Abstract
Mucins in the gastric mucus layer carry a range of glycan structures, which vary between individuals, can have antimicrobial effect or act as ligands for Helicobacter pylori. Mucins from various individuals and disease states modulate H. pylori proliferation and adhesin gene expression differently. Here we investigate the relationship between adhesin mediated binding, aggregation, proliferation and adhesin gene expression using human gastric mucins and synthetic adhesin ligand conjugates. By combining measurements of optical density, bacterial metabolic activity and live/dead stains, we could distinguish bacterial aggregation from viability changes, enabling elucidation of mechanisms behind the anti-prolific effects that mucins can have. Binding of H. pylori to Leb-glycoconjugates inhibited the proliferation of the bacteria in a BabA dependent manner, similarly to the effect of mucins carrying Leb. Furthermore, deletion of arsS lead to a decrease in binding to Leb-glycoconjugates and Leb-decorated mucins, accompanied by decreased aggregation and absence of anti-prolific effect of mucins and Leb-glycoconjugates. Inhibition of proliferation caused by adhesin dependent binding to mucins, and the subsequent aggregation suggests a new role of mucins in the host defense against H. pylori. This aggregating trait of mucins may be useful to incorporate into the design of adhesin inhibitors and other disease intervention molecules.
Similar content being viewed by others
Introduction
The mucus layer that covers mucosal surfaces is the first barrier that gastrointestinal bacteria encounter. The mucus layer in the stomach consists mainly of MUC5AC secreted from the surface mucosa and MUC6 secreted from the gland mucosa1. In half of the human population, the surface mucosa of the stomach is colonized by the pathogen Helicobacter pylori, which can cause gastritis, gastric and duodenal ulcers, adenocarcinoma and MALT lymphoma2,3. H. pylori adheres to highly glycosylated mucins via the blood group antigen binding adhesin (BabA) that binds to Lewis b (Leb) and related fucosylated structures, and the sialic acid binding adhesin (SabA) that binds mainly to sialyl-Lewis x (SLex) and sialyl-Lewis a (SLea)4,5,6. Adhesion of H. pylori to mucins and host cells is highly relevant for colonization, mucin-mediated defense, host cell response and development of disease7,8,9,10. The adhesion targets and the glycan environment that H. pylori is exposed to vary between individuals and change as the expression, glycosylation and spatial distribution of mucins change in response to H. pylori infection and disease development11,12,13,14,15,16,17. For example, in the rhesus monkey (Macaca mulatta) infection model there are time-dependent changes in Leb expression and in the same monkeys, as well as in mice, gerbils and humans, there is an induced expression of sialylated Lewis antigens upon infection with H. pylori16,18,19,20. However, the effect of a variably glycosylated environment on H. pylori is poorly understood.
We recently reported that the proliferation of H. pylori can be modulated differently by mucins from different individuals and disease states21. Mucins from some of the patients stimulated the proliferation of H. pylori, while mucins from other patients tended to have a growth repressing effect. The latter could not be explained solely by the presence of terminal α1,4-linked N-acetylglucosamine (α1,4-GlcNAc), which is a carbohydrate structure with antimicrobial activity present on mucins in the gastric glands22, as this structure was not detected in all mucin samples that repressed H. pylori proliferation. The binding ability of H. pylori to mucins is also of importance for proliferation, as H. pylori strains with different mucin binding abilities vary in their proliferative response to mucins21,23. Furthermore, interaction with mucins can modify the H. pylori expression of adhesin genes21. Taken together, this demonstrates that mucins can regulate the behavior of H. pylori beyond acting merely as a physical barrier and attachment site.
In the present study, we further investigated the relationship between binding and response to mucins. We used H. pylori strains with different binding abilities, including deletion mutants of the babA and sabA adhesin genes, and studied their binding in relation to proliferation and gene expression in response to mucins and glycoconjugates with the ligands Leb and SLex. In addition, we included an isogenic mutant lacking the histidine kinase sensor protein ArsS, as an arsS deletion mutant has been shown to have an increased expression of sabA24,25, and this mutant thus represents a model with potentially increased SabA dependent binding. Combining measurements of optical density, bacterial metabolic activity and live/dead stains enabled elucidation of mechanisms behind the anti-prolific effects of mucins, and we can conclude that H. pylori ligand binding via BabA causes a decrease in proliferation, which appears related to the extent of aggregation caused by binding.
Results
Accurate interpretation of H. pylori proliferation and viability requires careful method selection
Optical density is a widely used measure of bacterial growth, and has been used both by us and other research groups previously with the interpretation that the OD value of bacteria cultured with mucins is directly related to the cell count21,22. Although we have verified by CFU counts that for a subset of mucin-bacterial samples this appears accurate, we have now found that several cultures of bacteria with strong binding to the added mucins resulted in high OD560 values (reading performed after vigorous manual shaking), despite low CFU counts (Fig. 1A, B and E). Therefore, we investigated the relationship of these parameters to develop a method for accurate assessment of proliferation and viability. We found that adhesin dependent bacterial binding to mucins isolated from a gastric tumor (positive for both Leb and SLex) and from surface mucosa of normal gastric tissue (normal mucin 3, positive for Leb) results in the formation of aggregates (Skoog et al.21 and Fig. 1D) along with high OD560 readings (Fig. 1A and C). Dispersion of the aggregates by thorough pipette mixing reversed the increased OD in the H. pylori culture with the tumor mucin, thereby demonstrating that the aggregates were the cause of the increased OD (Fig. 1C). In contrast, when H. pylori J99 were cultured with a mucin rich in the anti-microbial glycan structure α1,4-GlcNAc, the OD560 correlated with the CFU count and indicated fewer bacteria than without mucins, even though cultures were only mixed by shaking before OD560 measurement (Fig. 1C). Microscopy analysis of bacteria cultured with mucins indicated that aggregation is caused by specific adhesin-ligand interaction, as most H. pylori J99 wt were present in large clusters whereas the vast majority of J99ΔbabAΔsabA were unassociated (Fig. 1D). Aggregates may cause inaccurate results if not fully dispersed prior to the plating for CFU, as several bacteria in one spot would appear as one colony. Thus, as a measure of bacterial count, aggregation may cause an enhancing error in the OD560 measurements, but a diminishing error by the CFU counting method. The metabolic activity (which can be used as a measure of cellular viability) can be measured by adding alamarBlue to the cultures26. Both the metabolic activity (alamarBlue signal) and the OD560 readings after vigorous pipetting were lower after culture with the tumor mucin compared to culture without mucins (p < 0.05, Fig. 1C), and the alamarBlue thus seems to accurately measure H. pylori proliferation even though cultures were only mixed by shaking before and after the addition of alamarBlue. Using a mucin that was positive for Leb and devoid of α1,4-GlcNAc (normal mucin 5), the OD560 increased, but was reversed after vigorous pipetting, and both the alamarBlue signal and the CFU counts decreased in cultures with J99 wt, whereas none of these parameters were affected in cultures with J99ΔbabAΔsabA (Fig. 1E). The relationship between the alamarBlue signal and CFU counts is similar to the relationship between OD560 and CFU counts in the absence of mucins (Fig. 1F): within the concentrations the experiments were performed, a % change in alamarBlue approximately equals the same % change in CFU (Y = 0.9399*X + 1.774, Fig. 1G). In summary, both OD and CFU counts, as a measure of bacterial proliferation, can be misleading when molecules that aggregate bacteria are present. Extrapolating metabolic activity into viability and proliferation appears more accurate in this context although one cannot separate proliferation and viability from each other. Microscopic examination of stains determining the number of live vs. dead cells can add additional information, but are time consuming and impractical if large sample numbers are to be analyzed simultaneously. Therefore, we mainly used alamarBlue to assay proliferation of H. pylori throughout the rest of this study, with microscopic examination on a subset of samples to visualize aggregation.
