Abstract
MYB transcription factors of the R2R3-MYB family have been shown to play important roles in many plant processes. A sugarcane R2R3-MYB gene (ScMYB2) and its two alternative forms of transcript (ScMYB2S1 and ScMYB2S2) were identified in this study. The deduced protein of ScMYB2S1 is a typical plant R2R3-MYB protein, while ScMYB2S2 encodes a truncated protein. Real-time qPCR analysis revealed that ScMYB2S1 is suppressed under PEG-simulated drought stress in sugarcane, while ScMYB2S2 is induced at later treatment stage. A senescence symptom was observed when ScMYB2S1 was injected into tobacco leaves mediated by Agrobacterium, but no symptom for ScMYB2S2. Further investigation showed that the expression levels of 4 senescence-associated genes, NtPR-1a, NtNYC1, NtCAT3 and NtABRE, were markedly induced in tobacco leaves after ScMYB2S1-injection, while they were not sensitive to ScMYB2S2-injection. Moreover, MDA and proline were also investigated after injection. Similarly, MDA and proline levels were induced by ABA and ScMYB2S1, while inhibited by ScMYB2S2. We propose that ScMYB2, by alternatively splicing two transcripts (ScMYB2S1 and ScMYB2S2), is involved in an ABA-mediated leaf senescence signaling pathway and play positive role in respond to drought-induced senescence in sugarcane. The results of this study provide information for further research in sugarcane stress processes.
Similar content being viewed by others
Introduction
Transcription factors are proteins that bind to specific DNA sequences1, thereby promoting or blocking the recruitment of RNA polymerase to specific genes2,3. A typical plant transcription factor contains a DNA-binding region, an oligomerization site, a transcription-regulation domain, and a nuclear localization signal4,5. According to the structural features of the DNA-binding domain, plant transcription factors can be divided into several families6, and many of them, including MYB/MYC, AP2/EREBP, bZIP, NAC and WRKY, have been implicated in abiotic stress tolerance7,8.
MYB transcription factors are defined by a highly conserved MYB DNA-binding domain (DBD) at the N-terminus, and have been found in a wide variety of eukaryotic organisms, including animals, plants, insects and fungi9,10. Animal MYB proteins are referred to as 3R-MYB for their DBDs, which generally consists of three tandem amino acid sequence repeats (motif, designated R1, R2, and R3) of about 50~53 amino acid residues in length, and forms a helix-turn-helix fold with three regularly spaced tryptophan residues11,12. Plant MYB transcription factors can be classified into four major groups based on the number and position of adjacent MYB motif repeats, namely 1R-MYB (R1-MYB), 2R-MYB (R2R3-MYB), 3R-MYB (R1R2R3-MYB) and 4R-MYB (R1R2R2R1/2)13,14, containing one, two, three and four MYB repeats, respectively15. In plants, R2R3-MYB genes are predominant16,17, and members of this family function in a variety of plant-specific processes18, as evidenced by their extensive functional characterization in Arabidopsis14.
As one of the largest plant transcription factor families, MYB proteins are known to play key roles in gene transcriptional regulatory networks that mediate a variety of developmental processes and defense responses, including cellular differentiation19,20,21, morphogenesis22, light-signaling pathways23, secondary metabolism24, hormone signal transduction25, disease resistance and abiotic stress tolerance26,27. The regulatory activities of plant MYB proteins are elaborately regulated at multiple steps14,28. In particular, accumulating evidence illustrates that post-transcriptional control of mRNA modulates the transcription factor activities during plant response to environmental stimuli28. Alternative splicing (AS), to regulate gene expression, is one subset of post-transcriptional processes29. Through effecting the production of mRNA isoforms with different exonic composition from a single gene, alternative splicing creates multiple mRNA transcripts30. Alternative splicing can affect protein function and influence protein diversity when AS events occur within the translated regions of mRNAs30. It is also common for AS to result in isoforms that contain a premature termination codon, which subsequently become targets for nonsense mediated decay30. By this way, instead of a truncated polypeptide, no protein is produced.
Plant leaf senescence is an age-dependent deterioration process and is also triggered by environmental stresses and phytohormones31,32. It has been recognized that senescence associated genes (SAGs) are induced by senescence32,33. Abscisic acid (ABA) is an important phytohormone and plays a critical role in regulating plant development and responses to various stress signals, such as drought32. It is also well-known that ABA promotes leaf senescence32.
In the present work, the function of a sugarcane (Saccharum officinarum) R2R3-MYB gene ScMYB2 with its two alternative splicing transcripts, ScMYB2S1 and ScMYB2S2I, were investigated. We utilized real-time qPCR to evaluate the response of the alternative splicing transcripts to drought stress induced by PEG. A visible symptom of leaf senescence, i.e. de-greening, was observed when one alternatively spliced transcript of ScMYB2S1 was transiently over-expressed in tobacco (Nicotiana tabacum) leaves. To further investigate the role of ScMYB2S1 and ScMYB2S2 in senescence, the contents of malonaldehyde (MDA) and proline, and then the expression profiles of 4 previously reported senescence-associated genes (SAGs) are checked in tobacco leaves after ScMYB2S1- or/and ScMYB2S2-transiently-transformed. Results suggest that ScMYB2 is involved in the response to drought. The differential expression of two alternatively spliced transcripts during PEG stress could be one kind of drought tolerance molecular mechanism in sugarcane.
