic-N, pyridinic-N, or pyrrolic-N-type) as a result of fine-tuning the CVD parameters (Table 2)46. For instance, Li et al. obtained major pyrrolic-N-type NDG growth by setting a high substrate temperature (1000 °C), which inhibits the pyridinic-N-type configuration85.
To date, the areas of NDG films grown via CVD are on the scale of square nanometers or square micrometers26,32,81,99. Future studies should target the growth of wafer-scale NDG with structural and thickness uniformity33,99. In particular, the NDG growth process must be systematized to clarify the relationship between the process parameters and the N lattice sites as well as their bonding configuration26,32,33,56,76.
N-doped graphene characterization
To characterize NDG, researchers employ various analytical tools18,71. Unlike the previous reviews20,32,33,57,59,71,72, we constrained the characteristics of CVD-grown NDG to the number of layers, overall texture/structure, surface chemistry, and a few other physicochemical attributes (summarized in Table 3).
Optical spectroscopy
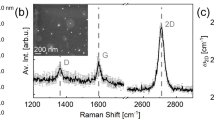
Raman spectroscopy is a powerful nondestructive tool that evaluates the crystalline quality, number of layers, and doping level of NDG100,101. Figure 3 presents a typical Raman spectrum of an NDG sample. The spectrum exhibits four peaks in 1000 to 3000 cm−1 range, one each in the D band (∼1351 cm−1), G band (∼1590 cm−1), D′ band (∼1629 cm−1), and 2D band (∼2696 cm−1)35,44,53,56,81. Some studies have reported a shoulder peak in the D′ band, associated with defects in the graphene lattice46,53. The NDG growth can be detected via Raman spectroscopy, as the G and D bands are sensitive to chemical doping101.
Raman spectra of NDG with different numbers of layers. The inset is a zoomed-in view of the 2D band (reproduced from56 with permission from the American Chemical Society).
The D band observed in graphitic C is a disorder-induced feature. The heteroatoms disrupt the graphene lattice symmetry, enhancing the intensity of the D band in NDG samples14,35,37,56,57,88 compared with that of pristine graphene75. In Raman mapping studies44, Luo et al. 46 and Xu et al. 17 reported an enhanced D peak resulting from increased defects in an NDG lattice over large areas (6 × 4 μm). Using Raman mapping, they revealed that the incorporated N formed a domain rather than a uniform distribution through the lattice. This finding was endorsed by time-of-flight secondary ion mass spectrometry scanned over a few tens of square micrometers.
In the literature, the D band to G band intensity ratio (ID/IG) of CVD-grown NDG ranges from 0.87 to 1.0 (Table 2), which is higher than that in CVD-grown graphene or graphite thin films4,73,74,75. Ito et al. demonstrated that the ID/IG ratio in NDG largely depends on the selected C and N source gases with or without the use of H286. They found lower ID/IG values at a relatively higher H2 flow (>100 standard cubic centimeter (minute)) or growth temperature (>800 °C), which they attributed to fewer C–N bonds and a more ordered graphene lattice. At low N-doping concentrations (<2 at.%), the G and 2D bands of the NDG samples are narrow and symmetric, similar to those of pristine graphene82.
Upshifted G and 2D bands are a common feature of substitutional N doping in graphene lattices46,81,85,101. In contrast, adsorbed n-type-doped heteroatoms feature a downshifted G band. This downshift was reported by several groups employing ex situ graphene processing17. Moreover, the full width at half maximum of the D and G bands was greater in the spectrum of pyrrolic-N NDG than in the spectra of graphitic-N56 and pyridinic-N-type NDG46,53, at the same doping concentration.
From the above studies, a higher-intensity D band is a valuable fingerprint of NDG. However, as other defect types can increase the D-band intensity, further confirmation of N doping via other techniques is required. Several groups have claimed NDG growth after measuring the Raman spectra at a few points on the sample, which is insufficient. Other studies have supported NDG growth using other characterization tools, which is highly recommended for confirming the layer number, crystalline quality, and N-doping content of NDG samples.
Other optical spectroscopic techniques, such as Fourier-transform infrared spectroscopy81, ultraviolet (UV)-visible spectroscopy81, and THz time-domain spectroscopy85, are seldom used in NDG characterization; thus, these are not discussed in detail, here.
Surface analysis techniques
To understand the pathway of N doping, we probe the elemental composition and chemical bonding of the doped N atoms in NDG. The most powerful tool for this purpose is XPS, which provides a surface-sensitive chemical analysis to depths of 1 to 10 nm. A typical XPS survey spectrum of an NDG sample is scanned over the 200 to 450 eV range of binding energies. The N content in the NDG is estimated from the intensity ratio of the N1s (~400 eV) and C1s (~284 eV) peaks. The N configurations can be determined from high-resolution C1s and N1s spectra44,46,53,79,83. Different C–N chemical bonding configurations manifest as several shoulder peaks in the core-level spectra. The C1s peak of an NDG sample can usually be deconvoluted into three components: (i) the major peak at a binding energy of 284.4 eV (sp2-hybridized C atoms in graphene/graphite)73,75, (ii) a peak at 285–285.5 eV (sp2 C bonded with N), and (iii) a peak at 286.5 eV (sp3 C atoms bonded with N)46,53. However, many NDG reports do not present the C1s core-level spectra86.
The N1s core-level spectrum also deconvolutes into several component peaks. Three main types of N-doping configurations are frequently reported76. Pyridinic-N and pyrrolic-N may lie at the edge of the lattice, and graphitic-N forms by replacing C in the graphene lattice. The binding energies of these C–N bonding configurations were found at 398.1–399.3, 399.8–401.2, and 400.5–402.7 eV, respectively53,76. Nitrillic-, pyrazole-, and oxidized-N-type bonding have also been reported46,79. Lin et al. post-treated graphene/SiC samples in NH3 plasma and found that the insertion of N species depends on the graphene thickness (Fig. 4b)79. They reported that MW plasma yields a higher N doping in bilayer NDG than RF plasma. In addition, graphitic-N (3 at.%) dominated pyridinic-N (0.2 at.%) in bilayer graphene, whereas the pyridinic-N formation dominated in single-layer graphene. Thus far, NDG with pure pyridinic-N, pure pyrrolic-N, and pure graphitic-N doping configurations were reported by Luo et al. 46, Li et al. 85, and Wei et al. 56, respectively.
a Survey spectra and b, c High-resolution core-level C1s and N1s spectra of NDG obtained via thermal CVD (reprodcuced from56 with permission from the American Chemical Society), d N1s spectra of NDG samples treated with microwave (MW) and radio-frequency (RF) plasmas. The numbers state the N at.% deduced from spectral analyses of graphitic-N and pyridinic-N (reproduced from79 with permission from Elsevier).
