Abstract
Although the association of hyperuricemia and cardiovascular diseases is well established by previous research studies, the relationship between gout and deep vein thrombosis (DVT) remains unclear. We conducted a nationwide longitudinal cohort study to investigate the relationship between gout and DVT. We used the Taiwan National Health Insurance Research Database to identify patients with gout diagnosed in Taiwan during 2000–2011, and we followed up these patients to determine the incidence of DVT among them. The association between gout and DVT was analyzed by cox proportional hazard model. The study cohort included 35,959 patients with history of gout attack and 35,959 matched controls without gout attack. During the median follow-up of 7.5 ± 3.6 years, the incidence rate of DVT was significantly higher in patients with gout than that in control group (13.48 versus 9.77 per 104 person-years, p < 0.001). Kaplan-Meier analysis revealed a tendency toward DVT development in gout patients (log rank test, p < 0.001). In a Cox model, patients with gout were found to have a 1.38-fold (95% confidence interval [CI], 1.18 to 1.62, p < 0.001) higher risk of developing DVT. Hyperuricemia with gout attack could be a possible risk predictor for DVT, but these findings need to be confirmed in future clinical and experimental studies.
Similar content being viewed by others
Introduction
Venous thromboembolism (VTE), with an annual incidence of 1–3 cases per 1000 individuals, is a common cause of cardiovascular morbidity and mortality1. VTE constitutes a spectrum ranging from asymptomatic distal deep venous thrombosis (DVT) and sub-segmental pulmonary embolism, to limb-threatening DVT and fatal pulmonary embolism. DVT and its associated complications are sources of morbidity, including severe functional impairment due to post-thrombotic syndrome, and chronic thromboembolic pulmonary hypertension. However, DVT is often underdiagnosed, and the disease burden is underestimated2. Although DVT and arterial atherothrombotic disease are generally considered to be different diseases3, risk factors for venous and arterial thrombosis have been shown to overlap4. Venous thrombosis has been previously associated with red blood cells (red thrombus), and arterial thrombi are mainly composed of platelets (white thrombus). Recent epidemiological studies have suggested that patients with atherosclerosis or cardiovascular risk factors may be at increased risk of VTE5.
Uric acid is a byproduct of purine catabolism, of which the terminal steps are catalyzed by xanthine oxidase. High uric acid levels are often accompanied by metabolic syndrome, diabetes, hypertension, hyperlipidemia and chronic kidney disease6, which all contribute to the development of cardiovascular disease. Some studies have reported that hyperuricemia is considered to be associated with coronary artery disease (CAD), independently of traditional risk factors7,8,9, but others have argued that this association is confounded by the coexistence of cardiovascular risk factors7,10,11. Moreover, increased serum uric acid in humans is associated with systemic inflammation12, endothelial dysfunction13,14,15, cardiovascular disease (CVD), and cardiovascular mortality16,17. Therefore, high uric acid levels should be a link between endothelial dysfunction, pro-inflammatory, and pro-thrombotic states, and could be a possible independent risk factor for VTE. However, there are few reports on the relationship between gout attack and VTE18. We conducted a nationwide longitudinal cohort study to investigate the relationship between history of gout attack and DVT occurrence.
Results
Patient characteristics
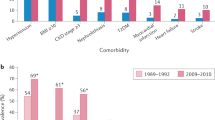
The study cohort included 35,959 patients with history of gout attack and 35,959 matched controls without gout (Table 1). Study subjects were almost equal in gender (73.7% men), and the mean age was 54.7 years (SD = 16.1 years). The prevalence of comorbidities such as cardiovascular risk factors and traditional risks factors for DVT was similar between the gout and control groups. The median follow-up period was 7.5 ± 3.6 years for the gout group and the range was 7.4 ± 3.6 years for the control group.
