Abstract
Plant-associated beneficial microbes have been explored to fulfill the imperative function for plant health. However, their impact on the host secondary metabolite production and nematode disease management remains elusive. Our present work has shown that chitinolytic microbes viz., Chitiniphilus sp. MTN22 and Streptomyces sp. MTN14 singly as well as in combination modulated the biosynthetic pathway of bacoside A and systemic defense mechanism against Meloidogyne incognita in Bacopa monnieri. Interestingly, expression of bacoside biosynthetic pathway genes (3-Hydroxy-3-methylglutaryl coenzyme A reductase, mevalonate diphosphate decarboxylase, and squalene synthase) were upregulated in plants treated with the microbial combination in the presence as well as in absence of M. incognita stress. These microbes not only augmented bacoside A production (1.5 fold) but also strengthened host resistance via enhancement in chlorophyll a, defense enzymes and phenolic compounds like gallic acid, syringic acid, ferulic acid and cinnamic acid. Furthermore, elevated lignification and callose deposition in the microbial combination treated plants corroborate well with the above findings. Overall, the results provide novel insights into the underlying mechanisms of priming by beneficial microbes and underscore their capacity to trigger bacoside A production in B. monnieri under biotic stress.
Similar content being viewed by others
Introduction
Exploitation of beneficial microbes is well known to promote productivity, ameliorate nutrient supply and defend host from the various stresses. Among the wide range of microbes present in the soil, chitinolytic microbes have received extensive attention for their potential in plant growth and phyto-disease management. As a source of biocontrol, bacterial chitinases have been widely established for obstructing the growth of pathogen1,2. Chitinolytic microbes have been cited as antagonistic agents, which either act directly on nematodes egg shells or instigate increased plant resistance towards disease3. Therefore, we were interested in the development of a compatible microbial combination that effectively functions to protect disease mediated by plant parasitic nematode. Few studies have previously attempted to elucidate the relative quantitative and qualitative contributions of microbes to the production of secondary metabolites in plants4,5. However, the information regarding the mechanism involved is still unknown. Therefore, the use of microbes for boosting in planta secondary metabolite production under biotic stress could be a better and sustainable approach.
Utilization of medicinal plants for health consideration has become extremely popular with the indiscriminate use of synthetic drugs. Bacopa monnieri (L.) Pennell (family Scrophulariaceae), is second in the list of most essential Indian medicinal plants with ample number of therapeutically important bacosides6. Bacoside A is considered as a major active component known to have protective activities against morphine-induced cerebral toxicity, chemical-induced liver toxicity, and wound healing activity7,8. The remedial properties are because of the presence of bioactive saponins synthesized via mevalonic acid (MVA) pathway9. Globally, the requirement is met solely from the wild natural populations of B. monnieri resulting in its listing as a threatened plant10. In addition, the cultivation of B. monnieri faces stern damage owing to Meloidogyne incognita infestation11,12. To keep pace with the growing demand of this medicinal plant and considering its ability to grow under natural conditions, the present study was undertaken to develop strategies to improve yields of B. monnieri through beneficial microbial intervention. Previously, numerous methods have been employed to enhance plant immunity and the production of bioactive metabolites by the association between plant-beneficial microbes and their host plant (s), which suggests that the microbial partners could have a significant effect on host physiology13,14. However, till date, no information is available regarding the potential and possible mechanisms of the synergistic mode of microbes in the accumulation of secondary metabolites along with the modulation of the defense signaling pathway to endure biotic stress. Considering the above facts, efforts were made to examine the effect of synergistic chitinolytic microbial combination on induction of in planta bacoside A production and the possible mechanism adopted by B. monnieri plants under M. incognita stress.
Results
Chitinolytic microbes promote plant growth and effectively control M. incognita
To evaluate the effect of microbes on the growth and secondary metabolite production of B. monnieri plants under nematode stress, biomass, total bacoside content, and disease index were measured. Under greenhouse conditions, chitinolytic microbes viz., Chitiniphilus sp. MTN22 (KF699070) and Streptomyces sp. MTN14 (KF699062) alone and their combinational treatment showed a significant difference in growth parameters in the presence of M. incognita over untreated control (Fig. 1). After 5 weeks of nematode inoculation, the plants treated with chitinolytic microbes demonstrated their efficiency against M. incognita infestation by lessening the nematode number in the soil and roots as represented by the lower Rf values (Supplementary Table S1). M. incognita population in soil and roots were noticeably reduced (P ≤ 0.05) in alone Chitiniphilus (1.6 fold) and Streptomyces (1.5 fold) along with the combinational treatment (2.2 fold) inoculated with pathogen over untreated-M. incognita stressed plants. Among the various treatments, the most efficient control of M. incognita was observed in the microbial combination treated plants which (P ≤ 0.05) reduced the root galling index (RGI) by 2.8 fold, followed by alone Chitiniphilus sp. and Streptomyces sp. treatments which demonstrated reduction by 1.7 and 1.5 fold, respectively against untreated M. incognita control (Supplementary Table S1). The total bacoside content found in different treatments is given in Fig. 2. Plants treated with Chitiniphilus sp. and Streptomyces sp. without pathogen showed the greatest increment in bacoside A content (1.5 fold) than untreated pathogen-inoculated control (Fig. 2, Supplementary Fig. S1). Application of microbes noticeably increased bacoside A content (1.1 to 1.5 fold) as compared to untreated challenged control (Supplementary Table 1). In addition, there was a significant difference between the rhizospheric colonization of the microbial combination plants with and without pathogen over the single microbe treated plants (Supplementary Table S2). In the present experiment, chitinase activity showed a significant rise in dual microbes treated soil with respect to single treatments. The results showed that the chitinase activity was significantly raised in all the chitinolytic microbes treated soil. Further, analysis of the data revealed that higher activity was found in the microbial combination treated plants with pathogen followed by alone treatments compared to untreated M. incognita stressed plants (Supplementary Table S2).