As a complementary technique to XPS, ultraviolet photoelectron spectroscopy (UPS) probes the doping types in graphene17,46,81. Molecules absorb UV light and emit photoelectrons. The molecular orbital energies in the valence band are determined by measuring the kinetic energies of the released photoelectrons. Luo et al. probed the electronic structure of NDG via UPS46 and discovered that pyridinic-N effectively modifies the valence band structure in graphene. The density of the p-doping states increased near the Fermi level, and the work-function increased by 0.2 eV from that of graphene. Xu et al. also observed a work-function difference of ~0.2 eV between NDG and pristine graphene from UPS measurements17. They confirmed a similar work-function difference between NDG and graphene using Kelvin probe force microscopy (KPFM).
Angle-resolved photoemission spectroscopy (ARPES) probes the electronic structure of graphene at the Fermi level near the K point in the Brillouin zone18,75. The band structure of graphene can be determined from the energy dispersion as a function of momentum along the high-symmetry directions measured via ARPES75,98. The Fermi level is located at zero binding energy, and the Dirac point is located near the Fermi level in pristine or doped graphene75. For example, Lin et al. 79. reported a 450 mV Fermi-level shift in SiC n-type-doped NDG. Dopants (p- or n-type) in graphene are introduced from the doping and graphene substrate75 or polymer residues that remain after the transfer process102,103. Because the spectral densities of states measured via ARPES are extended in real space, this technique is insensitive to local resonances but determines the resonant behavior of N doping98.
The above studies confirm that XPS reveals the doping content and N-doping configuration type, whereas UPS and ARPES detect the Fermi-level shift due to N doping. Other characterization techniques are necessary to confirm the N-doping levels and their effect on the local structural and electronic properties of NDG. These techniques are discussed in the following subsection.
Electron microscopy
Scanning electron microscopy (SEM) is routinely used to examine the morphology of various materials, including NDG. A beam of highly energetic electrons (with a few keV of kinetic energy) is scanned over the sample surface, and the secondary electrons (~50 eV) emitted from the sample produce an SEM image. Similar to graphene or graphite films73,74,75, NDG is placed on a substrate (Cu, Ni, SiO2/Si, etc.) for SEM imaging. The number of NDG layers can be qualitatively correlated to the image color contrast74,81. Using SEM imaging, Ito et al. investigated the effects of different C and N precursors on NDG growth (Fig. 5)86. The NDG grew over a large area (a few cm2), similar to that of graphene; however, the nitroethane-grown surface was severely damaged. This technique is useful for global characterization, but the N-doping content and locations must be confirmed using the previously discussed techniques.
SEM images of N-doped graphene grown on Cu substrates using different precursor treatments (under an H2 flow during growth). Scale bar in each image is 20 µm: a CH4, b pyridine, and c nitroethane (reproduced from86 with permission from the American Chemical Society).
Local structural measurements (with areal coverages of squared nanometers to squared micrometers) can be performed with transmission electron microscopy (TEM). In preparation for TEM, the NDG is transferred to an amorphous C-coated Cu or Au TEM grid34,85. In analysing NDG, researchers have compared the structural differences between pristine graphene and NDG samples34. Son et al. directly observed substitutionally-doped N using atomically resolved high-resolution TEM (HR-TEM)26. They applied a bandpass filter to capture clear images of the N atoms in the vicinity of C atoms. The substitutional N atoms were clearly imaged as high-brightness points in the graphene plane (Fig. 6a). The height profile of the HR-TEM image (dotted red line in Fig. 6a) abruptly increased for N atoms (Fig. 6b). The selected area electron diffraction (SAED) patterns of the same sample revealed single-crystal graphene without lattice breakdown (Fig. 6c).
a Bandpass-filtered high-resolution TEM image, b height profile of the dotted red line in panel a, c selected area electron diffraction pattern, and d elemental mapping images using electron energy loss spectroscopy (reproduced from26 with permission from Elsevier).
The intensity of the diffraction spots of NDG was similar to that of pristine graphene, indicating that the crystalline quality was maintained after doping26. The low N content (<3 at.% in graphene) yielded NDG with high crystalline quality26,44,87,97. Techniques accompanying TEM imaging, usually elemental mapping via electron energy loss spectroscopy (EELS), confirm that N atoms are uniformly distributed in NDG samples (red spots in Fig. 6d)34,35. From the EELS data, the N-doping amount was estimated as ∼1.8 at.% (in a similarly grown sample, XPS yielded a similar N content of 1.8 at.%).
Graphene and NDG grown with plasma CVD result in ring patterns when examined by SAED, due to their polycrystalline structure (originates several hexagonal sets of spots)34. The diffraction rings of graphene correspond to (002), (111), and (100) orientated nanocrystallites with interplanar spacings of 0.33, 0.26, and 0.21 nm, respectively34. In the diffraction pattern of NDG, the absence of the ring corresponding to the (002) plane correlates with the decreased interplanar spacing of NDG34. More structural TEM analyses of NDG produced with precisely controlled N-doping levels are required.
Scanning probe methods
Atomic force microscopy (AFM) can confirm the thickness (number of layers) of an NDG sample, similarly to graphene samples81. The UPS results have rarely been supported by KPFM, as discussed17. This subsection focuses on scanning tunneling microscopy (STM) and scanning tunneling spectroscopy (STS), which provide significant information on the quality of an NDG material.
The local atomic structure of graphene around the N dopants can be scanned using STM76,84,98. This method also controls and locates substitutional N-doping sites44,84. Joucken et al. elucidated the electronic structure of NDG in a combined XPS, ARPES, and STM/STS analyses (Fig. 7a–e)98. As the doping concentration increased from 0 to 1.1 at. %, the Fermi-level shift increased from 0 to 450 eV.
a–e ARPES spectra of samples treated for 0, 7.5, 15, 30, and 90 min. The Fermi-level shift (μ = EF − ED) is stated on each spectrum. Insets present typical (10 nm × 10 nm) STM images of the corresponding samples. The N dopants appear as red protrusions, and the N concentration (c) is given on each image (reproduced from98 with permission from Springer Nature). f Low-temperature STM image acquired at 77 K. Several surface defects appear. Inset displays the three most abundant types of defects. Scale bars = 2 nm, I = 2.0 nA, V = 0.2 V (reproduced from76 with permission from Elsevier).
