Abstract
The West Indian fruit fly, Anastrepha obliqua, is an important agricultural pest in the New World. The use of pesticide-free methods to control invasive species such as this reinforces the search for genes potentially useful in their genetic control. Therefore, the study of chemosensory proteins involved with a range of responses to the chemical environment will help not only on the understanding of the species biology but may also help the development of environmentally friendly pest control strategies. Here we analyzed the expression patterns of three OBP genes, Obp19d_2, Obp56a and Obp99c, across different phases of A. obliqua development by qPCR. In order to do so, we tested eight and identified three reference genes for data normalization, rpl17, rpl18 and ef1a, which displayed stability for the conditions here tested. All OBPs showed differential expression on adults and some differential expression among adult stages. Obp99c had an almost exclusive expression in males and Obp56a showed high expression in virgin females. Thereby, our results provide relevant data not only for other gene expression studies in this species, as well as for the search of candidate genes that may help in the development of new pest control strategies.
Similar content being viewed by others
Introduction
The genus Anastrepha has over 235 species1 most of them endemic to the Neotropics. Only a few of these species, mostly in the fraterculus group, are agricultural pests, including the West Indies fruit fly Anastrepha obliqua (Diptera, Tephritidae). Because of the vast damage they inflict to several different fruit crops, they are of great economic importance, which elicit the development of several pest management strategies. Genetic control technique’s, as the Sterile Insect Technique (SIT) using gamma radiation, have been used to control natural populations of other tephritids such as Ceratitis capitata2. However, radiation leads to side effects3,4, decreasing the technique efficiency. Thus, the search of candidate genes that may lead to more competitive transgenic organisms may be an alternative for improvement of SIT programs.
A number of candidate genes has already been explored and promising results are reported for some, such as Astra and Astra-2 genes for the Caribbean fruit fly Anastrepha suspensa5. This encourages the search to identify genes related to reproduction and species recognition in this genus, which may be a priori good candidate genes for genetic control of pest populations. Because the olfactory system participates in important reproductive aspects, such as choosing a partner for mating and searching for oviposition sites6,7,8,9,10, genes that code for proteins involved in olfactory reception may prove useful in this regard as well.
From chemical stimulus to behavioral response, the olfactory process involves the capture, binding, transport and inactivation of odors; the activation of receptors and signal transduction; and the perception of odor by processing the signals at several levels of the central nervous system6,7. Thus, chemoreception participates in a range of responses to the chemical environment for animal survival, such as chemical cues for foraging behavior and food selection, predator avoidance, host plant recognition for oviposition and larval feeding, selection of mating partners, maternal behavior and kin recognition11. In insects, the beginning of the transduction cascade of olfactory signals is the solubilization and transport of the chemical signals through the aqueous lymph of insect’s sensilla to the olfactory receptors (ORs). The Odorant Binding Proteins (OBPs) mediate these processes12, making the interaction between the environment and the insect and determining the behavioral response. Hence, OBPs have been recognized as good candidates for genetic control programs of insect pests13,14 and hematophagous insect vectors15,16,17,18.
One important step in the process of prospecting genes for genetic control is the understanding of expression patterns of candidate genes in the target organism. Quantitative polymerase chain reaction (qPCR) has become an invaluable tool in studies of gene expression due to its sensitivity and precision, enabling the detection of different contrasts among RNA samples. Differential expression of some dipterans’ OBP family members has been studied by qPCR in different tissues14 and in different adult life stages13.
In this study, the goal was to assess by qPCR the expression profile of three OBPs genes, Obp19d_2, Obp56a and Obp99c, at different developmental stages on the West Indies fruit fly. These genes were chosen due to their differential expression between virgin and post-mating males in a previous in silico analysis, suggesting them as potential targets for further studies. However, there is a dearth of information regarding gene expression, as well as good reference genes for data normalization in qPCR expression studies in most animal species, let alone for A. obliqua. Thus, in addition to an analysis of OBPs’ expression, this study also aimed to determine a set of reliable reference genes for expression studies across different developmental stages for the West Indies fruit fly. In order to do so, we investigate patterns of gene expression for eight potential reference genes using qPCR: ribosomal protein L18 (rpl18), β-Tubulin (btub), elongation factor 1α (ef1a), ribosomal protein S17 (rps17), glyceraldehyde-3-phosphate dehydrogenase (gapdh), actin (act), Syntaxin (Syx) and Troponin C (TpnC) . Our results indicate that the genes rpl18, rps17 and ef1a show the highest expression stability throughout development, making them potentially reliable reference genes for gene expression studies in A. obliqua. Our results also show that some OBP genes are differentially expressed across different genders and reproductive stages, which make them good candidates for future application in genetic control.