Additionally, the nutrient uptake (NPK) status of B. monnieri was not much affected by the microbial inoculation (Supplementary Table S3). The maximum percentage of nitrogen content was noticed in the plants inoculated with dual microbes without (1.4 fold) and with (1.2 fold) pathogen, respectively while least nitrogen uptake was observed in the treatment of alone Chitiniphilus with pathogen (Supplementary Table S3). The phosphorus and potassium content were also found to be non significant among all the treatments in comparison to untreated pathogen-inoculated plants (Supplementary Table S3).
The impact of chitinolytic microbes on the expression of various genes
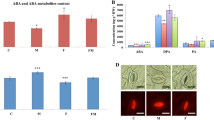
We next examined whether microbial inoculation enhanced bacoside A production through the modulation of biosynthetic pathway gene expression. The results indicated that plants primed with chitinolytic microbes showed the highest level of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR), mevalonate diphosphate decarboxylase (MDD), and squalene synthase (SQS) transcription as compared to the control plants (Fig. 3). Expectedly, the microbial combination of Chitiniphilus sp. and Streptomyces sp. treated plants showed induced level of HMGR (6.2 fold) and MDD (6.8 fold) transcription compared to the untreated pathogen stressed control plants (Fig. 3a and b). Most interestingly, SQS expression was up-regulated and had the highest level (6.4 and 5.1 fold) in dual microbes treated plants with and without pathogen, respectively (Fig. 3c). Moreover, there was also a significant increment in the PR1 (Pathogenesis-related protein 1) transcript levels in the plants treated with dual microbial treatment (4.2 fold) followed by Streptomyces (4.0 fold) and Chitiniphilus (3.5 fold) alone with pathogen (Fig. 3d).
Control: untreated-uninoculated; Control + Mi: untreated M. incognita-inoculated. Pathogenesis-related protein 1 (PR1), 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR), mevalonate diphosphate decarboxylase (MDD), and squalene synthase (SQS) transcripts were amplified by qRT-PCR using gene-specific primers. Total RNA was isolated from the B. monnieri leaves after 5 weeks of M. incognita inoculation. The untreated M. incognita inoculated plant was used as a control. Results were normalized to 18S-rRNA (reference transcript). Relative expression was determined using the equation; 2−ΔΔCt. Data are mean ± standard error (n = 3 biological replicates) and asterisk indicate significant between treated and untreated pathogen-inoculated control (LSD test *P < 0.05; **P < 0.01).
Influence of chitinolytic microbes on various enzymatic activities
The effect of microbes on primary metabolism and defense enzymes of the plants under pathogenic stress compared with infected healthy and control plants was evaluated. Content of chlorophyll a was enhanced significantly whereas chlorophyll b and carotenoids were not affected by the microbial treatments (Fig. 4a,b and c). The increment in chlorophyll a was observed by 1.8 fold in the microbial combination treated plants followed by Streptomyces (1.7 fold) and Chitiniphilus (1.6 fold) with pathogen than untreated pathogen-inoculated control (Fig. 4a). In addition, higher levels of phenolic and flavonoid content were observed in dual (2.3 and 2.6 fold, respectively) followed by alone Streptomyces (1.9 and 2.1 fold, respectively) and Chitiniphilus (1.9 and 2.0 fold, respectively) treatments with pathogen (Fig. 4d and e). Furthermore, maximum peroxidase (PO), phenylalanine ammonia lyase (PAL) and polyphenol oxidase (PPO) activities were also recorded in the microbial combination (2.0, 1.5 and 3.5 fold, respectively) followed by Streptomyces (1.9, 1.4 and 3.3 fold, respectively) and Chitiniphilus (1.7, 1.4 and 2.8 fold, respectively) treatments in the presence of pathogen with respect to the pathogen-infected control plants (Fig. 4f,g and h).
Control: untreated-uninoculated; Control + Mi: untreated M. incognita-inoculated. (a) Chlorophyll a; (b) Chlorophyll b; (c) Carotenoids; (d) Total phenolic content (TPC); (e) Total flavonoid content (TFC); (f) Peroxidase (PO) activity; (g) phenylalanine ammonia lyase (PAL) activity; and (h) polyphenol oxidase (PPO) activity. Error bars indicate the standard error of six replicates. Different letters indicate significant difference between treated and untreated pathogen-inoculated control (LSD test P < 0.05).