Recently, Lv et al. reported the topography of individually doped N atoms in a highly resolved STM image of graphene treated in situ using NH3 in a two-step process (Fig. 7f)84. They performed STM observations of samples transferred onto SiO2/Si with unique double-substitutional N-doping sites (N2AA). In density functional theory (DFT) calculations, they further confirmed the occurrence of localized states in the conduction band induced by N2AA type doping. Thus with the use of STM technique, N-doping at atomic scale was evidenced.
STM locates the N doping with an atomic resolution over a sample surface scanned over areas in square nanometers76. However, this technique not suitable to confirm whether the dopant is uniformly dispersed over large areas (approximately centimeters squars).
Modeling
DFT simulations are another critical tool for studying the properties of NDG, particularly the geometry and electronic properties. According to various theoretical studies, N doping can effectively increase the electron density of graphene while adjusting the Fermi energy (Ef) level. Moreover, chemical properties can be adjusted via N doping80.
The semiconducting behavior of NDG depends on the configuration type of N doping. Pyridinic-N and pyrrolic-N mainly form at the defects and edges of NDG, whereas graphitic-N forms at the replacement sites of C atoms. Therefore, NDG maintains high electron mobility similar to that of graphene104. As the bond lengths of C–N and C–C are similar (1.41 and 1.42 Å, respectively), the pyridinic-N and graphitic-N doping types have little effect on the graphene structure, but the pyrrolic-N doping type undergoes a structural change from the original hexagonal lattice. Pyridinic-N is the most stable type for monovacancy, whereas pyridinic-N and graphitic-N energetically favor Stone–Wales and divacancy defects105. In graphitic-N-type NDG, three σ-bonds form between N and its neighboring C atoms; one is involved in the formation of the π bond, and the last bond is partially involved in the π* state of the conduction band. The resultant doping in graphene is n-type106. In contrast, pyridinic-N and pyrrolic-N-type NDG form defect sites that withdraw electrons from graphene, inducing p-type doping107.
Applications
Among the many possible applications of NDG (Fig. 1), we focus on FET and battery technologies, which directly use CVD-grown NDG for nanoelectronics and storage, respectively. Both applications require further improvements in capacity and performance. Other applications require growth scaling and architectural modifications of CVD-grown graphene. These fields are excluded because listing all these would be excessively exhaustive. 2D NDG has been used in several studies on FETs17 and battery devices (Table 2)53. The FETs require proper N-doping locations and low N content to avoid a drastic decrease in electron mobility and open a suitable bandgap, whereas battery applications benefit from a specific type of N-doping configuration, as detailed below19,53.
Field-effect transistors
In reciprocal space, the valence and conduction bands of graphene intersect at two inequivalent points, providing a unique electric field effect and high conductivity. Graphene exhibits an ambipolar behavior where both the hole and electron carriers are transported perpendicular to its surface9,11,13,17. The first high-speed FET based on graphene was prepared via the micromechanical cleavage of pyrolytic graphite108. Since then, graphene and doped graphene-based nanoelectronics have been extensively researched. The effects of doping on the electrical properties of graphene can be investigated via electrical measurements in FET geometry (Fig. 8)25,97. The NDG-based FET devices have a bandgap and exhibit a larger on/off current ratio and lower turn-on fields than pristine graphene35,38,57.
a, b Schematic of a field-effect transistor (FET) device and I–Vg characteristics of a NDG-based FET (reproduced from26 with permission from Elsevier); c, d Ids versus Vds plots of graphene and NDG FET devices for various Vg, where Vg, Ids, and Vds denote the gate voltage, source-drain current, and source-drain voltage, respectively. Insets at the upper left of panels c and d are the presumed band structures of graphene and NDG FET devices, respectively (reproduced from56 with permission from the American Chemical Society).
The FETs are usually fabricated from CVD-grown NDG graphene transferred onto a SiO2/Si substrate17,25,35,83. The Cu or Au electrodes are deposited onto the patterned NDG film via thermal or e-beam evaporation9,17. The NDG provides a conducting channel between the source and drain electrodes9. Xu et al. reported high mobility and a Dirac point shift of −140 V in NDG grown via an ex situ treatment of CVD graphene (surface-charge transfer type doping)17, whereas NDG grown using substitutionally-doped N exhibited lower mobility values56,58. Wei et al. prepared bottom-gated FETs from NDG (with a significant graphitic-N configuration) and pristine graphene56. They reported relatively lower mobility and a higher on/off ratio in NDG than in graphene56. The lower mobility in NDG can be attributed to the disrupted symmetry of the graphene lattice due to the incorporated N atoms. The generated defects behave as scattering centers, reducing mobility and conductivity58. The mobilities of NDG have reached values of 200 to 450 cm2/V s, which are lower than the commonly reported mobilities of CVD-formed graphene (1000 to 5000 cm2/V s)9,75,109,110 (Table 3). The mobility of NDG exceeds that of graphene doped with other heteroatoms via CVD methods. For example, the mobility of graphene doped with 4 at.% of S is 270 cm2/V s29.
Pyrrolic-N and pyridinic-N configurations generally have high defect densities, resulting in a lower graphene carrier conductivity and mobility than those of the graphitic-N configuration46,83. Recently, Xu et al. developed a higher-mobility (1150 cm2/V s) FET device in which the N dopants were adsorbed on bilayer graphene17. They postulated that the low defect densities in NDG substantially weaken the short-range scattering and improve mobility.
The bandgap in the semiconductor channel of conventional FETs should be at least 0.4 eV111. The NDG band structure has been calculated in DFT and ab initio simulations. Mukherjee et al. showed that 12.5 at.% N doping in graphene reduces the Dirac cone shift below the Ef by 1.5 eV with finite splitting112. Below Ef, the π, and π* bands are contributed by the pz orbitals of C and N, respectively (Fig. 9a). By increasing the N-doping concentration from 3.13 to 12.5 at.%, Olaniyan et al. increased the bandgap of NDG from 0.21 to 0.6 eV113, meeting the requirements of FET channel materials (Fig. 9b, c). As N atoms are more electron-rich than the resident C atoms, the Fermi level moves significantly above the Dirac point, resulting in n-type semiconductor materials.
a Energy band structure and density of states of NDG (reproduced from112 with permission from Springer Nature); b, c optimized structures, energy band structures, and density of states of NDG at different N concentrations (3.13 and 12.50 at.%). Red and black balls represent N and C atoms, respectively (reproduced from113 with permission from Elsevier).