Results
Cloning and sequence analysis of ScMYB2
Two rounds of PCR were performed, and gel electrophoresis of the inner PCR products showed one fragment (Supplementary Fig. S1). The amplicons of about 1,100 bp long were separated and recovered from the agarose gel, which were subsequently used for T-A cloning and transformed into Escherichia coli. Ten randomly positive clones were picked and sequenced, and two R2R3-MYB-like cDNA sequences were obtained, designated as ScMYB2S1 (GenBank Accession Number KM387410) and ScMYB2S2 (GenBank Accession Number KM387411), respectively. ScMyB2S1 had a full length of 1, 066 bp, with an ORF of 687 bp, 5′ UTR (untranslated region) of 40 bp, and 3′UTR of 339 bp (Fig. 1). The deduced protein of ScMYB2S1 was a typical plant R2R3-MYB protein, containing two MYB DNA-binding domains (R2 and R3 repeats) at the N-terminal. Within the R2 and R3 repeats, the highly conserved tryptophan (W) residues implicated in DNA-binding were spaced by the 19 or 18 amino acid residues, respectively. The first W of R3 repeat in ScMYB2S1 protein was replaced by a methionine (M) (Fig. 1).
Overlapping the full-length sequences of ScMYB2S1, 968-bp long ScMYB2S2 transcript contained an additional 29-bp-sequence inserting into the corresponding location of the ORF, which interrupted the reading frame of a subsequent region behind the start codon and caused frameshift mutation of the sequence. Thus, compared with the amino acid sequence of ScMYB2S1, the first MYB DNA-binding domain (R2) in the amino acid sequence of ScMYB2S2 was missing, which resulted in the residue part starting with the first methionine (M) of R3 repeat, thereafter sharing 100% homology to the ScMYB2S1. Cloning a genomic sequence of the gene was also performed to identify whether the two transcripts, ScMYB2S1 and ScMYB2S2, were produced by alternative splicing of the same gene. The genomic sequence of the ScMYB2 gene (GenBank Accession Number KM387409) displayed at least two alternatively spliced isoforms (Fig. 2): a typical plant R2R3-MYB transcription factor gene ScMYB2S1 and ScMYB2S2 encoding a truncated protein starting at a methionine in the R3 repeat.
The genomic sequence of the ScMYB2 had a highly conserved splicing arrangement with three exons and two introns (126 bp and 76 bp). The 126 bp intron appeared to consist of two short tandem intron-like sequences, 29-bp sequence mentioned above at the 5′-terminal and the other 97 bp sequence at the 3′-terminal. All of them conformed to the GT-AG rule (Fig. 2).
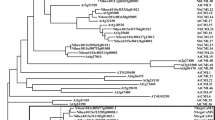
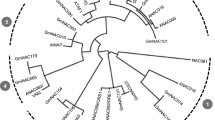
Following the methods described by Matus et al.34 and Lin-Wang et al.24, we constructed a phylogenetic tree with ScMYB2S1, ScMYB2S2 and the other 131 Arabidopsis MYB proteins using Mega5.05 software. Figure 3 indicated that ScMYB2S1 and ScMYB2S2 were close to AtMYB48 and AtMYB49, two members from Arabidopsis described as alternative splicing/non-canonical intron subgroup34.
Expression profiles of ScMYB2S1 and ScMYBS2 under drought stress
To further examine the function of the alternatively spliced transcripts of ScMYB2, their responses to drought stress were performed. When treated with 25.0% PEG, the expression level of ScMYB2 rapidly decreased at 3 h (Fig. 4) and stayed at the relatively low level during the periods from 3 h to 24 h, but increased in the later periods (48 h and 72 h). By using primers specific for each splice variant, the level of ScMYB2S1 expression decreased steadily after 3 h following the treatment and maintained at a low expression level up to 72 h. In contrast, the expression of the ScMYB2S2 increased dramatically at 48 and 72 h.
ScMYB2 represents a pool of both variants. The other two are splice variant specific. Each value is the average of three replicate experiments ± standard error (n = 3). The different lowercase showed the significance difference of ScMYB2, ScMYB2S1 and ScMYB2S2 expression levels under p-value < 0.01 level.
Agrobacterium-mediated transient expression in tobacco leaves
To gain insights into the role of ScMYB2 in sugarcane, transient expression of pGreenII0229-ScMYB2S1, pGreenII0229-ScMYB2S2 and pGreenII0229 (control) were tested for their effect on tissue-cultured tobacco (N. tabacum) leaves via A. tumefaciens injection. The effect of over-expressing ScMYB2S1, ScMYB2S2 or control was recorded at 24 h after injection. The whole leaf over-expressing ScMYB2S1 changed color from green to yellow (Fig. 5a), when compared with control (Fig. 5c). Meanwhile, there was no obvious change in leaf color when the ScMYB2S2 was over-expressed (Fig. 5b).
Physiological measurement
MDA and proline content in tobacco leaf samples were detected 48 h after ABA-treatment or injection. Figure 6 showed that ScMYB2S1-injection induced both MDA and proline levels obviously, while ScMYB2S2-injection and mixed-injection (with ScMYB2S1 and ScMYB2S2 volume ratio of 1:1) had limited effect on MDA or proline levels. Predictably, both MDA and proline contents were increased significantly after ABA-treatment (Fig. 6).
1: ABA-treatment; 2: Injected with recombinational Agrobacterium containing empty vector; 3. Injected with recombinational Agrobacterium containing the target gene of ScMYB2S1; 4. Injected with recombinational Agrobacterium containing the target gene of ScMYB2S2; 5: Injected with the mixture of the two recombinants containing ScMYB2S1 and ScMYB2S2. (A) MDA level (nmoL/g); (B) Proline contents (μg/g). The different lowercase indicated the significance difference of MDA and proline contents under p-value < 0.01 level.