(A) OD560 of liquid cultures of H. pylori J99 cultured for 60 h with purified human gastric mucins from several individuals. H. pylori were still in the growth phase at this time point (i.e. had not entered the stationary phase). (B) CFU count of H. pylori J99 cultured for 60 h with human mucins. (C) Comparison between metabolic activity, measured as reduced alamarBlue, and OD560 of H. pylori J99 cultured for 24 h with or without the normal mucin sample 1 (does not induce formation of aggregates, but carries the α1,4-GlcNAc structure that has antibiotic like properties) and the tumor mucin sample (induce formation of aggregates and does not carry α1,4-GlcNAc). Cultures were shaken or pipette-mixed prior to OD560 measurement. Values are shown as percentage of J99 cultured without mucins (represents 100%), n.a. = not analyzed. (D) LIVE/DEAD BacLight staining of the J99 wt and J99ΔbabAΔsabA deletion mutant after culture with Leb-positive mucins isolated from a normal stomach (normal mucin 3). Live bacteria are colored green and dead bacteria are colored red. (E) Comparison between OD560 measurements, metabolic activity and CFU counts for J99 wt and J99ΔbabAΔsabA deletion mutant after culture with Leb-positive mucin sample 5. Values are shown as percentage of each strain cultured without mucins (represents 100%). (F) The relationship between the alamarBlue signal, CFU counts and OD560 in the absence of mucin. (G) The relationship between the % change in alamarBlue signal at OD560, CFU counts and OD560 in the absence of mucin (100% corresponds to the highest value in (F). All values are presented as mean ± S.E.M, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001: statistical tests were performed with ANOVA with Dunnett’s post hoc test, except the CFU data in panel E, which was analyzed with the Mann-Whitney U test.
Binding of H. pylori to Leb-glycoconjugates inhibits the proliferation of the bacteria, similarly to the effect of mucins carrying Leb
H. pylori binding to mucins that carry Leb and SLex, can inhibit proliferation (Fig. 1B,C and E)21. However, mucins carry a large repertoire of glycans and may also carry structures that can enhance growth21. Furthermore, strains may potentially vary in how they respond to glycan elements due to differences in genetic composition and thereby glycan response machinery. To study proliferation of H. pylori in response to individual glycans, we focused on strains J99 and P12. Both strains carry the babA gene, and J99 also carry the sabA gene. Strain P12 carries two sabB alleles27, which is a result from gene conversion with sabA28. SabB has an unknown function and thus P12 lacks the SabA mediated binding. Binding of both J99 and P12 was more pronounced to Leb than to SLex (p < 0.001, Fig. 2). Furthermore, both J99 and P12 bound better to the mucin derived from a healthy stomach that carried Leb (but not SLex) than to the tumor derived mucin that carried both Leb and SLex (p < 0.001, Fig. 2A). To study proliferation in response to individual glycans, we cultured strains J99 and P12 with Leb and SLex conjugated to human serum albumin. Since adhesion to the SLex-conjugate was either low or absent, the SLex-glycoconjugate represents a glycan attached to the same carrier as the Leb-glycoconjugate but that does not cause major aggregation. Measurements of the metabolic activity of H. pylori by alamarBlue reduction demonstrated that Leb-conjugates decreased the metabolic activity of J99 and P12 (p < 0.05, Fig. 2B). Thus, binding of H. pylori to Leb-glycoconjugates inhibits the proliferation of the bacteria, similarly to the effect of mucins carrying these glycans. In contrast, the metabolic activity of P12 was enhanced in the presence of SLex, which might reflect growth stimulation in response to glycans via an unknown mechanism that is independent of SabA and adhesion, as a similar growth enhancement was obtained in response to mucins in the absence of binding with this strain (below).
(A) Binding of J99 and P12 to Leb- and SLex-glycoconjugates and to the tumor and normal mucin sample analyzed using the microtiter plate based assay (n = 4). (B) Analysis of the metabolic activity as the percentage of reduced alamarBlue in relation to parallel cultures without glycoconjugates (represents 100%) demonstrated a loss of viability in the cultures with Leb-glycoconjugates (n = 6). Values are mean ± S.E.M, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. ANOVA with Bonferroni’s post hoc test or Student t-test, aap ≤ 0.01, aaap ≤ 0.001 ANOVA with Bonferroni’s post hoc test, compared to proliferation in the absence of glycoconjugates. The results in the graphs have been reproduced at least twice with the same outcome.
The proliferation of the babA deletion mutants is not inhibited by Leb-positive mucins and glycoconjugates
To verify that H. pylori proliferation is inhibited by the glycan binding event specifically, we used adhesin mutants. These were cultured with glycoconjugates as well as with mucins to provide a more complex, in vivo like, glycan environment. In line with the genetic modification, the J99 and P12 babA deletion mutants (ΔbabA) bound less to Leb-glycoconjugates and less to mucins isolated from a healthy stomach than the isogenic wt strains (Fig. 3). P12 binding to SLex was very low as expected due to the lack of sabA, and no difference in binding to SLex was detected with a P12 mutant where the sabB allele in the sabA locus had been deleted (Fig. 3B and D). Binding of J99 wt to SLex was also very low, but although no decrease in binding to SLex was detected with the J99 sabA deletion mutant (ΔsabA) using the microtiter based assay, a decrease was detected when binding was measured in solution in the presence of isotopes (RIA) (Fig. 3A and C). Thus, both strains mainly interact with mucins and glycoconjugates via BabA, but we still kept the J99ΔsabA in the below assays as an additional control to verify that the differences between wt and ΔbabA strains were not due to procedures associated with the genetic manipulations or clone selection.