Gout attack and the risk of DVT
During the mean 7.4 years of the follow-up period, the incidence rate of DVT was 13.48 per 104 person-years for the gout cohort and 9.77 per 104 person-years for the matched control cohort (Table 2). Compared with the matched control cohort, the gout cohort had a significant risk for DVT (HR = 1.38, 95% CI, 1.18–1.62, p < 0.001). The cumulative incidence of DVT for the two groups is shown in Fig. 1. In the sensitivity analysis, after excluding traditional risk factors for DVT and other potential confounding factors, history of gout attack still significantly increased the risk of DVT (Table 3). As shown in Fig. 2, the association between gout and DVT was also consistent in subgroup analysis.
Discussion
The role of uric acid in VTE, and particularly DVT, remains largely unknown. To the best of our knowledge, this is the first large-scale, nationwide, population-based analysis study that aimed to elucidate the relationship between gout and DVT. Our study, which included 35,959 patients with at least one episode of gout attack, demonstrated a significantly increased risk of DVT (HR = 1.38, 95% CI, 1.18–1.62, p < 0.001) after a median 7.5-year follow-up period. This association between gout and DVT was also consistent in subgroup analysis after adjustment for risk factors for atherosclerosis and thrombophilic conditions. These findings provide novel evidence that history of gout attack may be a risk predictor for DVT, and deserves a prospective study to investigate whether reducing uric acid levels could decrease DVT occurrence.
DVT is a potentially dangerous condition that can lead to considerable morbidity and severe functional impairment. Accumulating evidence has indicated that risk factors for VTE may overlap with those for arterial thrombosis. A high proportion of patients with VTE were shown to have endothelial dysfunction and an increased risk of subsequent development of cardiovascular disease19,20. Prandoni et al. reported an association between asymptomatic carotid artery atherosclerosis and DVT in a case-control study21, which suggests a close interaction between atherosclerosis and DVT. Furthermore, analysis of a secondary end-point of the JUPITER (Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin) trial revealed that treatment with a statin (rosuvastatin, 20 mg/day), a lipid-lowering agent, significantly reduced the risk of VTE in apparently healthy people with high levels of C-reactive protein and normal levels of low-density lipoprotein cholesterol22. These results suggest that atherosclerosis can induce venous thrombosis and these two conditions share common risk factors.
High uric acid concentration has been reported to be linked to endothelial dysfunction, increased free radical generation, insulin resistance and high levels of systemic inflammatory markers (such as C-reactive protein)12,14,23. In this nationwide cohort study, we identified the previous history of gout attack as a risk predictor of DVT occurrence, even after adjusting for traditional risk factors for cardiovascular disease and thrombophilic conditions. One particular strength of this study is the nationwide, cohort study design with age- and comorbidity-matched controls, which allows a powerful conclusion to be drawn. These findings provide clinical evidence that history of gout attack could increase DVT risk, with the potential to be a new therapeutic target for DVT prevention. However, further prospective clinical and experimental studies are needed to prove the concept.
The mechanism underlying the link of gout/hyperuricemia and DVT remains unclear. Potential mechanisms linking high uric acid levels to DVT include endothelial dysfunction, enhanced reactive oxidative stress, and pro-inflammatory and pro-thrombotic conditions in endothelial injury or dysfunction which play a critical role in VTE and atherosclerosis. Clinical studies showed that endothelial function as determined by flow-mediated dilation is inversely correlated with serum uric acid levels in subjects with asymptomatic hyperuricemia24,25. Uric acid has also been found to attenuate nitric oxide production in cultured endothelial cells, probably through induction of intracellular oxidative stress and inflammation25. In one retrospective study, the risk of left atrial thrombus increased in patients with non-valvular atrial fibrillation and hyperuricemia26. Additionally, reducing uric acid levels by administration of allopurinol, a xanthine oxidase inhibitor, improves endothelial function in different clinical conditions27.
Low-grade chronic inflammation, as indicated by levels of inflammatory markers such as C-reactive protein, was shown to play a critical role in atherosclerotic disease progression and VTE formation28. Ruggerio et al. reported that there is a positive and significant association between the levels of uric acid and several inflammatory markers in a large population-based sample of older persons and in a sub-sample of participants with normal uric acid concentrations12. Moreover, in experimental studies, uric acid stimulates the release of chemokines and synthesis of inflammatory cytokines29,30. These results strongly suggest high uric acid concentration might contribute to a pro-inflammatory state, which is an important factor for atherosclerosis progression and thrombus formation in the venous system. Our findings provide novel evidence that hyperuricemia could be associated with DVT occurrence, but further studies are warranted to clarify how uric acid causes micro-thrombus formation in the circulatory system.