Expression profiles of SAGs in tobacco leaves
Real-time qPCR was used to examine the expression profiles of four SAGs, NtNYC1, NtPR-1a, NtCAT3 and NtABRE, in tobacco leaves under different treatments. All these genes were induced after ScMYB2S1 injection, with an expression level about 2.17, 3.80, 5.14 and 2.48 times higher than that of the control, respectively (Fig. 7). Conversely, after injection of ScMYB2S2, the expression of NtNYC1 and NtPR-1a were decreased with 0.81 and 0.75 times, respectively, lower than that of the control (Fig. 7). While at the same time, the expression level of NtCAT3 and NtABRE were about 1.54 and 1.12 times higher than control (Fig. 6). Overall, however, the expression levels of these genes were between the two former situations after mix-injection of ScMYB2S1 and ScMYB2S2 (Fig. 7).
1: Injected with recombinational Agrobacterium containing empty vector; 2. Injected with recombinational Agrobacterium containing the target gene of ScMYB2S1; 3. Injected with recombinational Agrobacterium containing the target gene of ScMYB2S2; 4: Injected with the mixture of the two recombinants containing ScMYB2S1 and ScMYB2S2. (A) NtNYC; (B) NtPR-1a; (C) NtCAT3; (D) NtABRE. The different lowercase indicated the significance difference of 4 SAGs expression levels under p-value < 0.01 level.
Discussion
In the present study, a R2R3-MYB gene was isolated from sugarcane, designated as ScMYB2, showing to produce two alternatively spliced transcripts: ScMYB2S1 and ScMYB2S2. Sequence analysis showed that the 126-bp-intron of the genomic sequence of the ScMYB2 gene consisted of two short tandem “GT-AG” structures, which provided the structural basis for alternative splicing. Further sequence analysis revealed that ScMYB2S1 was a functional protein, containing two complete MYB DNA-binding domains (R2 and R3 repeats). ScMYB2S2, with the first MYB DNA-binding domain (R2 repeat) missing in the N-terminal amino acid sequence, might limited its function on directly involved in transcriptional regulation. Similarly, two Arabidopsis R2R3-type MYB genes, AtMYB59 and AtMYB48, both were found to have four distinctively spliced transcripts that encoded either MYB-related proteins or R2R3-MYB proteins35. Interestingly, ScMYB2, AtMYB59 and AtMYB48 were found to be within the same phylogenetic subgroup in this study (Fig. 3).
Alternative splicing occurs widely in eukaryote and provides the main source of transcriptome and proteome diversity in an organism36. Recent studies suggested that the signal transduction related genes associated with various stress responses seemed to be particularly prone to alternative splicing in plants and animals35,37. The R2R3-MYB transcription factor superfamily has been showed to play an important role in many plant processes including abiotic stress responses18. It had been demonstrated that several MYB genes were alternatively spliced, and encoded proteins with regulatory functions35,38,39.
Previous studies have shown that alternative splicing also presents in sugarcane MYB transcription factor genes. Prabu et al.40,41 identified an inducible alternatively spliced R2R3-MYB transcription factor gene ScMYBAS1 (EU670236) in S. officinarum based on a cDNA suppression subtractive hybridization library. Subsequently, semi-quantitative RT-PCR analysis revealed that the alternatively spliced transcripts, ScMYBAS1-2 and ScMYBAS1-3, were constitutively expressed at high level when subjected to water deficit and salt stress treatments, however no sequence information was provided40,41. In GenBank the genomic DNA of MYBAS1 (HM136779) from Saccharum hybrid cultivar Co740, and three alternatively spliced variants: MYBAS1V1 (HM136780), MYBAS1V2 (HM136781) and MYBAS1V3 (HM136782), are noted as being induced by water deficit stress based on semi-quantitative RT-PCR analysis. Sequence analysis shows that MYBAS1V1 is only 2 bases different from ScMYBAS1 (Data not showed).
Many members in plant R2R3-MYB family have been reported as abiotic-stress-induced transcription factors40,41,42,43. Moreover, over-expressing the R2R3-MYB genes improved the resistance to freezing, drought, and salt stresses in transgenic plants44,45,46. In this study, ScMYB2S2, one alternatively spliced transcript of ScMYB2, was induced at the late periods of PEG-simulated drought stress. Contrary to expectation, the expression of ScMYB2S1, the other alternatively spliced transcript of ScMYB2, was suppressed constitutively by PEG-simulated drought stress. This shows that highly specific probes or primers are crucial to identify the expression pattern of a certain gene for alternatively spliced transcripts or allelic genes which are highly homologous to each other.
It was reported that leaf senescence was affected by drought stress47. In this study, the de-greening symptom displayed when one alternatively spliced transcript of ScMYB2S1 was transiently over-expressed in tobacco leaves, suggesting the function of ScMYB2S1 might relate to leaf senescence regulation. The stress hormone ABA promotes leaf senescence in an ethylene-independent pathway and induces compatible solutes (e.g. malondialdehyde and proline) accumulation in leaf48,49. Within those compatible solutes, MDA and proline levels are commonly known as markers of stress50. To further investigate the role of ScMYB2S1 and ScMYB2S2 in leaf senescence, the contents of MDA and proline in tobacco leaves were detected in this study. The results showed that both MDA and proline levels were significantly induced by ABA treatment (Fig. 6). Similarly, the two compatible solutes were also obviously increased after ScMYB2S1-injection (Fig. 6). On the contrary, ScMYB2S2 -injection had less effect on them (Fig. 6). From those results, we can concluded that it is ScMYB2S1 rather than ScMYB2S2, lead to the accumulation of MDA and proline in tobacco leaves.