A significant scope remains for enhancing FET results by controlling the percentage and configuration of the N dopant. In future studies, the uniformity of N doping must be confirmed in large-area mobility mapping of NDG samples75. Moreover, most experimental NDG FET results have been overshadowed by several additional effects, such as NDG growth and transfer and post-processing during the device fabrication and measurements. More studies employing both experimental and theoretical approaches would help validate these results.
Energy storage: batteries
Renewable energy generation and electric vehicles require high-capacity, fast-charging batteries. Since discovering monolayer graphene in 2004, 2D materials and their 3D networks have attracted broad interest as alternatives to bulk graphite or graphitic carbon-based hybrid flakes/sheets108. However, the current research/applications of battery applications are dominated by powdered-form graphene/graphene derivatives and other carbon-based nanomaterials (e.g., carbon nanotubes, carbon fibers, and carbon black) and hybrids and composites based on these materials8,63. As a battery material, 2D graphene has been overshadowed by other materials with different shapes, sizes, defects, and impurities33,57,72,114.
According to some reports, pristine graphene cannot be applied as the anode in magnesium- or potassium-ion batteries because it has poor adsorption ability88,115. The hexagonal carbon sheet of graphene presents a high-energy barrier to the solid diffusion process required in battery processes because graphene sheets are closely stacked, and their performance is limited at high voltages. Conversely, N doping alters the electronic structure of graphene and produces active sites in the graphene/graphite lattice while increasing the interlayer distance19,88,116. Thus, NDG as the anode material enhances the battery capacity by improving the adsorption, substitution, and intercalation capabilities19,53,88.
Batteries based on CVD-grown NDG are rarely reported53,117. Reddy et al. studied the reversible Li-ion intercalation properties in a Li-ion battery (LIB) assembly using directly grown NDG (N = 9 at.%) or graphene on a Cu current collector (Fig. 10)53. The reversible discharge capacity of the NDG-based LIB was almost double that of the graphene-based LIB. They emphasized that the higher capacity resulted from the surface defects introduced via N doping. Moreover, the direct growth process ensured good electrical contact between the NDG electrode and the current collector. This assembly reduced the electronic resistance and enhanced the electrode rate capability53.
a Cyclic Li-ion battery voltammograms of the NDG/Cu foil electrode and b charge/discharge voltage profiles for the NDG electrode (reproduced from53 with permission from the American Chemical Society); c K-ion battery: capacity comparison of graphene, NDG, and graphite in battery cells, with a schematic of the staging and defect storage mechanism in few-layer NDG (reproduced from88 with permission from the American Chemical Society).
Because Li is neither abundant nor well distributed in the Earth’s crust, it is considerably more expensive than other Earth-abundant alkali metals (Na and K) and Al. Moreover, the demand for batteries is expected to increase in the future. Accordingly, the research focus in the past decade has shifted to Na, K, and Al batteries118,119. Share et al. demonstrated high K-ion storage capacity in K-ion batteries (KIBs) with few-layer NDG as the anode material88. More specifically, the maximum theoretical storage capacity increased from 278 mA h/g in the graphite-based KIB to 350 mA h/g in the KIB based on N-doped few-layered graphene. The higher capacity of N-doped few-layer graphene than that of pristine few-layer graphene was attributed to the defective N-doping sites and formation of the first-stage intercalation compound (KC8). The pyridinic-N configuration imposes a local electron deficiency that draws electrons from nearby K atoms, enhancing KIB storage properties19.
Thus, N doping can overcome the anode limitations in batteries by providing additional active sites and increasing the storage capacity88,116. The NDG with pyrrolic-N and pyridinic-N configurations is suitable for LIBs and KIBs19,53, but precisely controlling the configuration, distribution, and doping content of N over large areas is a difficult task. In addition, defects and distortions are inevitably introduced by the NDG synthesis process34,35. Although these defects decrease the mobility of NDG, they are beneficial for the catalytic reaction in LIBs53. Controlled NDG growth on porous substrates (catalytic or noncatalytic) could be the way forward for large-scale battery applications72.
The electrode materials of alkali-metal-ion batteries can be evaluated via DFT simulations. The insertion voltage of alkali-metal atoms in electrode materials and the diffusion barrier and diffusion path of alkali-metal atoms on the structure can be investigated through the DFT. Moreover, the theoretical capacity of the electrode material can be determined by studying the maximum number of alkali-metal atoms adsorbed on the structure. Cao et al. 120. theoretically investigated the adsorption mechanisms of Li atoms on pyridinic-N, pyrrolic-N, and graphitic-N-type NDG. The adsorption energy of Li atoms was the highest for pyridinic-N (∼40% higher than that for pristine graphene). In this configuration, the formation of Li clusters was limited, and the storage capacity was boosted to 1262 mA h/g.
Yu et al. also studied different NDG configurations as the electrode material for LIBs (Fig. 11a)105. Their results indicate that not all N-doping configurations can increase the capacity of LIBs. On N-substituted graphene, the adsorption energy of a Li atom is positive, meaning that this NDG configuration is an unsuitable electrode material for LIBs. In contrast, N-modified single- or double-vacancy defects, especially pyridinic-N2V2 defects, can significantly increase the reversible battery capacity. Therefore, pyridinic-N and pyrrolic-N-doped configurations are promising electrode materials for LIBs and can improve the performance of Na-ion batteries. Kimal et al. 121. investigated the performance of different types of NDG as the Na-ion battery. They found that after adsorbing six Na atoms, all NDG configurations and pristine graphene become asymmetric, and the adsorption configurations significantly change (Fig. 11b). Furthermore, the Na storage capacity in pyridinic-N or pyrrolic-N-doped graphene is improved by the electron effect originating from the vacancy sites or electron-deficient dopants (Fig. 11b).
a Optimized structures of a Li atom adsorbed on pristine graphene and N-substituted graphene (N1, NAA2, NAB2, NAB’2,) N1V1, pyridinic-N3V1, pyrrolic-N3V1, pyridinic-N2V2, and pyridinic-N4V2 defects, where NxVy denotes a configuration with x N atoms and y vacancies in graphene. The gray, blue, and pink balls represent C, N, and Li atoms, respectively (reproduced from105 with permission from the Royal Society of Chemistry). b Optimized structures of six Na atoms adsorbed on pristine graphene and graphitic-N, pyridinic-N, and pyrrolic-N-type NDG. (reproduced from121 with permission from Elsevier).
Overall, this review can guide performance improvement in CVD-grown NDG in FET and battery applications. For example, graphitic-N can maintain high carrier mobility in FET devices, whereas pyridinic-N and pyrrolic-N types of NDG are essential in batteries.