Results
Efficiency of candidate reference genes
A quantitative study of relative gene expression requires normalization of data for target genes against a set of reference genes that are used as internal standards and are evenly expressed among tested conditions. Therefore, we investigated gene expression patterns of eight potential reference genes in different A. obliqua developmental stages. To determine the best primer concentrations, we tested three different concentrations in several primer combinations. The optimal concentration was 0.3 μM compared to 0.1 μM and 0.15 μM. For sensitivity assays, all genes tested, with the exception of syx and TpnC, showed efficiency values between 95% to 105% and standard curve correlation coefficients of 0.99 (Table 1; Supplementary Fig. S1 online; Supplementary Table S1 online), meaning that the amount of PCR product approximately doubles each cycle19. For all genes, the melting curves showed one single peak, indicating that assays led to specific amplifications (Supplementary Fig. S1 online). Because syx and TpnC failed to show acceptable amplification efficiency (>105%), they were disregarded as reference genes, leaving six genes that were further evaluated.
Candidate genes with relative expression stability across life stages
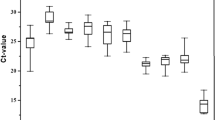
The Cq (quantitative cycle) values generated from the six candidates for reference genes were used to estimate stability of gene expression across different life stages. Figure 1A,B reveals different patterns of expression and variation among candidate genes. The difference in average Cqs among the candidate genes was only 2.18 cycles, with the lowest Cqs values (19.23) for ef1a gene and the highest (21.41) for rpl18 gene (Fig. 1A). The investigation of variation across different life stages revealed two different groups (Fig. 1B): one group consisted of rpl18, rps17, ef1a and btub with standard deviation (SD) < 1.0; and another group consisted of gapdh e act with SD > 1.0. There are several different algorithms that investigate patterns of expression to identify the best reference genes which are available for qPCR studies. In this study, by using three tools (NormFinder, BestKeeper and RefFinder) available online, we identified the most stable genes as being rpl18, rps17 and ef1a (Table 2). It is worth noting that all three algorithms indicated that act exhibited the largest variation and lowest stability and is therefore not suitable as a reference gene for the experimental conditions used in this study.
Gene expression stability of candidate reference genes.
(A) Gene expression across different Anastrepha obliqua life stages. The bars show mean Cq values for each life stage from three biological replicates. (B) Variation in expression levels of candidate reference genes. Box-plots show medians (lines), means (dots), 95th percentile (boxes) and ranges (whiskers); (n = 24). The variation in expression level for each candidate gene was estimated across eight different life stages from Anastrepha, each stage with three biological replicates. The genes and their respective acronyms are: ribosomal protein L18 (rpl18), β-Tubulin (btub), elongation factor 1α (ef1a), ribosomal protein S17 (rps17), glyceraldehyde 3-phosphate dehydrogenase (gapdh) and actin (act). LII - larval stage II; LIII - larval stage III; PI - pupal stage I; PII - pupal stage II; MVF - mature virgin female; MVM - mature virgin male; PMF - post-mating female; PMM - post-mating male.
Investigation on target genes involved in reproduction
We used the results generated from differential expression of transcripts of virgin mature and post-mating males of Anastrepha fraterculus transcriptome to select some differentially expressed genes. The rationale was that this difference indicates genes potentially involved in the reproductive process. Of the differentially expressed genes obtained in our in silico analysis, three OBPs were selected to represent a gene family already associated with the reproduction process8,20. Table 3 shows the differentially expressed genes selected from the Anastrepha transcriptome. Based on the fold-change differences, two OBP genes were chosen for being supposedly up-regulated (Obp19d_2 and Obp99c) and one down-regulated (Obp56a) by mating.