Plant senescence is regulated by senescence associated genes (SAGs)33. Stress-induced senescence signals are perceived and then transferred via SAGs51. To date, a series of SAGs have been identified from various plant species33,52. In this study, 4 SAGs genes were selected randomly to investigate whether they were involved in the co-expression network regulating by ScMYB2. Among these four SAGs, pathogenesis-related 1a (PR1a) and catalase (CAT) have been identified as marker gene of biotic stress51. Non-yellow coloring (NYC1) gene, encoding the membrane-spanning isoform of Chl b reductase was expressed during leaf senescence in rice53, and its function on chlorophyll degradation also has been proved in Arabidopsis33. Recent studies have shown that abscisic acid responsive element (ABRE)-binding factor gene (ABRE) also involved in responding to ABA-induced leaf senescence52,54. An ABRE-binding factor has been identified as a positive regulator of abiotic stress and ABA signaling both in Arabidopsis and in rice55. Collectively, the function of all these four genes was identified to accelerate leaf senescence33,56. Previous studies have shown that both endogenous ABA and exogenously applied ABA can induce the expression of SAGs and promote leaf de-greening and senescence in general52. The present study showed that these 4 SAGs were significantly induced by ScMYB2S1-injection, while ScMYB2S2-injection didn’t have much effect (Fig. 7). We concluded that when transiently over-expressing in tobacco leaf, it is ScMYB2S1 rather than ScMYB2S2 playing an ABA-like function, induced the expression of the four SAGs.
ScMYB2S1 was down-regulated in responding to drought stress during the whole processing period in sugarcane. Combined analysis of its performance in de-greening and promoting both compatible solute contents and the expression levels of SAGs in tobacco (Figs 5, 6 and 7) suggested that ScMYB2S1 could act as a negative regulator and play a positive role in response to drought-induced senescence process. Meanwhile, the expression level of ScMYB2S2 in sugarcane was up-regulated by drought stress at the later stage. It seems that ScMYB2 tends to generate the isoform of ScMYB2S2 by alternative splicing during the later stage. Different with ScMYB2S1, ScMYB2S2 has limited effect on leaf de-greening (Fig. 5), MDA and proline contents (Fig. 6) and the expression of SAGs. It needs to further investigate whether this limited influence is due to its incomplete functional domain. And, recent studies have shown that alternative splicing of some transcription factor genes generates small interfering peptides (siPEPs), which negatively regulates the target transcription factors28. Further studies are also needed to substantiate whether the two transcripts are existed an interaction at nucleic acid level or protein level.
In conclusion, a hypothesis is proposed here that ScMYB2, by alternatively splicing two transcripts (ScMYB2S1 and ScMYB2S2), might involve in an ABA-mediated leaf senescence signaling pathway and play positive role in respond to drought-induced senescence in sugarcane. The differential expression of two alternatively spliced transcripts during PEG stress could be one kind of drought tolerance molecular mechanism in sugarcane. The alternative splicing of the transcription factor gene ScMYB2 may be pivotal to the molecular defense mechanism of sugarcane during drought-induced senescence processes.
Methods
Materials and treatments
3′-Full RACE Core Set Ver.2.0 Kit, TaKaRa LA PCRTM in vitro Cloning Kit, PrimeScript RT-PCR Kit, DNA markers were purchased from TaKaRa (Dalian, China). RQ1 RNase-Free DNase was obtained from Promega Corporation (USA), SYBR® Green PCR Master Mix Kit was purchased from Applied Biosystems TM (USA), and the instrument used in the real-time qPCR analysis was the ABI PRISM7500 real-time PCR system (USA). Plant Malondialdehyde (MDA) assay Kit and Proline assay Kit were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Sugarcane cultivar Badila (S. officinarum), tobacco (N. tabacum) variety K326 seedlings and tissue culture plantlets used in this study was provided by the Key Laboratory of Sugarcane Biology and Genetic Breeding, Ministry of Agriculture, Fuzhou, China. According to Guo et al.57, uniform plantlets of an elite sugarcane cultivar Badila were grown in 1/4 Hongland nutrient solution for one week and then subjected to PEG8000 (25.0%) treatment. The sampling times were 0 h, 3 h, 6 h, 12 h, 24 h, 48 h and 72 h after the start of treatment.
Agrobacterium-infiltrated transient transformation of tobacco was carried out according to Lin-Wang described24. For phenotype observation assay, tobacco tissue culture plantlets were used for transiently transformed assay. Approximately 150 μL of each recombinant Agrobacterium culture was infiltrated at four points into a tender leaf. Tobacco seedlings grown in a greenhouse with environmental control systems were used for physiological measurement and gene expression assay. Two kind of stress treatments were applied to 8-week-old plants before flowering: ABA treatment (sprayed 100 μM ABA on leaf at 48 h) and Agrobacterium injection (using a suspension of recombinational Agrobacterium introduced into tobacco leaf by direct injection). As for the latter, approximately 300 μL of Agrobacterium containing the target gene or empty vector control were infiltrated at four points into the leaf. After 48 h treatment, all the leaf samples were collected and divided into three groups and assayed, respectively. One group was fixed in liquid nitrogen immediately and stored in a refrigerator at −85 °C until RNA extraction. According to the methods from Plant Malondialdehyde (MDA) assay Kit and Proline assay Kit, the other two groups were grinded in buffer solution immediately and then measured, respectively. All of the treatments were repeated independently three times. The data were analyzed with DPS v7.05 directly and the significance difference of the MDA and proline contents were marked using different lowercase in figure.