Summary and perspectives
A good number of articles on the growth and application of NDG have been published in recent years. In addition, several synthesis and characterization techniques have been investigated. However, the literature reveals considerable differences in the properties and device efficiencies of NDG films. Many recent studies have been performed on 3D networks of 2D-doped graphene flakes, which contain a variety of undetermined defects and doping sites. As reviewed, well-defined doping configurations N at.% quantities are difficult to obtain, explaining the widely disparate results in the field. Ideally, before proceeding to 3D, the NDG materials should be thoroughly studied in their 2D form in order to understand the underlying formation mechanisms and role of each N-doping configuration and their useful concentration threshold for a given application. However, a method to produce a wafer-scale with a controlled number of NDG layers and uniform N doping is lacking. Among the possible options, CVD is the most promising to overcome this challenge.
The key advantages of the 2D form of NDG are as follows: (i) easy and reliable control of N doping; (ii) unambiguous characterization of C–N bonding types using either XPS or TEM techniques; and (iii) avoidance of the effect of N inclusion at the interlayer (intercalation) or voids or at functional/defect sites, such as that of 2D networks of 3D graphene. In future work, we recommend CVD-grown 2D NDG as a model system (Fig. 12) for controlling the N-doping level and configuration, to understand and engineer the doping effects in target applications. Moreover, these studies could be highly beneficial when implementing 3D networks of 2D NDG (Fig. 12) for particular applications (e.g., electrochemistry) and interpreting device performance.
Two-dimensional N-doped graphene (2D NDG) system that can be used as a model to control the N-doping at.%, location, and device performance effects (field-effect transistor). The results could be applied to control N-doping processes and device performance of 3D-NDG materials (e.g., electrochemistry applications).
We recommend the following critical pathways for CVD-based NDG growth:
-
Growth
-
The development of a standard growth process would help achieve controlled growth of high-quality NDG at the wafer-scale. Thus far, the process parameters and activation sources of NDG growth in the literature are widely variable.
-
Pretreated catalytic substrates (e.g., Cu, Ni, or Cu/Ni alloy) under a proper C or N source prior to graphene growth to precisely control the C and N quantities need to be researched in greater detail.
-
Limited substrates (catalytic and dielectric) for NDG growth have been explored thus far. Various other substrates (e.g., GaN for photodetector applications) must be studied.
-
The vacancies created via doping, especially those in pyridinic- and pyrrolic-N, must be controlled, and their effects on applications must be studied and modeled simultaneously.
-
Once NDG growth over squared centimeter samples with controlled quality is achieved, large-area graphene growth CVD systems (e.g., roll-to-roll CVD systems) could be adapted to achieve wafer-scale NDG growth.
-
Characterization
-
Both global and local characterization techniques are required to determine the quality of the as-produced NDG material. In some reports, this complementarity is overlooked compromising the quality of the analytical study and subsequent conclusions.
-
Experimental and theoretical studies of each type of N-doping configuration and its effect on target applications are required. For example, in FET applications, whether 2 at.% of N, predominantly of graphitic-N-type, opens a suitable bandgap while retaining the requisite of high charge carrier mobility must be determined.
-
Applications
-
To achieve reliable and reproducible results for 3D constructs, first, the properties (e.g., electrical, optical, and mechanical) and applications (e.g., FET, battery, sensing, and catalysis) of NDG in the 2D form must be explored in greater detail.
-
The N-dopant location (substitutional or intercalated) in multilayer NDG and its effect on the performance of a specific application (e.g., battery) must also be explored in greater detail.
These studies would facilitate the adoption of NDG as an active material for nanoelectronics, energy storage devices, and many other technologies. Then, and thinking beyond this, 3D networks of NDG could be assembled with better control and reproducible responses.
Data availability
All relevant data are available from the corresponding author.
References
Morozov, S. V. et al. Giant intrinsic carrier mobilities in graphene and its bilayer. Phys. Rev. Lett. 100, 016602 (2008).
Balandin, A. A., Ghosh, S., Nika, D. L. & Pokatilov, E. P. Thermal conductivity of graphene: the first measurements and theoretical analysis. Therm. Cond. 30, 97 (2010).
Stoller, M. D., Park, S. J., Zhu, Y. W., An, J. H. & Ruoff, R. S. Graphene-based ultracapacitors. Nano Lett. 8, 3498–3502 (2008).
Deokar, G. et al. Wafer-scale few-layer graphene growth on Cu/Ni films for gas sensing applications. Sensor Actuat B-Chem. 305, 127458 (2020).
Penezic, A. et al. Carbohydrate-lectin interaction on graphene-coated surface plasmon resonance (SPR) interfaces. Plasmonics 9, 677–683 (2014).
He, Q. Y., Wu, S. X., Yin, Z. Y. & Zhang, H. Graphene-based electronic sensors. Chem. Sci. 3, 1764–1772 (2012).
Novodchuk, I., Bajcsy, M. & Yavuz, M. Graphene-based field effect transistor biosensors for breast cancer detection: a review on biosensing strategies. Carbon 172, 431–453 (2021).
Huang, X., Zeng, Z. Y., Fan, Z. X., Liu, J. Q. & Zhang, H. Graphene-based electrodes. Adv. Mater. 24, 5979–6004 (2012).
Wei, W. et al. Graphene FETs with aluminum bottom-gate electrodes and its natural oxide as dielectrics. IEEE Trans. Electron Dev. 62, 2769–2773 (2015).
Bi, L. Q., Dai, S. S., Wu, Y. B. & Guo, D. H. Graphene field effect transistors and their applications in terahertz technology: a review. Carbon 145, 772–772 (2019).
Bae, S. et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 5, 574–578 (2010).
Chen, Y., Li, J. Z., Li, T., Zhang, L. K. & Meng, F. B. Recent advances in graphene-based films for electromagnetic interference shielding: review and future prospects. Carbon 180, 163–184 (2021).
Hossain, S. et al. Nanostructured graphene materials utilization in fuel cells and batteries: a review. J. Energy Storage 29, 101386 (2020).
Yang, W. et al. Nitrogen plasma modified CVD grown graphene as counter electrodes for bifacial dye-sensitized solar cells. Electrochim. Acta 173, 715–720 (2015).
Qiu, Y. C. et al. High-rate, ultra long cycle-life lithium/sulfur batteries enabled by nitrogen-doped graphene. Nano Lett. 14, 4821–4827 (2014).
Cai, D. D. et al. Superhigh capacity and rate capability of high-level nitrogen-doped graphene sheets as anode materials for lithium-ion batteries. Electrochim. Acta 90, 492–497 (2013).
Xu, W. et al. N-doped graphene field-effect transistors with enhanced electron mobility and air-stability. Small 10, 1999–2005 (2014).