Expression profiles of OBPs in different life stages
Despite the important role of OBPs in the reproductive behavior of Diptera, we still lack greater knowledge about the function and expression patterns of these proteins at different developmental stages of insects. Thus, we used qPCR to determine the expression profiles of the three selected OBPs in A. obliqua larvae, pupae and mature virgin and post-mating adults of both sexes. In order to generate OBP sequences from A. obliqua, we used A. fraterculus contig sequences to create primers for cloning and sequencing these genes from A. obliqua. This procedure generated full OBP sequences from A. obliqua from which we designed qPCR primers to study A. obliqua expression. Sequences from A. obliqua were very similar to OBPs from A. fraterculus, sharing 100% similarity for Obp19d_2, 97% for Obp56a and 93% for Obp99c at the amino acid level. Furthermore, the region we used to derive the qPCR primers had 100% similarity between the species, so it is likely that these primers could be used to investigate expression studies in both species, though we have only tested them for A. obliqua. The sensitivity of each primer set determined by standard curves with seven dilutions is shown in Table 4. These primers showed efficiency close to 100% and standard curve correlation coefficients of 0.99 (Supplementary Fig. S2 online; Supplementary Table S2 online). The specificity of each primer set was confirmed by single melting peaks (Supplementary Fig. S2 online). For gene expression analysis, reactions were performed with three technical replicates of three biological replicates for each life stage. The relative normalized expression was calculated by  method21 in contrast with three validated reference genes expression (rpl18, rps17 and ef1a).
method21 in contrast with three validated reference genes expression (rpl18, rps17 and ef1a).
The relative transcript abundance of OBPs in larvae II, larvae III, early pupae, late pupae, mature virgin and post-mating female and mature virgin and post-mating male is exhibited in Fig. 2. All three OBP genes showed low expression during immature stages (pupae and larvae) compared to adult phases. We failed to detect significant differences in Obp19d_2 expression between virgin and post-mating male or female adults. However, we observed a higher expression of this OBP in post-mating males when compared to post-mating females p (<0.05). As for Obp56a, a 2-fold decrease in abundance (unpaired t test, p < 0.01) was observed in post-mating females compared to virgin females, whereas no significant change was detected in males among different profiles. The levels of expression of this OBP in virgin and post-mating males were as low as that observed for post-mating females, but significant difference was observed only between post-mating males and females (p < 0.05). On the other hand, Obp99c had low expression values for mature virgin and post-mating females, comparable to the expression observed in the early stages of development. However, we observed a significantly higher expression in virgin and post-mating males in comparison to virgin and post-mating females (2-fold and 3-fold increase, respectively) (unpaired t test, p < 0.001).
Discussion
Normalization is an essential step for the quantitative study of relative gene expression, which means that the relative expression of a target gene must be determined against a reference gene, whose expression levels should be stable and not influenced by the experimental procedure22. Since levels of expression in reference genes may vary depending on the species being studied, or even on environmental or developmental conditions, no reference gene should be considered universal23. Therefore, every qPCR experiment should begin with the identification and validation of a set of reference genes that can be reliably used according to a specific condition.
To our knowledge, this is the first study for suitable reference genes for gene expression studies in the genus Anastrepha across different life stages. Eight potential reference genes were analyzed and ranked according to qPCR efficiency and expression stability. From them, three (rpl18, rps17 and ef1a) were reliable as reference genes because they showed appropriate efficiency values and constitutive expression levels across the different stages we contrasted. In fact, the efficiency and the expression stability of these three genes have already been reported for other insects. The gene rpl18 was the most stable for the bed bug Cimex lectularius and the Colorado potato beetle Leptinotarsa decemlineata24,25. For the mosquito Aedes aegypti, rps17 was the most stable gene across different ages26 and other experimental conditions27,28,29. On the other hand, rps17 was not reliable as reference gene in three Calliphorid flies30. The ef1a gene was analyzed on Bombus terrestris, Bombus lucorum, Bactrocera dorsalis, Cimex lectularius and Leptinotarsa decemlineata24,25,31,32,33 and among these species ef1a was reported as a good reference gene only for B. lucorum. A range of mixed results for the same potential reference gene in different studies and experimental conditions reinforces the importance of search and normalization of suitable reference genes for qPCR studies. Indeed, even species from the same genus as B. terrestris and B. lucorum, have required different reference genes for qPCR studies31, which emphasizes the need of normalization even for closely related species.
The wide range of expression levels shown by act in our experiments with A. obliqua also highlights the importance of normalization and species-specific studies for qPCR. This gene is widely used as reference in qPCR experiments in a range of organisms, because it is assumed to be constitutively expressed. For instance, act is considered one of the most stable gene in dipterans such as the tephritid B. dorsalis33 and D. melanogaster34. However, our data showed that, among the potential reference genes tested, act had the widest range in expression values across different stages of development in A. obliqua, making it inappropriate as a reference gene in the conditions we considered. We should emphasize that even though rpl18, rps17 and ef1a have been confirmed as appropriate reference genes for A. obliqua across the developmental stages we considered, any condition other than the ones tested here may require new validations.