Molecular cloning, sequencing and bioinformatics analysis
A sugarcane R2R3-MYB EST was obtained based on the bioinformatics analysis using the data from the previous RNA-Seq experiments (not yet published). Two nested gene-specific 3′ RACE primers was designed according to the EST sequence information, and one pair of gene-specific primer was designed to amplify the given target regions using genomic DNA as a template. Primer sequences are as follows:
3′ RACE GSP1: 5′-ACATAGTGGCTTCTTCTCCC-3′;
3′ RACE GSP2: 5′-TATCCAAGGTAGAAGCGAGCAA-3′;
ScMyb2 F: 5′-TATCCAAGGTAGAAGCGAGCAA-3′ (same to 3′ RACE GSP2);
ScMyb2 R: 5′-CCATAAGCATACCTCCAGTGTT-3′.
The method used in 3′ RACE was followed to the specifications of the 3′-Full RACE Core Set Ver.2.0 Kit (Takara). A full-length R2R3-MYB homolog gene of sugarcane (named ScMYB2) was identified by Blastx (http://blast.ncbi.nlm.nih.gov/Blast.cgi) with two Myb-like DNA-binding domains (pfam00249). The open reading frame (ORF) of the full-length cDNA sequence of ScMYB2 was predicted using the ORF Finder online tool from NCBI (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). Sequence alignment was performed using DNAMAN 5.2.2 software.
Expression profiles of ScMYB2 under PEG-simulated drought stress
Total RNA isolation was performed using the TRIzol® Reagent (Invitrogen, USA). The removal of DNA from RNA samples was realized by RQ1 RNase-Free DNase (Promega, USA). The reverse transcription was realized by following the specifications of the PrimeScript® RT reagent Kit (Takara, China). Finally, the SYBR® Green PCR Master Mix (AB, USA) was employed in real-time qPCR reaction.
The 25 S rRNA (BQ536525) gene was chosen as an internal control in real-time qPCR analysis57,58 and the forward and reverse primers for 25 S rRNA were 5′-GCAGCCAAGCGTTCATAGC-3′and 5′-CCTATTG GTGGGTGAACAATCC-3′58. Based on the common sequences of ScMYB2S1 and ScMYB2S2, a pair of real-time qPCR primers was designed using the Primer Express 3.0 software and the forward and reverse primers for ScMYB2 were 5′-ACCGTCGTTGGGACTTCATT-3′ (MYB2qF) and 5′-CAGGCTTGGTATGTCAGTGAGGAG-3′ (MYB2qR), respectively (Fig. S2). Moreover, pairing with common reverse primer (MYB2qR), two sequence-specific forward primers were designed for ScMYB2S1 and ScMYB2S2 with the sequences 5′-CATTGCCCAAGTCTCAGGCC-3′ (MYB2S1qF) and 5′-CATTGCCCAAGTCTCAGGTT-3′ (MYB2S2qF), respectively (Supplementary Fig. S2).
The real-time qPCR reaction was realized with following conditions: 2 min at 50 °C, 10 min at 95 °C, and then 40 cycles of 94 °C for 15 s, and 60 °C for 60 s. Each assay was repeated by three times. When the reaction was completed, a melting curve was obtained. The 2−ΔΔCT method was adopted to analyze the real-time qPCR results59. The data were analyzed with DPS v7.05 directly and the significance difference of the gene expression were marked using different lowercase in figure.
Binary vectors construction
To study the function of ScMYB2S1 and ScMYB2S2 in tobacco, PCR was performed using the clone containing ScMYB2S1 or ScMYB2S2 as template to obtain the ScMYB2S1 or ScMYB2S2 ORF with matched sites. The primer sequences were MYB2S1F: 5-CCCAAGCTTATGGTGACCGTGAG-3, MYB2S2F: 5-CCCAAGCTTATGTCACCACAAGAA-3 and MYB2SR: 5-CGGAATTCTCACATCATGATTTCT-3′ (Hind III and EcoR I sites are underlined). The 50 μL PCR reaction mix contained 5.0 μL 10 × PCR buffer; 4.0 μL deoxynucleotide triphosphates (dNTPs) (2.5 mM); 2.0 μL each of forward and reverse primers (10 μM); 2.0 μL plasmid DNA (100 ng); and 0.25 μL Ex-Taq enzyme (5 U/μL). The ddH2O was added as supplement. The PCR amplification program consisted of pre-denaturation for 5 min at 94 °C; denaturation for 30 s at 94 °C, annealing for 30 s at 55 °C, and extension for 45 s at 72 °C for 30 cycles; and final extension for 10 min at 72 °C. The ScMYB2S1 or ScMYB2S2 ORF with Hind III and EcoR I sites was subcloned into intermediate vector pSIM35Scassett (Hind III - EcoR I sites) in the E. coli strain DH5α to generate the putative recombinants. The intermediate vector containing the gene expression cassette of ‘35S promoter-ScMYB2S1 (or ScMYB2S2)-CaMV polyA’ was digested with EcoR V and these fragments were ligated into the equally digested EcoR V pGreen II0229 (Supplementary Fig. S3). A clone with a recombinant plasmid was validated by PCR, double digestion and sequencing, and was termed as pGII0229-ScMYB.