Liu, H. T., Liu, Y. Q. & Zhu, D. B. Chemical doping of graphene. J. Mater. Chem. 21, 3335–3345 (2011).
Wu, X. et al. Advanced carbon-based anodes for potassium-ion batteries. Adv. Energy Mater 9, 1900343 (2019).
Ikram, R. et al. Recent advancements of N-doped graphene for rechargeable batteries: a review. Crystals 10, 1, (2020).
He, C. Y., Wang, R. H., Fu, H. G. & Shen, P. K. Nitrogen-self-doped graphene as a high capacity anode material for lithium-ion batteries. J. Mater. Chem. A 1, 14586–14591 (2013).
López-Urías, F., Lv, R., Terrones, H. & Terrones, M. Doped Graphene: Theory, Synthesis, Characterization, and Applications. https://doi.org/10.1002/9781118691281.ch9. In book: Graphene Chemistry (pp.183–207). https://www.researchgate.net/publication/277705007_Doped_Graphene_Theory_Synthesis_Characterization_and_Applications (2013).
Lee, S. J. et al. Heteroatom-doped graphene-based materials for sustainable energy applications: a review. Renew. Sust. Energ. Rev. 143, 110849 (2021).
Lee, H., Paeng, K. & Kim, I. S. A review of doping modulation in graphene. Synth. Met. 244, 36–47 (2018).
Wang, X. N-doping of graphene through electrothermal reactions with ammonia. Science 329, 1467–1467 (2010).
Son, M. et al. High-quality nitrogen-doped graphene films synthesized from pyridine via two-step chemical vapor deposition. Carbon 159, 579–585 (2020).
Late, D. J. et al. Characteristics of field-effect transistors based on undoped and B- and N-doped few-layer graphenes. Solid State Commun. 150, 734–738 (2010).
Ma, X. L., Ning, G. Q., Qi, C. L., Xu, C. G. & Gao, J. S. Phosphorus and nitrogen dual-doped few-layered porous graphene: a high-performance anode material for lithium-ion batteries. Acs Appl. Mater. Inter. 6, 14415–14422 (2014).
Zhou, J. H. et al. Growth and properties of large-area sulfur-doped graphene films. J. Mater. Chem. C. 5, 7944–7949 (2017).
Kalita, G., Wakita, K., Takahashi, M. & Umeno, M. Iodine doping in solid precursor-based CVD growth graphene film. J. Mater. Chem. 21, 15209–15213 (2011).
Feng, W., Long, P., Feng, Y. & Li, Y. Two-dimensional fluorinated graphene: synthesis, structures, properties and applications. Adv. Sci. 3, 1500413 (2016).
Granzier-Nakajima, T., Fujisawa, K., Anil, V., Terrones, M. & Yeh, Y. T. Controlling nitrogen doping in graphene with atomic precision: synthesis and characterization. Nanomaterials 9, 425 (2019).
Wang, H. B., Maiyalagan, T. & Wang, X. Review on recent progress in nitrogen-doped graphene: synthesis, characterization, and its potential applications. Acs Catal. 2, 781–794 (2012).
Zhai, Z. H., Shen, H. L., Chen, J. Y., Li, X. M. & Jiang, Y. Direct growth of nitrogen-doped graphene films on glass by plasma-assisted hot filament CVD for enhanced electricity generation. J. Mater. Chem. A 7, 12038–12049 (2019).
Gao, H. et al. A simple method to synthesize continuous large area nitrogen-doped graphene. Carbon 50, 4476–4482 (2012).
Sturala, J., Luxa, J., Pumera, M. & Sofer, Z. Chemistry of graphene derivatives: synthesis, applications, and perspectives. Chemistry 24, 5992–6006 (2018).
Plutnar, J., Pumera, M. & Sofer, Z. The chemistry of CVD graphene. J. Mater. Chem. C. 6, 6082–6101 (2018).
Terrones, H., Lv, R. T., Terrones, M. & Dresselhaus, M. S. The role of defects and doping in 2D graphene sheets and 1D nanoribbons. Rep. Prog. Phys. 75, 062501 https://doi.org/10.1088/0034-4885/75/6/062501 (2012).
Olanrele, S. O., Lian, Z., Si, C. W. & Li, B. Halogenation of graphene triggered by heteroatom doping. Rsc Adv. 9, 37507–37511 (2019).
Karlicky, F., Datta, K. K. R., Otyepka, M. & Zboril, R. Halogenated graphenes: rapidly growing family of graphene derivatives. Acs Nano 7, 6434–6464 (2013).
Kashid, R. V. et al. Field emission characteristics of pristine and N-doped graphene measured by in-situ transmission electron microscopy. J. Appl. Phys. 113, 214311 (2013).
Karimzadeh, A., Hasanzadeh, M., Shadjou, N. & de la Guardia, M. Optical bio(sensing) using nitrogen doped graphene quantum dots: recent advances and future challenges. Trac Trend Anal. Chem. 108, 110–121 (2018).
Wang, Y., Shao, Y. Y., Matson, D. W., Li, J. H. & Lin, Y. H. Nitrogen-doped graphene and its application in electrochemical biosensing. Acs Nano 4, 1790–1798 (2010).
Feng, S. M. et al. Ultrasensitive molecular sensor using N-doped graphene through enhanced Raman scattering. Sci. Adv. 2, e1600322 (2016).
Wang, R. et al. Preparation of nitrogen-doped TiO2/graphene nanohybrids and application as counter electrode for dye-sensitized solar cells. Acs Appl. Mater. Inter. 6, 2118–2124 (2014).
Luo, Z. Q. et al. Pyridinic N doped graphene: synthesis, electronic structure, and electrocatalytic property. J. Mater. Chem. 21, 8038–8044 (2011).
Zhao, Y., Nakamura, R., Kamiya, K., Nakanishi, S. & Hashimoto, K. Nitrogen-doped carbon nanomaterials as non-metal electrocatalysts for water oxidation. Nat. Commun. 4, 2390 (2013).
Liu, X. & Dai, L. M. Carbon-based metal-free catalysts. Nat. Rev. Mater. 1, 16064 (2016).
Bagley, J. D., Kumar, D. K., See, K. A. & Yeh, N. C. Selective formation of pyridinic-type nitrogen-doped graphene and its application in lithium-ion battery anodes. Rsc Adv. 10, 39562–39571 (2020).
Chu, F. Q. et al. Nitrogen-doped three-dimensional graphene-supported platinum catalysts for polymer electrolyte membrane fuel cells application. Funct. Mater. Lett. 11, 1850015 (2018).