Binding of strain J99 (A) and P12 (B) to Leb- and SLex-glycoconjugates, the tumor derived and normal mucin coated onto microtiter plates (n = 4). The results in the graphs have been reproduced at least twice with the same outcome. Values are mean ± S.E.M. Stars indicate statistical relationship of mutant binding compared to the binding of the isogenic wt to the same ligand, One-way ANOVA with Bonferroni’s post hoc test, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, whereas the letter a indicates that the binding (depicted after subtraction of the background signal) is statistically different (p < 0.05) from the background signal. Binding of strain J99 (C) and P12 (D) to Leb- and SLex-glycoconjugates in solution using RadioImmuno assay (n = 9–10). Values are median ± interquartile range. Stars indicate statistical relationship of mutant binding compared to the binding of the isogenic wt to the same ligand, Kruskal-Walllis One-way ANOVA on ranks with Dunn’s multiple comparisons test, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
J99ΔsabA as well as J99 wt formed aggregates in the presence of both the Leb-glycoconjugate (Fig. 4) and the tumor mucin sample (tumor mucin referred to as P1 TS in Skoog et al.21) resulting in an increase in OD (Figs 1C and 5B). When the bacteria were mixed by pipetting to break aggregates, the ODs at the end point of these cultures were instead decreased (Figs 1C and 5B), which corresponded to a decreased metabolic activity as measured by alamarBlue reduction (p < 0.001, Fig. 5C and E). The loss of proliferation in response to the Leb-glycoconjugate and the tumor mucin sample was reversed by deletion of babA, which also resulted in a decrease in formation of aggregates (Figs 4 and 5A,C,E: an image of the aggregates formed after culture of J99 with the tumor mucin has been published ref. 21). These results are in line with the OD and metabolic activity of J99 wt and J99ΔbabAΔsabA after culture with the normal mucin 5 in Fig. 1E. Similarly, the decreased metabolic activity of strain P12 wt in the presence of Leb, was reversed by deletion of babA (Fig. 5D). The tumor mucin did not have negative effects on the growth on strain P12, however, deletion of babA lead to enhanced growth in the presence of the mucin and Leb (Fig. 5F), further indicating that this strain has a positive growth response to mucin glycans, which is suppressed by the BabA dependent binding to the mucin. Thus, this mucin has no negative effect on proliferation other than that caused by BabA dependent binding to Leb (and similar structures) present on the mucin.
(A,B) OD560 of J99ΔbabA and J99ΔsabA during culture in the presence Leb- and SLex-glycoconjugates. Endpoints of cultures were mixed by pipetting to break aggregates. (C,D) Viability of H. pylori after 24 h culture in the presence of the glycoconjugates as measured by alamarBlue reduction. (E,F) Viability of bacteria after 24 h culture in the presence of the tumor mucin sample as measured by alamarBlue reduction. All values are mean ± S.E.M, **p ≤ 0.01, ***p ≤ 0.001, Student’s t-test, n = 6. The results in the graphs have been reproduced at least twice with the same outcome.
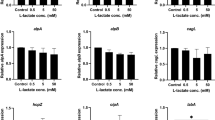
Binding to Leb affect adhesin gene expression
We have previously shown that H. pylori adhesin expression can be differentially affected in culture with different mucins21. Here we investigated the relationship between binding to mucins and expression of the BabA and SabA adhesins in H. pylori J99 after co-culture with mucins, including mucins isolated from 10 individuals, and found that the Pearson product-moment correlation coefficient tended to be negative (p = 0.068, r = −0.597 and p = 0.085, r = −0.571, Fig. 6A). All samples contained MUC5AC and some also MUC6, and all but one sample were positive for Leb, while only two samples were positive for SLex 21. The very complex environment provided by the mucins carrying in the order of 100 different carbohydrate structures has potential to affect binding and stimulate the bacteria in multiple ways. Thus, even in this very complex surrounding, the overall effect of binding appears to be downregulating the adhesins. Therefore, we examined if the expression of the adhesins was affected by binding to its ligand after 24 h of culture with the SLex- and Leb-glycoconjugates in strain J99. babA mRNA levels decreased after culture in the presence of Leb (p < 0.05), but not in the presence of SLex (Fig. 6B), and expression of sabA trended towards being decreased after culture with Leb and SLex (p = 0.09, Fig. 6B).
(A) Correlation between mucin binding of J99 wt (as analyzed by microtiter based assay) to 10 different (individual) human mucin samples and gene expression of babA (p = 0.068, r = −0.597) and sabA (p = 0.085, r = −0.571, Pearson correlation) in J99 wt after 24 h culture with these mucins. 9 of the 10 mucins were positive for Leb 21. (B) Expression of babA and sabA in H. pylori J99 after 24 h culture with Leb- and SLex-glycoconjugates. Stars indicate change of expression compared to untreated bacteria confirmed by paired sample t-test, where Ct values of each experiment were paired to remove confounding factors of variable base levels between experiments. Fold expression compared to bacteria cultured without glycoconjugates or mucins are shown where expression is calculated as ΔCt in relation to expression of 16S rRNA as a housekeeping gene. Values are mean ± S.E.M. from 2 biological replicates, where each data point consists of the mean of 2 technical replicates with very similar result, *p ≤ 0.05.
Absence of ArsS reduces BabA dependent decreases in viability and aggregation
The level of SabA expression has previously been shown to be increased in a H. pylori strain with deleted arsS compared to its isogenic wt strain24. Consistent with these results, J99ΔarsS binding to SLex slightly increased compared to that of J99 wt (p < 0.05, Fig. 7A). Unexpectedly, binding to Leb was decreased by 80% in J99ΔarsS (p < 0.0001, Fig. 7A), and by 30% in strain P12ΔarsS (p < 0.01, Fig. 7B), as determined using the microtiter based assays. In line with these results, binding to the mucin derived from the gastric tumor (carrying both Leb and SLex) increased in J99ΔarsS compared to J99 wt strain (p < 0.05), and binding to mucins derived from a healthy stomach (carrying Leb) trended towards a decrease in the P12ΔarsS strain. That the differences are less distinct with the mucins may be explained by the presence of a multitude of other structures that might contribute to binding. The decrease in binding to Leb was accompanied by a slight decrease in babA gene expression in J99ΔarsS compared to wt (p < 0.05, Fig. 8A), but BabA protein expression was slightly increased in J99ΔarsS and tended to be decreased by a similar degree in P12ΔarsS (Fig. 8B and C, p < 0.05 and p < 0.08). In comparison, the level of SabA protein increased 6-fold in J99 ΔarsS compared to wt (Fig. 8D and E, p < 0.01). Together with that we found a decreased binding to Leb conjugates in solution with the P12ΔarsS compared to wt (i.e. consistent with the microtiter based assay) but not for J99ΔarsS (data not shown) and that ArsS has been shown to be involved in protein trafficking29, the results indicate that it is the topographical localization or presentation of the adhesin that determines the level of BabA-dependent binding on microtiter plates and aggregation in solution (Fig. 5), and not the amount of adhesin present.