Agrobacterium-mediated transient expression
A single colony of A. tumefaciens strain EHA105 was cultured overnight in 5 mL LB broth at 28 °C with 250 rpm shaking. Three mL of this culture was shifted to 1,000 mL LB medium and incubated at the same conditions until the OD600 was about 0.5. The culture was centrifuged at 3,500 × g for 15 min at 4 °C. The pellet was resuspended/washed with 15% glycerol in ultrapure water. After washing, the bacteria were pelleted again. All the steps were performed on ice. This process of washing was repeated three times. After final washing, the pellet was divided into 100 μL and stored at −80 °C.
Electroporation was carried out with an electric pulse of 2.0 kV/cm and 400Ω. After each transformation the bacterial cells were resuscitated by suspending in 1 mL of liquid LB media and subsequent incubation at 28 °C with 250 rpm shaking, before spreading on the LB plates. pGII0229, pGII0229-ScMYB2S1 and pGII0229-ScMYB2S2 were used for electroporation, respectively. Screening of positive clones was performed on LB medium containing 50 μg/mL kanamycin and 35 μg/mL rifampicin. The plasmid was isolated from the positive clones, and PCR was employed to confirm the transformation. Agrobacterium-infiltrated transient transformation of tobacco was carried out as previously described24.
Expression profiles of SAGs in tobacco leaves
Four verified senescence-associated genes (SAGs), NtPR-1a (ACC. No. X12737.1)51, NtCAT3 (Acc. No. Z36977.1)51, NYC1(XM016652882)33 and NtABRE (KF736850)33, were selected to verify their expression profiles in tobacco leaves after ABA treated or ScMYB2s-transiently-transformed, respectively. The forward and reverse primers for NtPR-1a were 5′-ATATCCCACTCTTGCCGTGCCCAA-3′ and 5′-GCTACCTGGTCGTCCCAGGTCAA-3′51. The forward and reverse primers for NtCAT3 were forward 5′-CTGCAGCTCCCAGTTAAC-3′ and 5′-GAGCATCAACCCATCTGCA-3′51. Using the Primer Express 3.0 software, real-time qPCR primers were designed according to NtNYC1 (ACC. No. XM016652882.1) and NtABRE (ACC. No. KF736850) sequences, respectively. The forward primers for NtNYC1 and NtABRE were 5′-TGACATTTGGGTAAACAACGCT-3′ and 5′-CTAATAGGAACACGGGTGAAACTG-3′, respectively. The reverse primers for NtNYC1 and NtABRE were 5′-ATCCATAGACAGCCGTTAGAGGG-3′ and 5′-ATCCATAGACAGCCGTTAGAGGG-3′, respectively. The elongation factor 1α (ELF-1α) (AF120093) gene was chosen as an internal reference gene for normalization of real-time RT-PCR in tobacco and the forward and reverse primers for EF-1a were 5′-TGAGATGCACCACGAAGCTC-3′and 5′-CCAACATTGTCACCAGGAAGTG-3′60. The real-time qPCR reaction was realized with following conditions: 2 min at 50 °C, 10 min at 95 °C, and then 40 cycles of 94 °C for 15 s, and 60 °C for 60 s. Each assay was repeated by three times. When the reaction was completed, a melting curve was obtained. The 2−△△CT method was adopted to analyze the RT-qPCR results60. The data were analyzed with DPS v7.05 directly and the significance difference of the gene expression were marked using different lowercase in figure.
Additional Information
How to cite this article: Guo, J. et al. A sugarcane R2R3-MYB transcription factor gene is alternatively spliced during drought stress. Sci. Rep. 7, 41922; doi: 10.1038/srep41922 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Latchman, D. S. Transcription factors: an overview. Int J Biochem Cell B. 29, 1305–1312 (1997).
Roeder, R. G. The role of general initiation factors in transcription by RNA polymerase II. Trends Biochem Sci. 21, 327–335 (1996).
Nikolov, D. B. & Burley, S. K. RNA polymerase II transcription initiation: A structural view. P Natl Acad Sci USA. 94, 15–22 (1997).
Liu, L., White, M. J. & MacRae, T. H. Transcription factors and their genes in higher plants functional domains, evolution and regulation. Eur J Biochem. 262, 247–257 (1999).
Yamasaki, K., Kigawa, T., Seki, M., Shinozaki, K. & Yokoyama, S. DNA-binding domains of plant-specific transcription factors: structure, function, and evolution. Trends Plant Sci. 11, e1117723 (2013).
Riechmann, J. L. & Ratcliffe, O. J. A genomic perspective on plant transcription factors. Curr Opin Plant Biol. 3, 423–434 (2000).
Century, K., Reuber, T. L. & Ratcliffe, O. J. Regulating the regulators: The future prospects for transcription-factor-based agricultural biotechnology products. Plant Physiol. 147, 20–29 (2008).
Sakuraba, Y., Kim, Y. S., Han, S. H., Lee, B. D. & Paek, N. C. The Arabidopsis transcription factor NAC016 promotes drought stress responses by repressing AREB1 transcription through a trifurcate feed-forward regulatory loop involving NAP. Plant Cell. 27, 1771–1787 (2015).
Lipsick, J. S. One billion years of Myb. Oncogene. 13, 223–235 (1996).
Du, H. et al. Genome-wide analysis of the MYB transcription factor superfamily in soybean. BMC Plant Biol. 12, 106 (2012).
Rosinski, J. A. & Atchley, W. R. Molecular evolution of the Myb family of transcription factors: evidence for polyphyletic origin. J Mol Evol. 46, 74–83 (1998).
Kanei-Ishii, C. et al. The tryptophan cluster: a hypothetical structure of the DNA-binding domain of the myb protooncogene product. J Biol Chem. 265, 19990–19995 (1990).