Wang, K. W. et al. In situ grown monolayer N-Doped graphene and ZnO on ZnFe2O4 hollow spheres for efficient photocatalytic tetracycline degradation. Colloid Surface A 618, 126362 (2021).
Bie, C. B., Zhu, B. C., Xu, F. Y., Zhang, L. Y. & Yu, J. G. In situ grown monolayer N-doped graphene on CdS hollow spheres with seamless contact for photocatalytic CO2 reduction. Adv. Mater. 31, 1902868 (2019).
Reddy, A. L. M. et al. Synthesis of nitrogen-doped graphene films for lithium battery application. Acs Nano 4, 6337–6342 (2010).
Kaur, M., Kaur, M. & Sharma, V. K. Nitrogen-doped graphene and graphene quantum dots: a review on synthesis and applications in energy, sensors and environment. Adv. Colloid Interfac. 259, 44–64 (2018).
Chen, Z. Y. et al. Nitrogen-doped interpenetrating porous carbon/graphene networks for supercapacitor applications. Chem. Eng. J. 409, 127891 (2021).
Wei, D. et al. Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett. 9, 1752–1758 (2009).
Yadav, R. & Dixit, C. K. Synthesis, characterization and prospective applications of nitrogen-doped graphene: a short review. J. Sci. Adv. Mater. Dev. 2, 141–149 (2017).
Capasso, A. et al. Nitrogen-doped graphene films from chemical vapor deposition of pyridine: influence of process parameters on the electrical and optical properties. Beilstein J. Nanotech. 6, 2028–2038 (2015).
Bie, C. B. et al. Design, fabrication, and mechanism of nitrogen-doped graphene-based photocatalyst. Adv. Mater. 33, 2003521 (2021).
Alazmi, A., Rasul, S., Patole, S. P. & Costa, P. M. F. J. Comparative study of synthesis and reduction methods for graphene oxide. Polyhedron 116, 153–161 (2016).
Maddi, C. et al. Nano-architecture of nitrogen-doped graphene films synthesized from a solid CN source. Sci Rep. 8, 3247 (2018).
Lin, Z. Y., Waller, G. H., Liu, Y., Liu, M. L. & Wong, C. P. 3D Nitrogen-doped graphene prepared by pyrolysis of graphene oxide with polypyrrole for electrocatalysis of oxygen reduction reaction. Nano Energy 2, 241–248 (2013).
Zhang, Z. et al. Verifying the rechargeability of Li-CO2 batteries on working cathodes of Ni nanoparticles highly dispersed on N-doped graphene. Adv. Sci. 5, 1700567 (2018).
Pham, T. V. et al. High areal capacitance of N-doped graphene synthesized by Arc discharge. Adv. Funct. Mater. 29, 1905511 (2019).
Panchokarla, L. S. et al. Synthesis, structure, and properties of boron- and nitrogen-doped graphene. Adv. Mater. 21, 4726 (2009).
Sun, L. et al. Nitrogen-doped graphene with high nitrogen level via a one-step hydrothermal reaction of graphene oxide with urea for superior capacitive energy storage. Rsc Adv. 2, 4498 (2012).
Long, D. H. et al. Preparation of nitrogen-doped graphene sheets by a combined chemical and hydrothermal reduction of graphene oxide. Langmuir 26, 16096–16102 (2010).
Bai, J. C. et al. Nitrogen-doped graphene as catalysts and catalyst supports for oxygen reduction in both acidic and alkaline solutions. Int. J. Hydrog. Energ. 38, 1413–1418 (2013).
Deng, D. H. et al. Toward N-doped graphene via solvothermal synthesis. Chem. Mater. 23, 1188–1193 (2011).
Zhang, L. L. et al. Single nickel atoms on nitrogen-doped graphene enabling enhanced kinetics of lithium-sulfur batteries. Adv. Mater. 31, 1903955 (2019).
Xu, H. F., Ma, L. B. & Jin, Z. Nitrogen-doped graphene: synthesis, characterizations and energy applications. J. Energy Chem. 27, 146–160 (2018).
Ullah, S. et al. Synthesis of doped porous 3D graphene structures by chemical vapor deposition and its applications. Adv. Funct. Mater. 29, 1904457 (2019).
Deokar, G. et al. Semi-transparent graphite films growth on Ni and their double-sided polymer-free transfer. Sci. Rep. 10, 14703 (2020).
Deokar, G., Genovese, A. & Costa, P. Fast, wafer-scale growth of a nanometer-thick graphite film on Ni foil and its structural analysis. Nanotechnology 31, 485605 (2020).
Deokar, G. et al. Towards high quality CVD graphene growth and transfer. Carbon 89, 82–92 (2015).
Fiori, S. et al. “Inside out” growth method for high-quality nitrogen-doped graphene. Carbon 171, 704–710 (2021).
Shi, B. et al. One-pot green synthesis of oxygen-rich nitrogen-doped graphene quantum dots and their potential application in pH-sensitive photoluminescence and detection of mercury(II) ions. Talanta 142, 131–139 (2015).
Balamurugan, J., Nguyen, T. T., Aravindan, V., Kim, N. H. & Lee, J. H. Flexible solid-state asymmetric supercapacitors based on nitrogen-doped graphene encapsulated ternary metal-nitrides with ultralong cycle life. Adv. Funct. Mater. 28, 1804663 (2018).
Lin, Y. P. et al. Efficient and low-damage nitrogen doping of graphene via plasma-based methods. Carbon 100, 337–344 (2016).
Yanilmaz, A. et al. Nitrogen doping for facile and effective modification of graphene surfaces. Rsc Adv. 7, 28383–28392 (2017).
Rybin, M. et al. Efficient nitrogen doping of graphene by plasma treatment. Carbon 96, 196–202 (2016).
Wei, D. C. et al. Low temperature critical growth of high quality nitrogen doped graphene on dielectrics by plasma-enhanced chemical vapor deposition. Acs Nano 9, 164–171 (2015).
Liu, B., Yang, C. M., Liu, Z. W. & Lai, C. S. N-doped graphene with low intrinsic defect densities via a solid source doping technique. Nanomaterials 7, 302 (2017).
Lv, R. et al. Nitrogen-doped graphene: beyond single substitution and enhanced molecular sensing. Sci. Rep. 2, 586 (2012).
Li, J. Y. et al. Scalable synthesis of pyrrolic N-doped graphene by atmospheric pressure chemical vapor deposition and its terahertz response. Carbon 62, 330–336 (2013).