(A,B) Binding of bacteria to Leb, SLex, tumor mucin and normal mucin, using the microtiter based assay (n = 4). (C,D) Viability of bacteria after 24 h culture in the presence of Leb, SLex, tumor mucin or normal mucin samples expressed as % change in alamarBlue reduction (n = 6). Results are shown as percent of reduced alamarBlue compared to each bacteria cultured without glycoconjugates/mucins, ap ≤ 0.05, aap ≤ 0.01, aaap ≤ 0.001 ANOVA with Dunnett’s post hoc test, *p ≤ 0.05 **p ≤ 0.01, ***p ≤ 0.001 Student’s t-test. All values are mean ± S.E.M. The results in the graphs have been reproduced at least twice with the same outcome.
(A) mRNA expression of babA in J99 wt and J99ΔarsS. Expression of babA after 24 h of culture in liquid medium, measured as ΔCt in relation to 16 S rRNA as a housekeeping gene. Stars indicate difference in expression compared by paired sample t-test, *p ≤ 0.05, where Ct values of each experiment were paired to remove confounding factors of variable base levels between experiments (n = 3). (B) Immunoblot detecting BabA in bacterial lysates after growth on plate. AlpB was used as a loading control. (C) Quantification of the BabA immunoblot, normalized to AlpB (n = 5–7). (D) Immunoblot detecting SabA in J99 lysates. AlpB was used as a loading control. (E) Quantification of the SabA immunoblot, normalized to AlpB (n = 5–8). Data are presented as median with interquartile range, and analyzed with the Mann-Whitney U-test (*p ≤ 0.05, **p ≤ 0.01).
When cultured with Leb or the tumor or normal mucin sample, the metabolic activity of J99 wt decreased (p < 0.05) whereas that of J99ΔarsS was not affected (Fig. 7C). This could be explained by the lesser formation of aggregates (Fig. 4), causing less hindrance to proliferation. In contrast, neither J99 wt nor J99ΔarsS proliferation was affected by SLex. Similarly, the metabolic activity of P12 wt, but not of P12ΔarsS, decreased when cultured with Leb, and proliferation of P12ΔarsS was higher than that of the wt when cultured with mucins too (Fig. 7D). In line with that glycans can act stimulatory on this strain in the absence of babA dependent inhibition (Fig. 5F), the mucins enhanced growth of P12ΔarsS. The amplitude of the effects of deleting arsS on binding and proliferation differed between the strains, but decreasing BabA mediated aggregation via arsS deletion provides a second line of evidence for that adhesion dependent aggregation leads to decreased proliferation.
Discussion
Here, we demonstrated that BabA dependent binding of H. pylori to Leb-glycoconjugates inhibits proliferation of the bacteria due to formation of aggregates, similarly to the effect of human gastric mucins carrying Leb, and that these events are regulated by ArsS. Furthermore, binding to Leb decreases babA gene expression, indicating a negative feedback loop of the process. Thus, the results show that mucin-pathogen binding can have effects beyond merely inhibiting bacterial adhesion to the epithelial cell surface. The inhibition of proliferation caused by mucin binding and the subsequent aggregation suggests a new role of mucins in the host defense against H. pylori.
H. pylori have been observed by others to grow in aggregates within the human gastric mucus layer30. Growth limiting aggregation caused by mucin binding provides an additional manner for the host to control pathogen numbers alongside with that binding facilitate washing away the pathogen with mucus shedding. Formation of H. pylori aggregates in liquid cultures with mucins has also been reported by others31, who discuss that bacteria in aggregates may be protected from the outside environment. Similarly, Pseudomonas aeruginosa, has been shown to grow in aggregates within mucin gels, where aggregated bacteria are more resistant to antibiotics than non-aggregated bacteria of the same strain32. Thus, aggregation may also be beneficial for the bacteria protecting it from antibiotics, gastric acid and other harmful factors in their surroundings. Antibiotic resistance of H. pylori is a pressing issue33, and bacteria in aggregates may be exposed to subtherapeutic concentration of antibiotics, preventing fast eradication and enabling time to develop resistance. Disrupting aggregates formed in the gastric mucosa during eradication therapy might lead to a higher sensitivity to antibiotics and slower development of resistance.
As the majority of the bacteria were alive after culture with aggregate-causing mucins and glycoconjugates, as demonstrated by live/dead staining, there appears to be little or no direct antimicrobial activity caused by binding. The explanation for this might be that the binding causes formation of aggregates that slow down the proliferation due to physical hindrance or inter-bacterial communication. However, mucins are highly complex molecules, carrying in the order of 100 different glycan structures with potential to cause a multitude of effects. e.g. the ratio of live/dead bacteria was reduced in cultures with some mucins samples independent of aggregation, accompanied by a lower CFU count and reduced OD. These mucin samples are thus able to inhibit the proliferation via antimicrobial mechanisms, in analogy with the previously described “natural antibiotic” effect of terminal α1,4-linked N-acetylglucosamine22, which is present on some mucins. In addition, some mucin samples used in our previous studies increased the OD in the absence of noteworthy aggregation formation21. These samples are likely able to stimulate proliferation as suggested, similarly to the effect on strain P12, that we here show grows faster in the presence of glycoconjugates and mucins, when the growth inhibiting BabA dependent binding to Leb is absent. The enhancement in growth could possibly be explained by the use of mucin glycans as a nutrient source or by activation of growth stimulating signaling pathways, although no such abilities have yet been described for H. pylori. H. pylori can thus be differently affected by mucins with different glycosylation. The majority of the patient mucins that we have investigated cause effects on growth ranging from −40 to +40%. Although these may appear to be marginal changes compared to for example antibiotics, H. pylori is a slow growing bacteria, present in relatively low numbers in most stomachs. Since infection often is present for 15–20 years before symptoms arise, these effects are likely to have major impact over time, in analogy to compound interest. Stimulation and inhibition of H. pylori proliferation by mucins from different individuals might be contributing factors to the outcome of the infection.
Another novel finding was that deleting arsS lead to a decreased aggregate formation. Growth of the arsS mutants were not inhibited in the presence of Leb-positive mucin and glycoconjugate compared to the corresponding isogenic wt strains, further supporting that it is the aggregation per se that causes the inhibition. There was only a 15% decrease in babA expression in J99ΔarsS compared to wt (p < 0.05), which does not seem proportional to the loss in aggregation, and similarly there were only marginal effects on BabA protein levels. Alternative mechanism for the decrease in binding and aggregation remains to be investigated, but our opinion is that altered surface presentation or accessibility due to changes in outer membrane protein composition (such as the increased expression of SabA) are the likely causes for the decreased aggregation. arsS deletion may also interfere with downstream signaling in response to aggregation.