Phenolics compounds in leaves of B. monnieri
Considering that microbes act as an elicitor to enhance the defense parameters, we hypothesized that microbes might interfere with the phenylpropanoid and shikimate pathway. We thus investigated the effect of chitinolytic microbes on the phenolic status of the host plants. As expected, the variation in phenolics compounds was also found in the leaves of treated plants, under the influence of different microbial treatments (Table 1, Supplementary Fig. S2). The level of gallic acid varied from 5.3 to 22.8 μg g−1, syringic acid 0.6 to 5.1 μg g−1, ferulic acid 0.9 to 1.6 μg g−1, and cinnamic acid 0.2 to 2.5 μg g−1 dry weight (DW) in microbes treated plants (Table 1). The most effective treatment was dual combination of microbes where gallic acid accumulation was 1.4 fold, ferulic acid 1.8 fold, and cinnamic acid 2.5 fold higher as compared to the control treatment challenged only with the pathogen. However, maximum gallic acid was accumulated in dual microbial treatment in the presence of M. incognita (1.6 fold more) compared to untreated stressed plants. Further, syringic acid accumulation in leaves was highest in Streptomyces treatment inoculated with the pathogen. Interestingly, cinnamic acid and syringic acid were not detected in leaves of control treatment challenged with pathogen, M. incognita (Table 1, Supplementary Fig. S2).
Lignin and callose deposition
To envisage the effect of microbial inoculation on the lignification and callose deposition, stem and leaves sections were visualized, respectively. B. monnieri plants uprooted from various treatments illustrated variation in the deposition of lignin when compared with untreated pathogen-inoculated plants (Fig. 5a). The maximum and uniform lignin deposition indicated as blue ring was found in the microbial combination with and without pathogen-treated plants followed by treatment having single microbial inoculation. In the control treatment challenged with pathogen, intermittent lignin deposits were observed (Fig. 5a). Additionally, callose deposition in leaves was found to be preferentially deposited in the interveinal region cells of host leaves as an intense blue-green fluorescence (Fig. 5b). Treatments with combination of microbes, along with alone Chitiniphilus and Streptomyces led to an increase in callose deposition in host leaves, with maximum enhancement in the microbial combination over untreated pathogen-inoculated treatment.
The results from the present study were further validated by principal component analysis. The formation of six different groups among the treatments: first forming only control, second cluster consisted of control challenged only with the pathogen, third having Chitiniphilus and Streptomyces with pathogen, fourth having dual microbes with pathogen, fifth having Chitiniphilus and Streptomyces treatments without pathogen and sixth having microbial combination treatment without pathogen. The microbial combination in the presence and absence of pathogen formed an individual group where all the morphological and physiological parameters along with bacoside content were enhanced. PCA denoted 83.2% of the total variance (PC1 denoted 57.4%, and PC2 denoted 25.8%) (Supplementary Fig. S3).
Discussion
This study clearly demonstrated that when applied singly and in combinations, Chitiniphilus and Streptomyces act as antagonistic agents against M. incognita in terms of reducing galling index, reproduction factor and also ameliorated plant resistance along with the enhancement in secondary metabolites of plants. The enhanced disease protection could be possibly because of the chitinase producing microbes which might have effectively colonized the rhizospheric zone owing to the presence of easier nutrient sources in the soil. Also, the function of synergistic cooperation cannot be overlooked as our supposition is in conformity with the earlier findings, where the relationship between colonization and presence of chitinase in soil was highlighted15. The observations recorded are also in accordance with the previous studies of Krechel et al.1 and Oka et al.16 who demonstrated effective control of plant parasitic nematodes by chitinases producing rhizospheric microbes.
Along with the disease protection and plant growth promoting abilities of the beneficial microbes, induced synthesis of secondary metabolites is also reported12. In the present study, induction of bacoside A content via microbial application can thus be owed to the differential modulation in the expression of various genes involved in the bacoside pathway such as HMGR, MDD and SQS. It has been established that B. monnieri has two independent biosynthetic pathways; MVA occurring in the cytosol and the methyl-D-erythritol 4-phosphate (MEP) pathway in the plastid for triterpenoid saponins17. The MVA pathway initiates with the acetyl-coenzyme A, which condenses into the acetoacetyl-CoA by the catalyzing action of acetyl-CoA acetyltransferase. Acetyl-coenzyme A is converted to isopentenyl diphosphate (IPP) through MVA, and HMGR (HMG-CoA reductase) which finally supplies carbon for the bacoside biosynthesis17,18. In the present investigation, both the microbes upregulated the expression of HMGR, a post-transcriptionally as well as post-translationally regulated gene responsible for catalyzing the conversion of HMG-CoA to mevalonate19. In a previous study, the role of HMGR in isoprene biosynthesis was identified as loss of function of HMGR1 lead to dwarfism and early senescence in Arabidopsis20.