Ito, Y. et al. Chemical vapor deposition of N-doped graphene and carbon films: the role of precursors and gas phase. Acs Nano 8, 3337–3346 (2014).
Jin, Z., Yao, J., Kittrell, C. & Tour, J. M. Large-scale growth and characterizations of nitrogen-doped monolayer graphene sheets. ACS Nano 5, 4112–4117 (2011).
Share, K., Cohn, A. P., Carter, R., Rogers, B. & Pint, C. L. Role of nitrogen-doped graphene for improved high-capacity potassium ion battery anodes. Acs Nano 10, 9738–9744 (2016).
Bao, J. F., Kishi, N. & Soga, T. Synthesis of nitrogen-doped graphene by the thermal chemical vapor deposition method from a single liquid precursor. Mater. Lett. 117, 199–203 (2014).
Prasanthi, T. N., Sudha, C., Saroja, S. & Vijayalakshmi, M. Diffusion of nitrogen in FCC-Fe and FCC-Ni lattices: molecular dynamic simulations. In 11th International Conference on High Nitrogen Steels (Indian Institute of Metals, 2012).
Deng, B., Liu, Z. F. & Peng, H. L. Toward mass production of CVD graphene films. Adv. Mater. 31, 1800996 (2019).
Yang, R. T., Goethel, P. J., Schwartz, J. M. & Lund, C. R. F. Solubility and diffusivity of carbon in metals. J. Catal. 122, 206–210 (1990).
Li, X. S., Cai, W. W., Colombo, L. & Ruoff, R. S. Evolution of graphene growth on Ni and Cu by carbon isotope labeling. Nano Lett. 9, 4268–4272 (2009).
Reina, A. et al. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 9, 30–35 (2009).
Deokar, G., Codron, J.-L., Boyaval, C., Wallart, X. & Vignaud, D. In 4th Graphene Conference, Graphene 2014 (2014).
Prasanthi, T. N., Sudha, C., Saroja, S. & Vijayalakshmi, M. Simulation of nitrogen diffusion in Ni vis-a- vis Fe - Identification of better structural material for neutron detectors. Results Phys. 4, 96–100 (2014).
Zhang, C. H. et al. Synthesis of nitrogen-doped graphene using embedded carbon and nitrogen sources. Adv. Mater. 23, 1020–1024 (2011).
Joucken, F. et al. Charge transfer and electronic doping in nitrogen-doped graphene. Sci. Rep. 5, 14564 (2015).
Zan, R. & Altuntepe, A. Nitrogen doping of graphene by CVD. J. Mol. Struct. 1199, 127026 (2020).
Ferrari, A. C. et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 97, 187401 (2006).
Ferrari, A. C. Raman spectroscopy of graphene and graphite: disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun. 143, 47–57 (2007).
Pirkle, A. et al. The effect of chemical residues on the physical and electrical properties of chemical vapor deposited graphene transferred to SiO2. Appl. Phys. Lett. 99, 122108 (2011).
Kumar, K., Kim, Y. S. & Yang, E. H. The influence of thermal annealing to remove polymeric residue on the electronic doping and morphological characteristics of graphene. Carbon 65, 35–45 (2013).
Wang, X. et al. Heteroatom-doped graphene materials: syntheses, properties and applications. Chem. Soc. Rev. 43, 7067–7098 (2014).
Yu, Y. X. Can all nitrogen-doped defects improve the performance of graphene anode materials for lithium-ion batteries? Phys. Chem. Chem. Phys. 15, 16819–16827 (2013).
Jalili, S. & Vaziri, R. Study of the electronic properties of Li-intercalated nitrogen doped graphite. Mol. Phys. 109, 687–694 (2011).
Callahan, D. J. et al. Triple stimulus-responsive polypeptide nanoparticles that enhance intratumoral spatial distribution. Nano Lett. 12, 4 (2012).
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Buron, J. D. et al. Graphene mobility mapping. Sci. Rep. 5, 12305 (2015).
Acharya, S., Babu, A. V., Khadar, R. A. & Kottantharayil, A. Mobility improvement in CVD graphene by using local metal side-gate. Semicond. Sci. Technol. 35, 045027 (2020).
Schwierz, F. Graphene transistors. Nat. Nanotechnol. 5, 487–496 (2010).
Mukherjee, S. & Kaloni, T. P. Electronic properties of boron- and nitrogen-doped graphene: a first principles study. J. Nanopart. Res. 14, 1059 (2012).
Olaniyan, O. et al. A systematic study of the stability, electronic and optical properties of beryllium and nitrogen co-doped graphene. Carbon 129, 207–227 (2018).
Xu, J., Jiang, P., Liang, X. L., Tian, R. J. & Liu, Y. M. Nitrogen-doped porous carbon as cathodes for aluminum-ion batteries. Fuller. Nanotub. Carbon Nanostructures 29, 21–27 (2021).
Yao, F. et al. Diffusion mechanism of lithium ion through basal plane of layered graphene. J. Am. Chem. Soc. 134, 8646–8654 (2012).
Ju, Z. C. et al. Few layer nitrogen-doped graphene with highly reversible potassium storage. Energy Storage Mater. 11, 38–46 (2018).
Childress, A. et al. Effect of nitrogen doping in the few layer graphene cathode of an aluminum ion battery. Chem. Phys. Lett. 733, 136669 (2019).
Smajic, J. et al. Capacity retention analysis in aluminum-sulfur batteries. Acs Appl. Energ. Mater. 3, 6805–6814 (2020).
Smajic, J. et al. Mesoporous reduced graphene oxide as a high capacity cathode for aluminum batteries. Small 14, 1803584 (2018).
Ma, C., Shao, X. & Cao, D. Nitrogen-doped graphene nanosheets as anode materials for lithium ion batteries: a first-principles study. J. Mater. Chem. 22, 8911-8915 (2012).
Wasalathilake, K. C., Ayoko, G. A. & Yan, C. Effects of heteroatom doping on the performance of graphene in sodium-ion batteries: A density functional theory investigation. Carbon 140, 276–285 (2018).
Author information
Authors and Affiliations
Contributions
GD: Literature survey, conceptualization, original draft, writing (review and editing), JJ: DFT part literature survey, writing and editing, US: Supervision, writing (review and editing), project administration, funding acquisition, PMFJC: Supervision, writing (review and editing), project administration, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deokar, G., Jin, J., Schwingenschlögl, U. et al. Chemical vapor deposition-grown nitrogen-doped graphene’s synthesis, characterization and applications. npj 2D Mater Appl 6, 14 (2022). https://doi.org/10.1038/s41699-022-00287-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41699-022-00287-8