As mucins can affect the proliferation of H. pylori, a rational hypothesis is that mucins also can affect other aspects of the bacteria, such as the expression of genes relevant for colonization and virulence. Indeed, it has previously been shown that the attachment to mucins and host cells can regulate H. pylori virulence genes21,34,35. Furthermore, the expression of two virulence factors (cagA and ureA), that were increased in the J99 wt strain after culture with mucins, was not affected in the isogenic mutant lacking the babA and sabA adhesins21. In this study we have investigated how the binding to the Leb- and SLex-glycans affects the gene expression. Gene expression varies in different growth stages of the bacteria36, and thus a difference in growth rate between replicates and experiments may give varying results. When studying the response to mucins, and in particularly gene expression, the viability measurement by alamarBlue is useful when comparing different strains of bacteria to ensure that a difference between them is a cause of genetic strain differences and not a cause of occasional differences in growth. Here we showed that the correlation between binding to human gastric mucins with varying glycosylation and babA expression tended to be negative, and in the presence of Leb, the expression of the babA adhesin is reduced. In addition, our previous study showed a change in babA expression in response to mucins, with a lack of upregulation of babA only with mucins to which H. pylori bound via BabA21. Neither here nor in the previous study was there any significant change in babA expression level in response to the tumor mucin sample (referred to as P1 TS in ref. 21), to which there is only a low BabA-mediated binding, indicating that the degree of binding may be of importance for the regulation. Although a subset of human mucins may carry other glycan elements that stimulate BabA expression21, our results combined imply that BabA mediated binding represses H. pylori babA expression, inhibiting an increase in BabA in response to mucins where otherwise there may have been such increase, and decreasing it in the absence of such elements. The repression of babA expression in response to Leb binding may thus act as a negative feedback loop. An excessive binding to mucins would allow the bacteria to be washed away along with shedding mucus, decreasing the amount of adhesin expressed would be one way to enable long term colonization.
Although presence of babA in the infecting strain has been associated with a more severe clinical outcome, presence of its ligand Leb in the host has been associated with lower density of H. pylori in the stomach of infected individuals and lower level of gastritis, both in human children and in the rhesus monkey infection model16,37. These seemingly contradictory outcomes may be explained by a model where on the one hand binding to Leb on mucins decrease H. pylori growth and bound bacteria also are removed and disseminated with the shedding of the mucus, whereas on the other hand, BabA can provide intimate adherence to glycolipids on the epithelial cell surface. Indeed, it has been reported that low producers of BabA are associated with more severe clinical outcomes compared to high producers or BabA-negative strains38, indicating a fine tuned balance between these aspects of virulence and host defense.
In conclusion, mucin glycosylation can directly affect H. pylori binding repertoire and H. pylori adhesin expression. Host ligand presentation on mucins appear to undergo a constant host pathogen adaptation and response process; when H. pylori encounters the mucins that build up the mucus layer, the pathogen binding repertoire changes in response to the mucin glycans. The host responds to infection by changing its mucins and mucin glycans in a time dependent manner15,16,39,40, which in turn provoke further adaptations of the pathogen. Furthermore, mucins have the potential to influence H. pylori pathogenicity by affecting its growth and expression of virulence factors. The effect of mucins is mediated by its glycan composition, which may inhibit H. pylori proliferation by adhesion and aggregation of bacteria, as well as by antibiotic effects. Since glycosylation of mucins differs between individuals and varies with disease status, these effects may influence the outcome of the infection.
Methods
Ethics statement
One tumor sample was from our well characterized mucin library, and that sample was collected in 1983 at the IMIM-Hospital del Mar, Barcelona, Spain, before the hospital had an ethics committee. The other samples were obtained after written informed consent and approval of the local ethics committee (Lund University Hospital, Lund, Sweden). The methods applied to these samples were performed in accordance with the committee’s regulations.
Isolation of mucins
The main part of the study was performed on mucins isolated from two gastric specimens; one specimen was from a gastric adenocarcinoma tumor (intestinal type, hereafter referred to as tumor sample) and the other one from macroscopically normal antral mucosa of a tumor-affected stomach (hereafter referred to as normal sample), as evaluated by a clinical pathologist. In addition, a series of additional differentially glycosylated mucins were used for the results shown in Figs 1 and 6. Mucins were isolated using isopycnic gradient centrifugation and characterized for mucin and glycan content as previously described21,23,41. In this study we only used mucins present in the supernatant, where most of the MUC5AC and MUC6 molecules usually are found. The mucin content and carbohydrate structures present in the samples are summarized in Table 1.
Gradient fractions containing mucins were pooled together to obtain one sample for each specimen. All samples were extensively dialyzed in phosphate buffered saline (PBS) to remove guanidinium hydrochloride and cesium chloride (CsCl). Mucin concentration in pooled samples was determined by detection of carbohydrate as periodate-oxidisable structures in a microtiter-based assay: Flexible 96-well plates (BD Biosciences, Franklin Lakes, NJ, USA) were coated with mucin sample and left overnight at 4 °C. After washing three times with washing solution (5 mM Tris-HCl, 0.15 M NaCl, 0.005% Tween 20, 0.02% NaN3, pH 7.75), the carbohydrates were oxidized by treatment with 25 mM sodium metaperiodate in 0.1 M sodium acetate buffer, pH 5.5 for 20 min in room temperature. The plates were washed again and the wells were blocked with DELFIA blocking solution (50 mM Tris-HCl, 0.15 M NaCl, 90 μM CaCl2, 4 μM EDTA, 0.02% NaN3, 6% sorbitol, 0.1% BSA, pH 7.75) for 1 h. After further washing steps, the samples were incubated for 1 h with 2.5 μM biotin hydrazide in 0.1 M sodium acetate buffer, pH 5.5, followed by washing again. Europium-labeled streptavidin was diluted 1:400 in assay buffer (50 mM Tris-HCl, 0.15 M NaCl, 20 μM DTPA, 0.01% Tween 20, 0.02% NaN3, 1.5% BSA, pH 7.75) and was added to the wells. After 1 h incubation, the plates were washed six times and then incubated with enhancement solution (0.05 M NaOH, 0.1 M ftalat, 0.1% Triton X-100, 50 μM TOPO, 15 μM β-NTA) for 5 min on a shaker. The plates were measured using Wallac 1420 VICTOR2 plate reader with the Europium label protocol (PerkinElmer, Waltham, MA, USA). The concentrations were calculated from a standard curve of a fusion protein of MUC1, 16TR and IgG2a Fc starting at a concentration of 20 μg/mL and using seven 1:2 serial dilutions. This method of concentration determination was chosen as all mucins do not come into solution after freeze drying, and determining concentration by freeze drying therefore can contain large errors as well as remove mucin species selectively. Since this study focuses on the effects of carbohydrates, setting the concentration based on the carbohydrate content appear appropriate.