As a key regulatory enzyme of MVA pathway, mevalonate diphosphate decarboxylase (MDD) catalyzes the decarboxylation of the mevalonate 5- diphosphate (MVAPP) to the isopentenyl IPP, accompanied by the hydrolysis of ATP21. Likewise, squalene synthase (SQS) is a bifunctional enzyme which catalyzes reaction at an important regulatory point that controls carbon flux into sterol and triterpenoid biosynthetic pathway22. Upregulation of HMGR, MDD and SQS expression in microbes-inoculated plants may be the explanation for the higher bacoside A production. Our elucidation is also supported by the earlier findings where application of microbes over expressing biosynthetic genes, had elevated level of secondary metabolites as compared to the wild-type plants12,23. The findings further advocated that the bacoside synthesis is preferentially favored, by the significant expression of gene transcripts related to this pathway in chitinolytic microbes treated plants over the control. Augmentation in the bacoside content could be the result of upregulation of HMGR, MDD and SQS genes which was further substantiated by the high-performance liquid chromatography (HPLC) analysis. Thus, our findings strongly suggest that the applications of beneficial microbes are able to promote the biosynthetic potential of the plant for key secondary metabolites.
In the present investigation, the microbes not only reduced M. incognita infestation but also systemically provoked resistance without affecting the photosynthetic efficiency in terms of chlorophyll b and carotenoid content of the host plant. The principal groups of metabolites in plants known for their function in host plant resistance are phenolics and flavonoids. Collectively, improved PPO, PO, and PAL activities which are important defense enzymes of plants can be positively correlated with the bolstered up plant systemic resistance against the pathogen. In addition to the above defense enzymes, lignin plays an imperative role in protecting plants against pathogens and provides structural integrity to the host cell wall during the itinerary of normal tissue development24. Since, our results confirmed higher PPO activity in the dual microbe treated plants which is reported to catalyze phenols oxidation and finally lignification, we next studied lignin deposition pattern in the different treatments. Observations revealed disparity in lignin deposition in B. monnieri which could be accredited to the efficacy of the chitinolyitc microbes to prompt the lignification process.
Recently, there has been a terrific development concerning the utilization of chitinolytic microbes to enhance plant immunity25. The results demonstrated evidently that the production of phenolic compounds had a positive connection with disease diminution as less severity was observed in the treatments having high concentration of phenolic compounds. Furthermore, induced PAL activity which is a precursor of phenylpropanoid pathway may be the reason of elevated phenolic level in the dual microbe treatment. Presence of phenolic acids mainly gallic, ferulic, syringic and cinnamic acids in leaves indicated that the defense response induced by chitinolytic microbes is altered accordingly to the physiological state of the plant. The enhanced defense response also supports the reason behind improved plant growth in chitinolytic microbe treatments. In addition, the consistent increase of gallic acid in leaves of plants demonstrates that this compound, occurs constitutively, and its amount is modulated by the application of beneficial microbes. Elevated gallic acid level which is mainly responsible for crosslinking of suberin and lignin as well as other polyphenolic barriers in cell wall formed after pathogen infection might also be the reason for the presence of more lignified cells in the dual microbe treatment in comparison to other treatments26. Further, intensification in the defense response of plants was further validated by the enhanced callose deposition responsible for strengthening of the cell wall.
Ferulic and cinnamic acids formed through the shikimic acid pathway are well known antifungal and potent antioxidants phenolics27. A high amount of ferulic acid in the plants undeniably supports their function in reducing pathogenic stress. Sarma et al.28 and Takenaka et al.29 reported that the increased levels of ferulic acid in the host plants with beneficial microbes played a major role in reducing the biotic stress. Better disease protection efficiency of the Chitiniphilus and Streptomyces strains could also be possibly by the accumulation of antimicrobial cinnamic acid and syringic acid in treated plants challenged with the pathogen. However, at this stage possibility of participation of some other more complex molecular alterations in plants after the application of chitinolytic microbes resulting in the activation of defense responses through synthesis of certain enzymes linked with host resistance cannot be ruled out. Overall, the earlier findings of less mortality in host plants against various pathogens after application of beneficial microbes28,30 was further proved in the present study.
Results of the present study suggest that the presence of Chitiniphilus and Streptomyces in the rhizosphere of plant probably plays an important role in promoting the growth of the host plant by increasing chlorophyll a, defense enzymes, lignification, callose deposition along with increased bacoside A accumulation and disease suppression (Fig. 6). The absence of these microbes resulted in reduced growth and secondary metabolite production which further verified our above observations. Our study strongly highlights that the presence of chitinolytic microbes is able to promote the biosynthetic potential of the plant for some key secondary metabolites. Both Chitiniphilus and Streptomyces enhanced bacoside A content by modulating the expression of genes involved in biosynthesis pathway. For future applications, we suggest chitinase based formulations using chitinolytic microbes (that enhance plant growth rate, biomass and bacoside content) under natural conditions. Such microbial combination may not only provide a middle course by providing high productivity and protection coupled with high bacoside A but will also play a key role in dropping the cost of the expensive bacoside.