There were several limitations in the present study. First, the absolute values of uric acid were not available in the nationwide dataset. However, the levels of uric acid fluctuate significantly and are not constant, which make it difficult to determine the levels appropriately. In the present study, patients were regarded as having hyperuricemia only when they had at least one episode of a gout attack requiring long-term treatment with uric acid-lowering agents. Therefore, we focused on symptomatic hyperuricemia, and the results based on this definition may be more clinically relevant. Second, diagnoses of DVT that rely on administrative claims data registered by physicians or hospitals may be less accurate than diagnoses made according to standardized criteria. All of the DVT patients in our study had received image evaluation including CT scan or Doppler sonogram, but we couldn’t obtain image report data from our database. Third, some personal information, including body mass index and smoking status, was not available in the administrative data, preventing accurate assessment of the contributory and confounding effect of these factors. Most notable among these factors is obesity, which has been reported to increase the risk of DVT31. Cigarette smoking has been shown, although inconsistently, to have no effect on DVT development, as reported by Ageno et al. in a meta-analysis32. In addition, the duration of hyperuricemia before study enrolled date was not obtained in our current study. Last but not the least, the propensity score matching was done for factors known at baseline and changes in risk factors over time could be a source of residual confounding.
In spite of study limitations, our findings may have significant clinical and hypothetic implications. The large-scale nationwide population-based analysis study demonstrated that patients with gout attack had a significantly increased risk of DVT after a 7.5-year follow-up period. These findings provide novel evidence that history of gout attack could be a risk predictor for DVT occurrence, which may provide some new thoughts in prevention of DVT. However, these findings would have to be confirmed in future clinical and experimental studies, including evaluation the association of inflammatory and endothelial dysfunctional markers levels and uric acid levels.
Methods
Data Sources
Data were extracted from the Taiwan National Health Insurance (NHI) Research Database (NHIRD), which contains anonymized secondary data that is available for research purposes. Taiwan’s NHI program, launched in 1995, currently covers 99% of the population of 23 million people. The database comprises all registry and claims data from the NHI system, ranging from demographic data to detailed orders for ambulatory and inpatient care. Taiwan’s NHI Bureau is responsible for auditing medical payments by comprehensive review of medical records, examination reports, and results of imaging studies. If physicians fail to meet the standards for clinical practice, Taiwan’s NHI reserves the right to reject payment and can impose huge financial penalties. Disease diagnoses are coded according to the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM). The diagnostic accuracy for the major diseases in the NHIRD has been well validated33,34,35,36. The Longitudinal Health Insurance Database, a subset of the original NHIRD, which contains data from a random sample of 1 million NHI beneficiaries, was used in the current study.
Study Design
This nationwide, population-based, observational, retrospective cohort study was conducted to determine the association between gout and DVT. Two cohorts were enrolled in the study: the gout cohort and a matched cohort without gout attack. The gout cohort consisted of patients with at least one episode of a gout attack necessitating long-term treatment with uric acid-lowering medications, including allopurinol and probenecid33. Patients with the following characteristics were excluded: age <20 years, and with history of DVT. The index date was defined as the first day of a gout attack. The control cohort comprised all patients without a diagnosis of gout. The exclusion criteria for the gout cohort were also applied to the control cohort. Index dates for subjects in the control cohort were randomly assigned and corresponded to those of patients in the gout cohort. It is a retrospective follow-up study.
We used 1:1 propensity score matching and calculated propensity scores for the likelihood of gout using baseline covariates and multivariate logistic regression analysis (Supplementary Table 1). We matched 1 control patient with each patient in the gout cohort with a similar propensity score based on nearest-neighbor matching without replacement using calipers with a width equal to 0.1 standard deviation (SD) of the log of the propensity score.