Bacterial strains and culture conditions
H. pylori strains J99 and P12 were cultured on Brucella agar (Brucella Medium Base, Oxoid, Basingstoke, Hampshire, England) supplemented with 10% citrated bovine blood (Svenska Labfab, Ljusne, Sweden), 1% IsoVitox (Oxoid), 4 mg/L amphotericin B, 10 mg/L vancomycin and 5 mg/L trimethoprim in 5% O2 and 15% CO2 at 37 °C. Plates or broth were, when required, supplemented with streptomycin (100 mg/L), chloramphenicol (20 mg/L) or kanamycin (25 mg/L). Strain J99ΔarsS was created by transformation of a ΔarsS::rpsLCAT PCR fragment (P38/P43) generated by amplifying regions flanking the deletion by primers P38 with P40 and P41 with P43, the rpsLCAT cassette with P54 and P55 and adjoining these pieces with primers P38 and P43. Insertion and deletion of the 5′ region of the arsS gene was verified by PCR. The ΔarsS::rpsLcat PCR fragment was cloned in pUC19 and used as PCR template for making the other ΔarsS mutant strains described. H. pylori strains J99 wild type (wt), J99ΔbabA, J99ΔsabA and J99ΔbabAΔsabA were kindly provided by Prof. Thomas Borén, Umeå University, Sweden. The P12ΔsabAB and ΔbabA mutants were constructed by transformation of chromosomal DNA of J99 ΔsabA::Cm or J99 ΔbabA::Cm42. The mutants were verified by PCR (sabA, babA), Immunoblot analysis (α-SabA and α-BabA) and RIA assay (SLex and Leb). Primers used for PCR were the following; SabA-1 and SabA-R for sabA locus, A81 and A19 for babA locus in strain P12. All primer sequences are listed in Table 2.
RadioImmunoAssay (RIA)
Bacterial samples from at least five independent experiments, were collected from plate, washed twice in Sia buffer (1% periodate-treated BSA in PBS containing 0.05% Tween-20). Binding to soluble 125I-SLex- and Leb-receptor conjugates was performed as previously described43. Samples were assayed in duplicates using cocktail conjugate mixtures. The percentage of conjugate bound to bacteria was calculated for every sample.
SDS PAGE and immunoblot analysis
Bacterial samples from at least five independent experiments, were collected from plate, washed twice in Sia buffer. SDS Page analysis was made using NuPage Novex Bis-Tris protein gels (4–12% or 12%) using 1x MOPS SDS as running buffer (Life Technologies). The gels were either stained with PageBlue Protein stain (Thermo Scientific) or transferred to PVDF membrane using Trans-Blot SD Semi-Dry Transfer Cell (Bio Rad). Immunoblot analysis was performed as previously described44. Antibodies against AlpB (AK262)45, SabA (AK278) and BabA (AK277), were used in combination with secondary anti-rabbit IgG-HRP (P0160, DAKO A/S, Denmark). Blots were developed with SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL) and detected on High Performance Chemiluminescence film (GE Healthcare). BabA, SabA and AlpB protein densities were measured by ImageJ software (a public domain image processing program from the National Institutes of Health, Maryland, USA).The adhesin/AlpB density ratio for each sample was used to calculate the fold difference between the wt and the arsS deletion mutant.
Culture of H. pylori with mucins and glycoconjugates
H. pylori were harvested from agar plates (without chloramphenicol or kanamycin) into PBS and centrifuged at 2500 × g for 3 min. Bacteria were then resuspended and cultured in 6 replicates in 60% Brucella broth, 20% fetal bovine serum (FBS), 20% PBS containing mucins, with a final H. pylori starting concentration of OD600 0.2 and 50 μg/mL mucin. A control for normal proliferation was obtained by adding PBS without mucins to the culture medium. A second control was obtained by dialyzing mucin isolation buffer against PBS, parallel to the dialysis of the mucin, and this control gave very similar results to PBS alone. H. pylori were also cultured with 50 μg/mL of glycoconjugates of the carbohydrate structures Leb and sialyl-Lex coupled to human serum albumin (HSA) to create multivalency (IsoSep AB, Tullinge, Sweden). The control for normal proliferation in this assay was obtained by adding HSA (50 μg/mL) in PBS to the culture medium, or PBS alone, which gave very similar results. Bacteria were cultured in a total volume of 100 μL in 96-well plates for 24 or 48 hours at 37 °C under aerobic (20% O2, 5% CO2) or microaerobic (5% O2, 10% CO2) conditions with similar results. The strains used in this study grow well both under microaerobic and aerobic conditions. The comparisons between growth with different mucins or glycoconjugates were always performed on cultures run simultaneously, using the same starting inoculum, as slight variations in growth rate or length of lag phase may occur between experiments.
Proliferation assay
OD at 560 nm was measured at time points throughout the culturing of H. pylori with mucins and glycoconjugates. In addition, alamarBlue (Molecular Probes, Leiden, The Netherlands) was added to the wells to measure the metabolic activity of the bacteria. The plates were incubated for 1 hour at 37 °C with 10 μL of alamarBlue per well and measured at OD560 or at both OD540 and OD600 to calculate the relative amount of reduced alamarBlue according to the manufacturer’s instructions. At the end of the incubation, bacteria from a subset of wells were cultured on Brucella plates and CFU were counted after 5 days of incubation.
Fluorescence microscopy
Bacteria from two replicate wells were pooled after 24 and 37 h proliferation with mucin samples or glycoconjugates and stained with a LIVE/DEAD BacLight bacterial viability kit (Molecular Probes) according to the manufacturer’s instructions. Stained bacteria were applied to a microscopy slide and studied immediately under a fluorescence microscope for red and green fluorescence simultaneously.
RNA extraction, cDNA synthesis and Real-time PCR
After 24 h culture with mucins or glycoconjugates, 100 μL of RNAprotect Bacteria Reagent (Qiagen GmbH, Hilden, Germany) was added to each well and 6 wells per sample were pooled. RNA extraction was continued with Qiagen’s RNeasy kit, including DNase treatment with Qiagen’s RNase-Free DNase Set. cDNA was synthesized from 400 ng RNA using Quantitect Reverse Transcription kit (Qiagen) and real-time PCR was run as previously described21 with primer sequences listed in Table 3. Gene expression was calculated with 2−ΔCt and normalized against the 16S rRNA expression.