Methods
Microbes and pathogen inoculums
Chitiniphilus sp. and Streptomyces sp. were individually inoculated in Luria Bertani (LB) and Glucose Yeast Malt (GYM) broth, respectively and incubated at 30 ± 2 °C on a rotary shaker (120 rpm) for 1 day and 7 days, respectively for mass production. The centrifugation was done and the pellet density was maintained in 0.85% saline to 1 × 108 colony forming units (CFU) mL−1. We previously isolated Chitiniphilus and Streptomyces with high nematicidal and chitinase activity against M. incognita31.
M. incognita culture was isolated from Solanum melongena L. plants grown in greenhouse conditions. The intact plants were uprooted and egg masses were extracted using an extraction procedure32. The J2 juveniles were obtained from extracted eggs that were continuously oxygenated for 1 week using an aquarium pump33.
Treatment of plants with Chitiniphilus sp. and Streptomyces sp.
The B. monnieri (cv. CIM-Jagriti) runners of even length (6–8 cm) bearing at least 3–4 nodes along with roots were transplanted to the experimental pots containing 0.5 kg of sterilized sandy soil (pH 7.5). The pots consisted of autoclaved alkaline (pH 7.5, EC 0.30 dSm−1) sandy loam soil: vermicompost (3:1 w/w) and contained available nitrogen (142.00 kg ha−1), organic carbon (4.40 g kg−1), phosphorus (9.21 kg ha−1) and potassium (95.00 kg ha−1). Following treatments were used in the experiment: Chitiniphilus sp., Streptomyces sp. and microbial combination with pathogen along with two controls: untreated with and without pathogen. The plants were maintained in a greenhouse and all the sets were arranged in a completely randomized design with nine replicates. A separate set of treatments having chitinolytic microbes only (without pathogen) was also maintained. The microbial suspensions were applied at a dose of 10 mL per pot. In the combinational treatment, cell suspension of the two strains was mixed in equal ratio and vortexed to obtain homogenous mixture. Freshly hatched J2 juveniles of M. incognita (1000 J2) were inoculated in each of the pots after one week of microbial inoculation. The experiment was repeated twice and the results were collected 5 weeks after M. incognita inoculation. The pathogen population density was estimated in the soil by Cobb’s sieving and decanting technique followed by Baermann funnel33. The nematode and egg populations in the plant root system were analyzed by macerating 2 g aliquots of root tissues and the nematode population was calculated through reproduction factor Rf = Pf (final M. incognita population)/Pi (initial M. incognita population)34. The severity of root galling index (RGI) in root knot infested seedlings was assessed according to Oka et al.35 on 0–10 scale. The inoculated chitinolytic microbes density in the soil was calculated as colony forming unit (CFU) g−1. The chitinase activity was also measured in the rhizospheric soil from each treatment pot−1 by using 4-methylumbelliferyl-(GlcNAc)2 [4MU − (GlcNAc)2] (Sigma) according to Metcalfe et al.36. Nutrient uptake in dry matter of various treatments was determined based on NPK content by Jackson et al.37.
Extraction and estimation of bacoside A content
The leaves of different treatments were shade dried and methanolic extract was prepared from 100 mg of each sample. The combined methanolic extract was concentrated under vacuum for analysis. The process was repeated six times. The concentration of bacoside-A was measured by HPLC38.
Transcription analysis
Total RNA was isolated by trizol (Invitrogen) method and cDNA was reverse-transcribed with random primers kit (Thermo Fisher Scientific, United States). A gene 18S-rRNA was taken as the internal control. The primer sets for the genes PR1, HMGR, and MDD except SQS were designed using Primer 3 version 0.4.0. The effectiveness of the designed primer sets was assessed by measuring the PCR amplicons length in agarose gel. All the primers involved in the experiment are mentioned in Supplementary Table S4. The following reactions were carried out in the qRT-PCR for each of the target genes: 1 cycle of 10 min at 94 °C, followed by 40 cycles of 30 s at 94 °C, 30 s at 55 °C, and 30 s at 72 °C and at the end of the PCR cycles the amplicon dissociation curve was recorded. The reactions were repeated twice in triplicate. The mean expression of various genes was analyzed by the (2−ΔΔCt) comparative CT method39.
Phenolic compounds in the leaves of B. monnieri
For HPLC analysis, 100 mg of leaves was dried and extracted with 50% methanol (10 mL). The solvent was removed using rotary evaporator and the residue was dissolved in HPLC grade methanol and subjected to HPLC for the detection of specific phenolic compounds40. The process was repeated six times. The data (μg g−1 DW) was calculated by comparing the peak areas (λmax 254 nm) of the treated samples with their respective standards (Class VP series software, Shimadzu, Japan).
Enzymatic assays
The seedlings from each treatment were randomly uprooted and immediately frozen using liquid nitrogen. The root material (1 g) was homogenized using 10 mM sodium phosphate buffer (pH 6.0) with polyvinylpolypyrrolidone (1% w/v), phenylmethylsulfonyl fluoride (0.3 mM), and EDTA (1 mM) at 4 °C and was filtered through nylon filter. The centrifugation was done at 10,000 × g for 15 min and the crude extract was used for enzymatic activities41. All the assays were repeated six times.