Jin, H. & Martin, C. Multifunctionality and diversity within the plant MYB-gene family. Plant Mol Biol. 41, 577–585 (1999).
Dubos, C. et al. MYB transcription factors in Arabidopsis . Trends in Plant Sci. 15, 573–581 (2010).
Katiyar, A. et al. Genome-wide classification and expression analysis of MYB transcription factor families in rice and Arabidopsis . BMC Genomics. 13, 544 (2012).
Chen, Y. H. et al. The MYB transcription factor superfamily of Arabidopsis: expression analysis and phylogenetic comparison with the rice MYB family. Plant Mol Biol. 60, 107–124 (2006).
Martin, C. & Paz-Ares, J. MYB transcription factors in plants. Trends Genet. 13, 67–73 (1997).
Li, C. N., Ng Carl, K. Y. & Fan, L. M. MYB transcription factors, active players in abiotic stress signaling. Environ Exp Bot. 114, 80–91 (2015).
Raffaele, S., Rivas, S. & Roby, D. An essential role for salicylic acid in AtMYB30-mediated control of the hypersensitive cell death program in Arabidopsis. FEBS Lett. 580, 3498–3504 (2006).
Borg, M. et al. The R2R3 MYB transcription factor DUO1 activates a male germline-specific regulon essential for sperm cell differentiation in Arabidopsis. Plant Cell. 2011, 23, 534–549 (2011).
Wang, S. C. et al. Regulation of secondary cell wall biosynthesis by poplar R2R3 MYB transcription factor PtrMYB152 In Arabidopsis. Sci. Rep. 4, 5054 (2014).
Baumann, K. et al. Control of cell and petal morphogenesis by R2R3 MYB transcription factors. Development. 134, 1691–1701 (2007).
Cominelli, E. et al. Expression analysis of anthocyanin regulatory genes in response to different light quality in Arabidopsis thaliana . J Plant Physiol. 165, 886–894 (2008).
Lin-Wang, K. et al. An R2R3 MYB transcription factor associated with regulation of the anthocyanin biosynthetic pathway in Rosaceae . BMC Plant Biol. 10, 50 (2010).
Abe, H. et al. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell. 15, 63–78 (2003).
Singh, K. B., Foley, R. C. & Oñate-Sánchez, L. Transcription factors in plant defense and stress responses. Curr Opin Plant Biol. 5, 430–436 (2002).
Vannini, C. et al. Over expression of the rice Osmyb4 gene increases chilling and freezing tolerance of Arabidopsis thaliana plants. Plant J. 37, 115–127 (2004).
Seo, P. J., Park, M. J. & Park, C. M. Alternative splicing of transcription factors in plant responses to low temperature stress: mechanisms and functions. Planta. 237, 1415–1424 (2013).
McGlincy, N. J. et al. Regulation of alternative splicing by the circadian clock and food related cues. Genome Biol. 13, R54 (2012).
Barbazuk, W. B. A conserved alternative splicing event in plants reveals an ancient exonization of 5S rRNA that regulates TFIIIA. RNA Biol. 7, 397–402 (2010).
Uzelac, B. et al. Characterization of natural leaf senescence in tobacco (Nicotiana tabacum) plants grown in vitro . Protoplasma. 253, 259–275 (2016).
Zhao, Y. et al. The ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc Natl Acad Sci USA 113, 1949–1954 (2016).
Li, Z., Peng, J., Wen, X. & Guo, H. Gene network analysis and functional studies of senescence-associated genes reveal novel regulators of Arabidopsis leaf senescence. J Integr Plant Biol. 54, 526–539 (2012).
Matus, J. T., Aquea, F. & Arce-Johnson, P. Analysis of the grape MYB R2R3 subfamily reveals expanded wine quality-related clades and conserved gene structure organization across Vitis and Arabidopsis genomes. BMC Plant Biol. 8, 83 (2008).
Li, J. G. et al. A subgroup of MYB transcription factor genes undergoes highly conserved alternative splicing in Arabidopsis and rice. J Exp Bot. 57, 1263–1273 (2006).
Kazan, K. Alternative splicing and proteome diversity in plants: the tip of the iceberg has just emerged. Trends Plant Sci. 8, 468–471(2003).
Yang, S. M., Tang, F. & Zhu, H. Y. Alternative splicing in plant immunity. Int J Mol Sci. 15, 10424–10445 (2014).
James, A. B. et al. Alternative splicing mediates responses of the Arabidopsis circadian clock to temperature changes. Plant Cell. 24, 961–981 (2012).
Huang, W. J. et al. A R2R3-MYB transcription factor from Epimedium sagittatum regulates the flavonoid biosynthetic pathway. PLoS One. 8, e70778 (2013).
Prabu, G. R. & Kawar, P. G. Theertha Prasad D. Molecular cloning and characterization of an inducible alternatively spliced MYB transcription factor gene, ScMYBAS1 from sugarcane (Saccharum officinarum Linn.). Plant and Animal Genome XVII Conference Abstracts. San Diego, January 10–14, P067 (2009).
Prabu, G. R., Kawar, P. G., Pagariya, M. C. & Theertha Prasad, D. Identification of water deficit stress upregulated genes in sugarcane. Plant Mol Biol Rep. 29, 291–304 (2011).
Yang, A., Dai, X. Y. & Zhang, W. H. A R2R3-type MYB gene, OsMYB2, is involved in salt, cold, and dehydration tolerance in rice. J Exp Bot. 63, 2541–2556 (2012).