Primary Outcome Measures
The primary outcome was hospitalization or an out-patient visit for DVT (ICD-9 453.x) and pulmonary embolism (ICD-9 415.1) with oral anticoagulant use after discharge. All of the DVT patients received image evaluation including computerized tomography (CT) scan and Doppler sonogram and were identify for the diagnosis of DVT, but we cannot confirm that all patients were submitted to these image evaluations. Both cohorts were followed until patients were diagnosed with DVT or pulmonary embolism, died, or December 31, 2012.
Baseline Characteristics
Baseline demographic characteristics examined were age, sex, monthly income (NT$ [New Taiwan dollar] <19,100, NT$19,100–$41,999, and ≥NT$42,000), urbanization, and Charlson Comorbidity Index score. Urbanization levels in Taiwan are divided into 4 strata according to the Taiwan National Health Research Institute. Level 1 designates the most urbanized areas, and level 4 designates the least urbanized areas. The Charlson Comorbidity Index score is used to determine overall systemic health. Each increase in score indicates a stepwise increase in cumulative mortality37. We also identified other medications that potentially could confound the association between hyperuricemia and DVT risk.
Statistical Analysis
Descriptive statistics were used to characterize the baseline characteristics of the study cohorts. Baseline characteristics of the 2 groups were compared by standardized mean difference. Propensity scores of the likelihood of gout were determined by multivariate logistic regression analysis, conditional on baseline covariates (Supplementary Table 1). The incidence rates of DVT in the 2 groups were calculated using Poisson distribution. The cumulative incidence or risk of DVT was estimated by use of the Kaplan-Meier method, and differences between cohorts were evaluated with the log-rank test. Cox regression models with a conditional approach using stratification were used to calculate adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for the risk of DVT38. We performed sensitivity analysis with the inclusion of different criteria. The likelihood ratio test was used to examine the interaction between the occurrence of DVT subsequent to gout attack and the following variables: age, sex, Charlson Comorbidity Index score, and the presence of diabetes mellitus, hypertension, chronic kidney disease, heart failure, dyslipidemia, papalysis, estrogen use, antiplatelet agent use, and fracture. Subgroup analyses were also performed.
The SQL Server 2012 (Microsoft Corp, Redmond, WA, USA) was used for data linkage, processing, and sampling. Propensity scores were calculated with SAS version 9.3 (SAS Institute, Cary, NC, USA). All other statistical analyses were conducted with STATA statistical software (version 12.0; StataCorp, College Station, TX, USA). Statistical significance was defined as p < 0.05.
Additional Information
How to cite this article: Chiu, C.-C. et al. Association between previous history of gout attack and risk of deep vein thrombosis - a nationwide population-based cohort study. Sci. Rep. 6, 26541; doi: 10.1038/srep26541 (2016).
References
Zakai, N. A. et al. Racial and regional differences in venous thromboembolism in the United States in 3 cohorts. Circulation. 129, 1502–1509, doi: 10.1161/CIRCULATIONAHA.113.006472 (2014).
Spencer, F. A. et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med. 21, 722–727, doi: 10.1111/j.1525-1497.2006.00458.x (2006).
Franchini, M. & Mannucci, P. M. Venous and arterial thrombosis: different sides of the same coin? Eur J Intern Med. 19, 476–481, doi: 10.1016/j.ejim.2007.10.019 (2008).
Goldhaber, S. Z. Risk factors for venous thromboembolism. J Am Coll Cardiol. 56, 1–7, doi: 10.1016/j.jacc.2010.01.057 (2010).
Milan, M., Vedovetto, V., Bilora, F., Pesavento, R. & Prandoni, P. Further evidence in support of the association between venous thrombosis and atherosclerosis: a case-control study. Thromb Res. 134, 1028–1031, doi: 10.1016/j.thromres.2014.09.007 (2014).
Chu, N. F., Wang, D. J., Liou, S. H. & Shieh, S. M. Relationship between hyperuricemia and other cardiovascular disease risk factors among adult males in Taiwan. Eur J Epidemiol. 16, 13–17 (2000).
Yildiz, A. & Kaya, Z. Uric acid: a crucial marker of cardiovascular diseases? Int J Cardiol. 159, 158, doi: 10.1016/j.ijcard.2012.05.054 (2012).