Determination of chlorophyll and carotenoid content from powdered leaves tissue was done according to Arnon42 and Lichtenthaler et al.43, respectively. The powdered leaves (100 mg) samples were extracted with CH3OH:H2O (9:1, v/v) solution. The centrifugation was done at 8,000 × g for 5 min and absorbance was calculated at 663, 645, 480 and 510 nm. The total phenolic content (TPC) was expressed as mg gallic acid equivalent per g of dried sample44. The methanolic extract of leaves tissue (1 mL) was mixed with folin ciocalteu reagent (0.5 mL) along with sodium carbonate solution (7%; 2.5 mL) and incubated at room temperature for 2 h. The absorbance was recorded at 765 nm. Total flavonoid content (TFC) was calculated using rutin (mg g−1) as standard45. A 15% sodium nitrite solution (0.15 mL) was added to extract (0.5 mL). The 4% NaOH (2 mL) was added after 6 min of incubation and the volume of reaction mixture was made upto 5 mL which was further left for incubation for 15 min. The absorbance was calculated at 510 nm. PO activity was determined as unit mg−1 protein per min−1 at 420 nm46. The reaction mixture contained 100 μL of the diluted extract (10 fold) with 1.5 mL pyrogallol (0.05 mol L−1) and 0.5 mL H2O2 (1% v/v). The alteration in the absorbance was measured after 30 s intervals for 3 min at 420 nm. PAL activity was measured by adding enzyme extract (100 μL) in L-phenylalanine (6 μM; 900 μL) and Tris-HCl buffer solution as mentioned by Chen et al.46 and was denoted in mole trans-cinnamic acid g−1 protein h−1. The tubes were incubated for 70 min at room temperature in a water bath and the absorbance was measured spectrophotometrically at 290 nm. PPO activity was calculated by mixing enzyme extract (100 μL) in 700 μL of homogenization buffer according to Gauillard et al.47 and was measured as unit mg−1 protein per min−1. After the addition of catechol (0.2 M), the change in absorbency was measured for 1 min at 405 nm. For control, the reaction mixture without enzyme extract was also maintained.
Lignification and callose deposition
B. monnieri plants were harvested 5 weeks after the inoculation of pathogen, M. incognita. For imaging lignification pattern, the transverse sections of stem were examined under fluorescent microscope DMI 3000 B (Leica, Wetzlar, Germany) with a wide range of wavelengths (250–700 nm) to induce autofluorescence13. For callose detection, leaves were boiled in ethanol and placed in 0.01% aniline blue for 1 h. The samples were examined for the presence of cell wall deposition using a UV epifluorescence microscope48. Four biological repeats were performed for each test and at least 10 leaves were collected for the inspection from multiple seedlings in each experiment.
Statistical analysis
The significant differences between the various treatments and infected control were analyzed by LSD (least significant difference; P ≤ 0.05) using SPSS package (SPSS V16.0, SPSS Inc., Chicago, IL).
Additional Information
How to cite this article: Gupta, R. et al. Microbial modulation of bacoside A biosynthetic pathway and systemic defense mechanism in Bacopa monnieri under Meloidogyne incognita stress. Sci. Rep. 7, 41867; doi: 10.1038/srep41867 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Krechel, A., Faupel, A., Hallmann, J., Ulrich, A. & Berg, G. Potato-associated bacteria and their antagonistic potential towards plant-pathogenic fungi and the plant-parasitic nematode Meloidogyne incognita (Kofoid & White) Chitwood. Can. J. Microbiol. 48, 772–786 (2002).
Brzezinska, M. S., Jankiewicz, U., Burkowska, A. & Walczak, M. Chitinolytic microorganisms and their possible application in environmental protection. Curr. Microbiol. 68, 71–81 (2014).
Quecine, M. C. et al. Chitinolytic activity of endophytic Streptomyces and potential for biocontrol. Lett. Appl. Microbial. 47, 486–491 (2008).
Gupta, R. & Pandey, R. Microbial interference ameliorates essential oil yield and diminishes root-knot infestation in sweet basil under field conditions. Biocontrol Sci. Technol. 25, 1165–1179 (2015).
Gupta, R., Singh, A. & Pandey, R. Microbes based technology ameliorates glandular trichomes, secondary metabolites and antioxidant in Pelargonium graveolens L’Her. J. Sci. Food Agri. 96, 4151–4159 (2016).
Gohil, K. & Patel, J. A review on Bacopa monniera: current research and future prospects. Int. J . Green Pharm. 4, 1–9 (2010).
Russo, A. & Borrelli, F. Bacopa monniera, a reputed nootropic plant: an overview. Phytomedicine 12, 305–317 (2005).
Sharma, S. et al. In vitro rapid and mass multiplication of highly valuable medicinal plant Bacopa monnieri (L.) Wettst. Afri. J. Biotechnol. 9, 8318–8322 (2015).