Rahaie, M., Xue, G. P., Naghavi, M. R., Alizadeh, H. & Schenk, P. M. A MYB gene from wheat (Triticum aestivum L.) is up-regulated during salt and drought stresses and differentially regulated between salt-tolerant and sensitive genotypes. Plant Cell Rep. 29, 835–844 (2010).
Dai, X. Y. et al. Overexpression of an R1R2R3 MYB gene, OsMYB3R-2, increases tolerance to freezing, drought, and salt stress in transgenic Arabidopsis. Plant Physiol. 143, 1739–1751 (2007).
Shingote, P. R. et al. SoMYB18, a sugarcane MYB transcription factor improves salt and dehydration tolerance in tobacco. Acta Physiol Plant. 37, 217 (2015).
Wang, R. K., Cao, Z. H. & Hao, Y. J. Overexpression of a R2R3 MYB gene MdSIMYB1 increases tolerance to multiple stresses in transgenic tobacco and apples. Physiol Plantarum. 150, 76–87 (2014).
Wehner, G. G., Balko, C. C., Enders, M. M., Humbeck, K. K. & Ordon, F. F. Identification of genomic regions involved in tolerance to drought stress and drought stress induced leaf senescence in juvenile barley. BMC Plant Biol. 15, 125 (2015).
Wang, F. et al. Involvement of abscisic acid in PSII photodamage and D1 protein turnover for light-induced premature senescence of rice flag leaves. PLoS One. 11, e0161203 (2016).
de Zelicourt, A., Colcombet, J. & Hirt, H. The role of MAPK modules and ABA during abiotic stress signaling. Trends Plant Sci. 21, 677–685 (2016).
Ciceva, R. et al. Screening for drought tolerance in cultivars of the ornamental genus Tagetes (Asteraceae). PeerJ. 4, e2133 (2016).
Wehner, G., Balko, C., Humbeck, K., Zyprian, E. & Ordon, F. Expression profiling of genes involved in drought stress and leaf senescence in juvenile barley. BMC Plant Biol. 16, 3 (2016).
Gao, S. et al. ABF2, ABF3, and ABF4 promote ABA-mediated chlorophyll degradation and leaf senescence by transcriptional activation of chlorophyll databolic genes and senescence-associated genes In Arabidopsis. Mol Plant. 9, 1272–1285 (2016).
Kusaba, M. et al. Rice NON-YELLOW COLORING1 is involved in light-harvesting complex II and grana degradation during leaf senescence. Plant Cell. 9, 1362–1375 (2007).
Takasaki, H. et al. SNAC-As, stress-responsive NAC transcription factors, mediate ABA-inducible leaf senescence. Plant J. 84, 1114–1123 (2015).
Hossain, M. A. et al. The ABRE-binding bZIP transcription factor OsABF2 is a positive regulator of abiotic stress and ABA signaling in rice. J PlantPhysiol. 167, 1512–1520 (2010).
Golemiec, E., Tokarz, K., Wielanek, M. & Niewiadomska, E. A dissection of the effects of ethylene, H2O2 and high irradiance on antioxidants and several genes associated with stress and senescence in tobacco leaves. J Plant Physiol. 171, 269–275 (2014).
Guo, J. L. et al. A novel dirigent protein gene with highly stem-specific expression from sugarcane, response to drought, salt and oxidative stresses. Plant Cell Rep. 31, 1801–1812 (2012).
Iskandar, H. M. et al. Comparison of reference genes for quantitative real-time polymerase chain reaction analysis of gene expression in sugarcane. Plant Mol Biol Rep. 22, 325–337 (2004).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△CT method. Methods. 25, 402–408 (2001).
Schmidt, G. W. & Delaney, S. K. Stable internal reference genes for normalization of real-time RT-PCR in tobacco (Nicotiana tabacum) during development and abiotic stress. Mol Genet Genomics. 283, 233–241 (2010).
Acknowledgements
This work was supported by Scientific Research Fund of Fujian Provincial Education Department (JA12123) and the earmarked fund for the Modern Agriculture Technology of China (CARS-20). The authors are thankful to Prof. Andrew C Allan (The New Zealand Institute for Plant & Food Research Ltd, Mt Albert Research Centre, Auckland, New Zealand) for his great help in Figure 3 and his valuable suggestions while revising this manuscript. The authors especially thank Dr. Fengji Wang in College of Crop Science, Fujian Agriculture and Forestry University, Fuzhou, China, for providing the tobacco variety K326.
Author information
Authors and Affiliations
Contributions
J.G. and L.X. conceived the study. J.G., H.L., J.M., Y.C., Q.L., S.G. and H.W. performed the experiments. J.G., H.L., Y.S. and Y. Q. analyzed the data. J.G. and L.X. wrote the paper. J.G. and L.X. revised the final version of the paper. J.G. and L.X. approved the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Guo, J., Ling, H., Ma, J. et al. A sugarcane R2R3-MYB transcription factor gene is alternatively spliced during drought stress. Sci Rep 7, 41922 (2017). https://doi.org/10.1038/srep41922
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep41922
This article is cited by
-
Transcriptome and WGCNA reveal hub genes in sugarcane tiller seedlings in response to drought stress
Scientific Reports (2023)
-
Identification of Saccharum CaM gene family and function characterization of ScCaM1 during cold and oxidant exposure in Pichia pastoris
Genes & Genomics (2023)
-
Genome-wide analysis of R2R3-MYB transcription factors family in the autopolyploid Saccharum spontaneum: an exploration of dominance expression and stress response
BMC Genomics (2021)
-
Physiological, biochemical and molecular responses of lentil (Lens culinaris Medik.) genotypes under drought stress
Indian Journal of Plant Physiology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.