Zhang, J. W. et al. Association of serum uric acid and coronary artery disease in premenopausal women. Plos One. 9, e106130, doi: 10.1371/journal.pone.0106130 (2014).
Guo, W. et al. Hyperuricemia Is an Independent Predictor of Contrast-Induced Acute Kidney Injury and Mortality in Patients Undergoing Percutaneous Coronary Intervention. Angiology. 66, 721–726, doi: 10.1177/0003319714568516 (2015).
Culleton, B. F., Larson, M. G., Kannel, W. B. & Levy, D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 131, 7–13 (1999).
Feig, D. I., Kang, D. H. & Johnson, R. J. Uric acid and cardiovascular risk. N Eng J Med. 359, 1811–1821, doi: 10.1056/NEJMra0800885 (2008).
Ruggiero, C. et al. Uric acid and inflammatory markers. Eur Heart J. 27, 1174–1181, doi: 10.1093/eurheartj/ehi879 (2006).
Zoccali, C., Maio, R., Mallamaci, F., Sesti, G. & Perticone, F. Uric acid and endothelial dysfunction in essential hypertension. J Am Soc Nephrol: JASN 17, 1466–1471, doi: 10.1681/ASN.2005090949 (2006).
Patschan, D., Patschan, S., Gobe, G. G., Chintala, S. & Goligorsky, M. S. Uric acid heralds ischemic tissue injury to mobilize endothelial progenitor cells. J Am Soc Nephrol.: JASN 18, 1516–1524, doi: 10.1681/ASN.2006070759 (2007).
Khosla, U. M. et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 67, 1739–1742, doi: 10.1111/j.1523-1755.2005.00273.x (2005).
Meisinger, C., Koenig, W., Baumert, J. & Doring, A. Uric acid levels are associated with all-cause and cardiovascular disease mortality independent of systemic inflammation in men from the general population: the MONICA/KORA cohort study. Arterio Thromb Vasc Biol. 28, 1186–1192, doi: 10.1161/ATVBAHA.107.160184 (2008).
Kleber, M. E. et al. Uric Acid and Cardiovascular Events: A Mendelian Randomization Study. J Am Soc Nephrol.: JASN, doi: 10.1681/ASN.2014070660 (2015).
Yamada, N. et al. Risk factors for nonfatal pulmonary embolism in a Japanese population: A hospital-based case-control study. Angiology. 61, 269–274, doi: 10.1177/0003319709335907 (2010).
Mazzoccoli, G. et al. Arterial endothelial dysfunction and idiopathic deep venous thrombosis. J Biol Regul Homeost Agents. 25, 565–573 (2011).
Mazzoccoli, G. et al. Idiopathic deep venous thrombosis and arterial endothelial dysfunction in the elderly. Age. 34, 751–760, doi: 10.1007/s11357-011-9265-x (2012).
Prandoni, P. et al. An association between atherosclerosis and venous thrombosis. N Engl J Med. 348, 1435–1441, doi: 10.1056/NEJMoa022157 (2003).
Glynn, R. J. et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 360, 1851–1861, doi: 10.1056/NEJMoa0900241 (2009).
Krishnan, E., Pandya, B. J., Chung, L., Hariri, A. & Dabbous, O. Hyperuricemia in young adults and risk of insulin resistance, prediabetes, and diabetes: a 15-year follow-up study. Am J Epidemiol. 176, 108–116, doi: 10.1093/aje/kws002 (2012).
Erdogan, D. et al. Relationship of serum uric acid to measures of endothelial function and atherosclerosis in healthy adults. Int J Clini Pract. 59, 1276–1282, doi: 10.1111/j.1742-1241.2005.00621.x (2005).
Kang, D. H., Park, S. K., Lee, I. K. & Johnson, R. J. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol: JASN 16, 3553–3562, doi: 10.1681/ASN.2005050572 (2005).
Tang, R. B. et al. Serum uric acid and risk of left atrial thrombus in patients with nonvalvular atrial fibrillation. Can J Cardiol. 30, 1415–1421, doi: 10.1016/j.cjca.2014.06.009 (2014).