Vishwakarma, R. K. et al. Molecular cloning, biochemical characterization, and differential expression of an acetyl-CoA C-acetyltransferase gene (AACT) of Brahmi (Bacopa monniera). Plant Mol. Biol. Report 31, 547–557 (2013).
Ceasar, S. A., Maxwell, S. L., Prasad, K. B., Karthigan, M. & Ignacimuthu, S. Highly efficient shoot regeneration of Bacopa monnieri (L.) using a two-stage culture procedure and assessment of genetic integrity of micropropagated plants by RAPD. Acta Physiol. Planta. 32, 443–452 (2010).
Pandey, R., Kalra, A., Singh, S. C. & Gupta, M. L. Bacopa monnieri damaged by root-knot nematode and remedial measures. J. Med. Arom. Plant Sci. 4, 123–128 (2003).
Gupta, R. et al. Exploitation of microbes for enhancing bacoside content and reduction of Meloidogyne incognita infestation in Bacopa monnieri L. Protoplasma 252, 53–61 (2015).
Singh, A., Gupta, R., Srivastava, M., Gupta, M. M. & Pandey, R. Microbial secondary metabolites ameliorate growth, in planta contents and lignification in Withania somnifera (L.) Dunal. Physiol. Mol. Biol. Plants 22, 253–260 (2016).
Pandey, S. S. et al. Endophytes of opium poppy differentially modulate host plant productivity and genes for the biosynthetic pathway of benzylisoquinoline alkaloids. Planta 243, 1097–1114 (2016).
Jacquiod, S., Franqueville, L., Cécillon, S., Vogel, T. M. & Simonet, P. Soil bacterial community shifts after chitin enrichment: an integrative metagenomic approach. PLoS One. 8, e79699 (2013).
Oka, Y. et al. New strategies for the control of plant‐parasitic nematodes. Pest Manag. Sci. 56, 983–988 (2000).
Miziorko, H. M. Enzymes of the mevalonate pathway of isoprenoid biosynthesis. Arch. Biochem. Biophy. 505, 131–143 (2011).
Vishwakarma, R. K. et al. Squalene synthase gene from medicinal herb Bacopa monniera: molecular characterization, differential expression, comparative modeling, and docking studies. Plant Mol. Biol. Rep. 33, 1675–1685 (2015).
Kirby, J. & Keasling, J. D. Biosynthesis of plant isoprenoids: perspectives for microbial engineering. Annu. Rev. Plant Biol. 60, 335–355 (2009).
Suzuki, M. et al. Loss of function of 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase 1 (HMG1). In Arabidopsis leads to dwarfing, early senescence and male sterility, and reduced sterol levels. Plant J. 37, 750–761 (2004).
Abbassi, S. J., Vishwakarma, R. K., Patel, P., Kumari, U. & Khan, B. M. Bacopa monniera recombinant mevalonate diphosphate decarboxylase: Biochemical characterization. Int. J. Biol. Macromolec. 79, 661–668 (2015).
Tansey, T. R. & Shechter, I. Squalene synthase: structure and regulation. Progress Nucleic Acid Res . Mol. Biol. 65, 157–195 (2000).
Battini, F., Bernardi, R., Turrini, A., Agnolucci, M. & Giovannetti, M. Rhizophagus intraradices or its associated bacteria affect gene expression of key enzymes involved in the rosmarinic acid biosynthetic pathway of basil. Mycorrhiza 26, 699–707 (2016).
Conrath, U., Pieterse, C. M. & Mauch-Mani, B. Priming in plant–pathogen interactions. Trends Plant Sci. 7, 210–216 (2002).
Bashan, Y., de-Bashan, L. E., Prabhu, S. R. & Hernandez, J. P. Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998–2013). Plant Soil. 378, 1–33 (2014).
Lattanzio, V., Lattanzio, V. M. & Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochem. Adv. Res. 661, 23–67 (2006).
Mishra, R. P., Singh, R. K., Jaiswal, H. K., Kumar, V. & Maurya, S. Rhizobium-mediated induction of phenolics and plant growth promotion in rice (Oryza sativa L.). Curr Microbiol. 52, 383–389 (2006).
Sarma, B. K., Singh, D. P., Mehta, S., Singh, H. B. & Singh, U. P. Plant growth‐promoting rhizobacteria‐elicited alterations in phenolic profile of chickpea (Cicer arietinum) infected by Sclerotium rolfsii . J Phytopathol. 150, 277–282 (2002).
Takenaka, S., Nishio, Z. & Nakamura, Y. Induction of defense reactions in sugar beet and wheat by treatment with cell wall protein fractions from the mycoparasite Pythium oligandrum . Phytopathology 93, 1228–1232 (2003).
Mabrouk, Y. et al. Induction of phenolic compounds in pea (Pisum sativum L.) inoculated by Rhizobium leguminosarum and infected with Orobanche crenata . J. Phytopathol. 155, 728–734 (2007).
Gupta, R., Singh, A., Srivastava, M., Gupta, M. M. & Pandey, R. Augmentation of systemic resistance and secondary metabolites by chitinolytic microbes in Withania somnifera against Meloidogyne incognita . Biocontrol Sci Technol. 26, 1626–1642 (2016).