Binding assay
H. pylori were grown on agar plates for 24–48 h and harvested in PBS. The bacteria were centrifuged at 2500 × g for 3 min and then resuspended in Blocking Reagent for ELISA (Roche). Mucin samples were diluted in 4 M GuHCl and glycoconjugates of the carbohydrate structures Leb and sialyl-Lex coupled to human serum albumin were diluted in PBS to 4 μg/mL and coated in 4 replicates on 96-well polysorp plates (NUNC A/S, Roskilde, Denmark) overnight at 4 °C. The plates were washed three times with PBS 0.05% Tween and the wells were blocked for 1 hour with Blocking Reagent for ELISA containing 0.05% Tween (blocking buffer). After discarding the blocking buffer, bacteria with an OD600 of 0.1 were diluted 1:10 in blocking buffer and added to the plates, which then were incubated in a bacterial shaker at 37 °C for 2 hours. Then the plates were washed three times, which was repeated between every subsequent incubation step. The plates were incubated for 1 hour at room temperature with rabbit anti-H. pylori serum diluted 1:1000 in blocking buffer, and then another hour with HRP-conjugated donkey anti-rabbit IgG diluted 1:10,000 in blocking buffer. For detection, 100 μL of TMB substrate was added and the plates were incubated for 15 min before the reaction was stopped with an equivalent amount of 0.5 M H2SO4. The absorbance was measured in a microplate reader at 450 nm after color stabilization. Control wells without mucin coating but with all bacterial strains as well as wells with all mucin coatings but omitting the bacteria from the protocol above were included in all experiments. The signal in both types of control wells ranged from 0.06–0.1. Data in the figures are shown after subtracting this background signal.
Statistical analyses
Statistical analyses were performed using Graph Pad Prism 5.0 (GraphPad Software Inc.) software package. One-Way ANOVA followed by Bonferroni’s or Dunnett’s post hoc test and student’s t-test, or Kruskal-Walllis One-way ANOVA on ranks with Dunn’s multiple comparisons test and Mann-Whitney U-test (when appropriate) were used to compare between groups. The Pearson product-moment coefficient was calculated to analyze correlation. Grubb’s test was performed to verify an outlier. The level of statistical significance was set at p ≤ 0.05.
Additional Information
How to cite this article: Skoog, E. C. et al. BabA dependent binding of Helicobacter pylori to human gastric mucins cause aggregation that inhibits proliferation and is regulated via ArsS. Sci. Rep. 7, 40656; doi: 10.1038/srep40656 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Ho, S. B. et al. Expression cloning of gastric mucin complementary DNA and localization of mucin gene expression. Gastroenterology 109, 735–747 (1995).
Warren, J. R. & Marshall, B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1, 1273–1275 (1983).
Ernst, P. B. & Gold, B. D. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol 54, 615–640, doi: 10.1146/annurev.micro.54.1.615 (2000).
Linden, S., Boren, T., Dubois, A. & Carlstedt, I. Rhesus monkey gastric mucins: oligomeric structure, glycoforms and Helicobacter pylori binding. Biochem J 379, 765–775, doi: 10.1042/BJ20031557 (2004).
Lindén, S. et al. Strain- and blood group-dependent binding of Helicobacter pylori to human gastric MUC5AC glycoforms. Gastroenterology 123, 1923–1930. (2002).
Linden, S. K. et al. Listeria monocytogenes internalins bind to the human intestinal mucin MUC2. Arch Microbiol 190, 101–104, doi: 10.1007/s00203-008-0358-6 (2008).
Ishijima, N. et al. BabA-mediated adherence is a potentiator of the Helicobacter pylori Type IV secretion system activity. J Biol Chem, doi: 10.1074/jbc.M111.233601 (2011).
Linden, S. K. et al. MUC1 Limits Helicobacter pylori Infection both by Steric Hindrance and by Acting as a Releasable Decoy. PLoS Pathog 5, e1000617, doi: 10.1371/journal.ppat.1000617 (2009).
Yamaoka, Y. Roles of Helicobacter pylori BabA in gastroduodenal pathogenesis. World J Gastroenterol 14, 4265–4272 (2008).
Sheu, B. S., Sheu, S. M., Yang, H. B., Huang, A. H. & Wu, J. J. Host gastric Lewis expression determines the bacterial density of Helicobacter pylori in babA2 genopositive infection. Gut 52, 927–932 (2003).
Ho, S. B. et al. Mucin gene expression in normal, preneoplastic, and neoplastic human gastric epithelium. Cancer Res 55, 2681–2690 (1995).
Byrd, J. C. et al. Aberrant expression of gland-type gastric mucin in the surface epithelium of Helicobacter pylori-infected patients. Gastroenterology 113, 455–464 (1997).
Tsai, C. J. et al. Changes of gene expression in gastric preneoplasia following Helicobacter pylori eradication therapy. Cancer Epidemiol Biomarkers Prev 15, 272–280 (2006).
Sakamoto, J. et al. Expression of Lewisa, Lewisb, Lewisx, Sialyl-Lewisa, and Sialyl-Lewisx Blood Group Antigens in Human Gastric Carcinoma and in Normal Gastric Tissue. Cancer Research 49, 745–752 (1989).
Cooke, C. L. et al. Modification of gastric mucin oligosaccharide expression in rhesus macaques after infection with Helicobacter pylori. Gastroenterology 137, 1061–1071, 1071, e1061-1068, doi: 10.1053/j.gastro.2009.04.014 (2009).
Linden, S. et al. Role of ABO secretor status in mucosal innate immunity and H. pylori infection. PLoS Pathog 4, e2, doi: 10.1371/journal.ppat.0040002 (2008).
Linden, S. K., Wickstrom, C., Lindell, G., Gilshenan, K. & Carlstedt, I. Four modes of adhesion are used during Helicobacter pylori binding to human mucins in the oral and gastric niches. Helicobacter 13, 81–93, doi: 10.1111/j.1523-5378.2008.00587.x (2008).
Ohno, T. et al. Effects of blood group antigen-binding adhesin expression during Helicobacter pylori infection of Mongolian gerbils. J Infect Dis 203, 726–735, doi: 10.1093/infdis/jiq090 (2011).
Magalhaes, A. et al. Helicobacter pylori chronic infection and mucosal inflammation switches the human gastric glycosylation pathways. Biochim Biophys Acta 1852, 1928–1939, doi: 10.1016/j.bbadis.2015.07.001 (2015).
Gomes, J. et al. Glycophenotypic Alterations Induced by Pteridium aquilinum in Mice Gastric Mucosa: Synergistic Effect with Helicobacter pylori Infection. Plos One 7, e38353, doi: 10.1371/journal.pone.0038353 (2012).
Skoog, E. C. et al. Human Gastric Mucins Differently Regulate Helicobacter pylori Proliferation, Gene Expression and Interactions with Host Cells. PLoS One 7, e36378, doi: 10.1371/journal.pone.0036378 (2012).
Kawakubo, M. et al. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science 305, 1003–1006 (2004).