Kanbay, M. et al. Effects of allopurinol on endothelial dysfunction: a meta-analysis. Am J Nephrol. 39, 348–356, doi: 10.1159/000360609 (2014).
Libby, P., Ridker, P. M. & Maseri, A. Inflammation and atherosclerosis. Circulation. 105, 1135–1143 (2002).
Kanellis, J. et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 41, 1287–1293, doi: 10.1161/01.HYP.0000072820.07472.3B (2003).
Johnson, R. J., Rodriguez-Iturbe, B., Kang, D. H., Feig, D. I. & Herrera-Acosta, J. A unifying pathway for essential hypertension. Am J Hypertens. 18, 431–440, doi: 10.1016/j.amjhyper.2004.08.035 (2005).
Stein, P. D., Beemath, A. & Olson, R. E. Obesity as a risk factor in venous thromboembolism. Am J Med. 118, 978–980, doi: 10.1016/j.amjmed.2005.03.012 (2005).
Ageno, W., Becattini, C., Brighton, T., Selby, R. & Kamphuisen, P. W. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 117, 93–102, doi: 10.1161/CIRCULATIONAHA.107.709204 (2008).
Chao, T. F. et al. Hyperuricemia and the risk of ischemic stroke in patients with atrial fibrillation–could it refine clinical risk stratification in AF? Int J Cardiol. 170, 344–349, doi: 10.1016/j.ijcard.2013.11.011 (2014).
Chiu, C. C. et al. Increased risk of ischemic stroke in patients with systemic lupus erythematosus: a nationwide population-based study. Intern Med. 51, 17–21 (2012).
Lin, C. C., Lai, M. S., Syu, C. Y., Chang, S. C. & Tseng, F. Y. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc. 104, 157–163 (2005).
Cheng, C. L., Kao, Y. H., Lin, S. J., Lee, C. H. & Lai, M. L. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 20, 236–242, doi: 10.1002/pds.2087 (2011).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40, 373–383 (1987).
Austin, P. C. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med. 27, 2037–2049, doi: 10.1002/sim.3150 (2008).
Acknowledgements
This study was supported, in part, by the following research grants: the National Science Council, NSC 101-2314-B-075–038 and UST-UCSD International Centre of Excellence in Advanced Bio-engineering MOST 105-2633-B-009-003; V102A-026, VGH-V102B-016, VGHUST103-G7-2-1, VGH-V102E2-002 from Taipei Veterans General Hospital; a grant from the ministry of Health and Welfare (MOHW 104-TDU-B-211-113-003, and a grant from the Ministry of Education’s ‘Aim for the Top University’ Plan. Funding agencies had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
The study was designed by C.-C.C. (Chiu), Y.-T.C., S.-J.L. and P.-H.H. Y.-T.C. and S.-C.K. managed data collection. Data analysis was undertaken by C.-C.C. (Chiu), Y.-T.C. and P.-H.H., C.-C.C. (Chang), C.-C.H. and H.-B.L. reviewed the study protocol. C.-Y.H., S.-Y.L. and J.-W.C. reviewed the statistical data. C.-C.C. (Chiu) wrote the main manuscript. All authors contributed to reviewing relevant literature and writing, editing, and final approval of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chiu, CC., Chen, YT., Hsu, CY. et al. Association between previous history of gout attack and risk of deep vein thrombosis - a nationwide population-based cohort study. Sci Rep 6, 26541 (2016). https://doi.org/10.1038/srep26541
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep26541
This article is cited by
-
Prevalence and differences in the co-administration of drugs known to interact: an analysis of three distinct and large populations
BMC Medicine (2024)
-
Coagulation in gout: is there a link with disease activity?
Clinical Rheumatology (2022)
-
Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors
Nature Reviews Rheumatology (2020)
-
Uric acid and thrombotic risk: an emerging link
Internal and Emergency Medicine (2020)
-
Association between serum uric acid and cardiovascular risk in nonhypertensive and nondiabetic individuals: The Taiwan I-Lan Longitudinal Aging Study
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.