Herman, M., Hussey, R. S. & Boerma, H. R. Penetration and development of Meloidogyne incognita on roots of resistant soybean genotypes. J. Nematol. 23, 155–161 (1991).
Vos, C. et al. Mycorrhiza-induced resistance against the root–knot nematode Meloidogyne incognita involves priming of defense gene responses in tomato. Soil Biol. Biochem. 60, 45–54 (2016).
Sasser, J. N., Carter, C. C. & Hartman, K. M. Standardization of host suitability studies and reporting of resistance to root-knot nematodes (No. 11819). Department of Plant Pathology, Raleigh, North Carolina State University (1984).
Oka, Y., Shapira, N. & Fine, P. Control of root-knot nematodes in organic farming systems by organic amendments and soil solarization. Crop Prot. 26, 1556–1565 (2007).
Metcalfe, A. C., Krsek, M., Gooday, G. W., Prosser, J. I. & Wellington, E. M. H. Molecular analysis of a bacterial chitinolytic community in an upland pasture. Appl. Environ. Microbiol. 68, 5042–5050 (2002).
Jackson, N. E., Miller, R. H. & Franklin, R. E. The influence of vesicular-arbuscular mycorrhizae on uptake of 90Sr from soil by soybeans. Soil Biol. Biochem. 5, 205–212 (1973).
Gupta, A. P., Mathur, S., Gupta, M. M. & Kumar, S. Effect of the method of drying on the bacoside A content of the harvested Bacopa monnieri shoots using a high performance thin layer chromatography method. J. Med. Arom. Plant Sci. 20, 1052–1055 (1998).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001).
Singh, U. P., Sarma, B. K., Singh, D. P. & Bahadur, A. Plant growth-promoting rhizobacteria-mediated induction of phenolics in pea (Pisum sativum) after infection with Erysiphe pisi . Curr. Microbiol. 44, 396–400 (2002).
Ferrareze, J. P. et al. Jasmonic acid does not increase oxidative defense mechanisms or common defense-related enzymes in postharvest sugarbeet roots. Postharvest Biol. Technol. 77, 11–18 (2013).
Arnon, D. I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris . Plant Physiol. 24, 1 (1949).
Lichtenthaler, H. K., Ač, A., Marek, M. V., Kalina, J. & Urban, O. Differences in pigment composition, photosynthetic rates and chlorophyll fluorescence images of sun and shade leaves of four tree species. Plant Physiol. Biochem. 45, 577–588 (2007).
Ainsworth, E. A. & Gillespie, K. M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2, 875–877 (2007).
Zhishen, J., Mengcheng, T. & Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64, 555–559 (1999).
Chen, L. M., Lin, C. C. & Kao, C. H. Copper toxicity in rice seedlings: changes in antioxidative enzyme activities, H2O2 level, and cell wall peroxidase activity in roots. Bot. Bull. Acad. Sin. 41, 99–103 (2000).
Gauillard, F., Richardforget, F. & Nicolas, J. New spectrophotometric assay for polyphenol oxidase activity. Anal. Biochem. 215, 59–65 (1993).
Singh, A., Gupta, R. & Pandey, R. Rice seed priming with picomolar rutin enhances rhizospheric Bacillus subtilis CIM colonization and plant growth. PloS One 11, e0146013 (2016).
Acknowledgements
The authors are thankful to the Director, CSIR-CIMAP, for providing necessary facilities and Academy of Scientific and Innovation Research (AcSIR-CIMAP), India. RG acknowledges Mr. Kundan Wasnik for his help in the soil analysis.
Author information
Authors and Affiliations
Contributions
R.G., A.S. and R.P.: Conceived and designed the experiments, manuscript writing. R.G.: Performed and conceptualized the experiments. M.S.: Performed HPLC of Bacoside A.V.S.: Performed HPLC of phenolics. M.M.G.: HPLC data analysis. R.P.: Critical revision of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Gupta, R., Singh, A., Srivastava, M. et al. Microbial modulation of bacoside A biosynthetic pathway and systemic defense mechanism in Bacopa monnieri under Meloidogyne incognita stress. Sci Rep 7, 41867 (2017). https://doi.org/10.1038/srep41867
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep41867
This article is cited by
-
Development of amine-functionalized fluorescent silica nanoparticles from coal fly ash as a sustainable source for nanofertilizer
Scientific Reports (2024)
-
Integrative homology modeling and structural analyses of Cicer arietinum phenylalanine ammonia lyase (ca PAL) with co-expressed protein trans cinnamate 4 monooxygenase (C4H1) give clues to their interaction during stress response in chickpeas
Vegetos (2024)
-
Histological characterization of wild cucumber resistance to Meloidogyne species
Journal of Plant Diseases and Protection (2023)
-
Biotechnological production of bacosides from cell and organ cultures of Bacopa monnieri
Applied Microbiology and Biotechnology (2022)
-
Endophyte mediated activation of defense enzymes in banana plants pre-immunized with covert endophytes
Indian Phytopathology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.