Skoog, E. C., Lindberg, M. & Linden, S. K. Strain-Dependent Proliferation in Response to Human Gastric Mucin and Adhesion Properties of Helicobacter pylori are not Affected by Co-isolated Lactobacillus sp. Helicobacter 16, 9–19, doi: 10.1111/j.1523-5378.2010.00810.x (2011).
Goodwin, A. C. et al. Expression of the Helicobacter pylori adhesin SabA is controlled via phase variation and the ArsRS signal transduction system. Microbiology 154, 2231–2240, doi: 10.1099/mic.0.2007/016055-0 (2008).
Forsyth, M. H., Cao, P., Garcia, P. P., Hall, J. D. & Cover, T. L. Genome-wide transcriptional profiling in a histidine kinase mutant of Helicobacter pylori identifies members of a regulon. J Bacteriol 184, 4630–4635 (2002).
Ahmed, S. A., Gogal, R. M. Jr. & Walsh, J. E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J Immunol Methods 170, 211–224 (1994).
Fischer, W. et al. Strain-specific genes of Helicobacter pylori: genome evolution driven by a novel type IV secretion system and genomic island transfer. Nucleic Acids Res 38, 6089–6101, doi: 10.1093/nar/gkq378 (2010).
Talarico, S., Whitefield, S. E., Fero, J., Haas, R. & Salama, N. R. Regulation of Helicobacter pylori adherence by gene conversion. Mol Microbiol 84, 1050–1061, doi: 10.1111/j.1365-2958.2012.08073.x (2012).
Marcus, E. A., Sachs, G., Wen, Y., Feng, J. & Scott, D. R. Role of the Helicobacter pylori sensor kinase ArsS in protein trafficking and acid acclimation. J Bacteriol 194, 5545–5551, doi: 10.1128/jb.01263-12 (2012).
Hidaka, E. et al. Helicobacter pylori and two ultrastructurally distinct layers of gastric mucous cell mucins in the surface mucous gel layer. Gut 49, 474–480 (2001).
Jiang, X. & Doyle, M. P. Growth supplements for Helicobacter pylori. J Clin Microbiol 38, 1984–1987 (2000).
Caldara, M. et al. Mucin biopolymers prevent bacterial aggregation by retaining cells in the free-swimming state. Curr Biol 22, 2325–2330, doi: 10.1016/j.cub.2012.10.028 (2012).
De Francesco, V. et al. Worldwide H. pylori antibiotic resistance: a systematic review. Journal of gastrointestinal and liver diseases: JGLD 19, 409–414 (2010).
Kim, N. et al. Genes of Helicobacter pylori regulated by attachment to AGS cells. Infect Immun 72, 2358–2368 (2004).
Raghwan & Chowdhury, R. Host Cell Contact Induces Fur-dependent Expression of Virulence Factors CagA and VacA in Helicobacter pylori. Helicobacter, doi: 10.1111/hel.12087 (2013).
Thompson, L. J. et al. Gene expression profiling of Helicobacter pylori reveals a growth-phase-dependent switch in virulence gene expression. Infect Immun 71, 2643–2655 (2003).
Linden, S., Semino-Mora, C., Liu, H., Rick, J. & Dubois, A. Role of mucin Lewis status in resistance to Helicobacter pylori infection in pediatric patients. Helicobacter 15, 251–258, doi: 10.1111/j.1523-5378.2010.00765.x (2010).
Fujimoto, S. et al. Helicobacter pylori BabA expression, gastric mucosal injury, and clinical outcome. Clin Gastroenterol Hepatol 5, 49–58, doi: 10.1016/j.cgh.2006.09.015 (2007).
Styer, C. M. et al. Expression of the BabA Adhesin During Experimental Infection with Helicobacter pylori. Infect Immun 78, 1593–1600, doi: 10.1128/IAI.01297-09 (2010).
Marcos, N. T. et al. Helicobacter pylori induces beta3GnT5 in human gastric cell lines, modulating expression of the SabA ligand sialyl-Lewis x. J Clin Invest 118, 2325–2336, doi: 10.1172/JCI34324 (2008).
Nordman, H. et al. Gastric MUC5AC and MUC6 are large oligomeric mucins that differ in size, glycosylation and tissue distribution. Biochem J 364, 191–200 (2002).
Mahdavi, J. et al. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 297, 573–578, doi: 10.1126/science.1069076 (2002).
Aspholm, M. et al. SabA is the H. pylori hemagglutinin and is polymorphic in binding to sialylated glycans. PLoS Pathog 2, e110, doi: 10.1371/journal.ppat.0020110 (2006).
Backstrom, A. et al. Metastability of Helicobacter pylori bab adhesin genes and dynamics in Lewis b antigen binding. Proc Natl Acad Sci USA 101, 16923–16928, doi: 10.1073/pnas.0404817101 (2004).
Odenbreit, S., Kavermann, H., Puls, J. & Haas, R. CagA tyrosine phosphorylation and interleukin-8 induction by Helicobacter pylori are independent from alpAB, HopZ and bab group outer membrane proteins. Int J Med Microbiol 292, 257–266, doi: 10.1078/1438-4221-00205 (2002).
Acknowledgements
This work was supported by the Swedish Research council (521-2011-2370; 621-2014-4361) Formas (221-2011-1036 and 221-2013-590), the Åke Wibergs, Royal Society of Arts and Sciences in Gothenburg (KVVS), Lundgrens, Söderbergs, Ruth and Richard Julins foundations, the Swedish Cancer Society and Lion’s Cancer Research Foundation, Umeå University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Prof. Thomas Borén, Umeå University, Sweden, for providing us with H. pylori strains and mutants, as well as Carme de Bolós (IMIM-Hospital del Mar, Barcelona, Spain) for providing us with tumor tissue. We also thank Dr. János Tamás Padra, University of Gothenburg, Sweden for comments on the manuscript.
Author information
Authors and Affiliations
Contributions
E.S., M.P., A.Å., P.G., M.Q.H. and I.O. performed experiments and collected data. A.A., E.S., M.P., M.Q.H., A.Å. and S.L. analyzed data. E.S. and S.L. wrote the main manuscript text and M.P., A.A. and A.Å. wrote certain sections. All Authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Skoog, E., Padra, M., Åberg, A. et al. BabA dependent binding of Helicobacter pylori to human gastric mucins cause aggregation that inhibits proliferation and is regulated via ArsS. Sci Rep 7, 40656 (2017). https://doi.org/10.1038/srep40656
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep40656
This article is cited by
-
Nanohybrid-based immunosensor prepared for Helicobacter pylori BabA antigen detection through immobilized antibody assembly with @ Pdnano/rGO/PEDOT sensing platform
Scientific Reports (2020)
-
Helicobacter suis infection alters glycosylation and decreases the pathogen growth inhibiting effect and binding avidity of gastric mucins
Mucosal Immunology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.