An attribute that makes insects a group of great success is their sophisticated olfactory system, which is paramount to their survival and reproduction. Since we still know very little of the molecular events affecting behavior and biology of the West Indies fruit fly, information on the expression profiling of OBPs across different developmental stages and pre- and post-mating stages contributes relevant information about the role of these proteins on reproduction. Such knowledge might help towards the development of new and more environmentally friendly pest control strategies. Obp19d_2, Obp56a and Obp99c showed different levels of expression across development, even in immature stages (larvae and pupae), despite the very low relative transcript abundance compared to adult stages. Although several of the different expression levels here found are significant, some profiles show higher standard deviation, particularly virgin and post-mating adults in Obp19d_2 and virgin adults in Obp56a (Fig. 2). Because these deviations are consistent across technical replicates, we believe that they are due to variation in expression among individuals. OBPs belong to an epistatic network of pleiotropic genes that are plastic, dynamic and modulated by several factors, such as sex, physiological state, social relationships, as well as by the environment35. Though we may be able to control several of these aspects in the lab, we cannot control for population variation, since we performed our study in non-inbred populations. Variation at the population level for OBP genes and their expression might be adaptive, since it might enable different response to important environmental cues. The combination of population variation, epistatic interactions and environmental effects makes it a daunting task to investigate OBP expression, but our results still show relevant biological associations.
In D. melanogaster, microarray gene expression studies showed that Obp19d had an adult-biased expression, as observed in our data, whereas Obp56a had a larval-biased expression, which differs from our results. As for Obp99c, high expression levels were observed in both larvae and adults of D. melanogaster11, which differs from A. obliqua since the expression was very low in immature stages as well as in female adults. Overall, the low levels of expression of these OBPs in immature stages of A. obliqua lead us to speculate that, at least in larvae, these genes seem not be involved with some basic biological needs such as nutrient sensing. Furthermore, the adult-biased expression observed for these OBP genes in A. obliqua may be related to the search for partners and oviposition sites.
In adults, chemically-induced changes on females due to mating, mainly related to seminal fluid proteins, have been well characterized in D. melanogaster36,37,38; as well as mating consequences in males, like immunological sexual dimorphism39 and stimulation of male accessory glands proteins40. For A. obliqua, we found a difference in expression levels between virgin and post-mating adults only for Obp56a in females, with a significant lower level of expression before mating. This difference may indicate possible regulation by mating and it could contribute to changes on female’s behavior as it happens in D. melanogaster females that, after sperm contact due to mating, experience a decrease in attractiveness and receptiveness to other males37. In fact, Obp56a is regulated on females by sperm contact in D. melanogaster36. In C. capitata, females experience a switch on certain olfactory-mediated behaviors after mating: virgin females choose the odor of male pheromone instead of volatile host plant emissions and maintain this preference even without contact with males, whereas mated females have a stronger preference for host plant odors to oviposit41. Thereby, changes in chemoreception genes may be expected due to mating and, although the function of Obp56a is not known, the decrease of its abundance in A. obliqua post-mating females may suggest that this protein would be more related to perception of male pheromones, or other aspects related to mating, instead of the search for oviposition sites.
Furthermore, Obp56a also showed sexual dimorphism in expression, since it had a higher expression on post-mating males than on females. The same was observed on mated males for Obp19d_2 and Obp99c. This differential expression may be a consequence of the different gender response to the chemical environment. This sexual dimorphism is expected since males and females may have very different chemical cues. The higher expression of OBPs on post-mating males would suggest that these genes could be related to seeking partners, responding to female’s pheromone, while post-mating females gear their efforts to seeking oviposition sites and therefore may be more responsive to volatile host plant emissions. Obp19d is also known as pheromone binding protein-related protein (PBPRP2), indicating its role with reproduction in D. melanogaster42. On the other hand, Obp99c was significantly more expressed on virgin and mated males suggesting that this protein may be related to functions restricted to this gender. Genes in the Obp99 class have been associated with the smell of benzaldehyde in D. melanogaster43,44, which is common to several plants, especially in flowers and fruits45, making it a possible class of genes associated with host preference. Furthermore, benzaldehyde increased attraction of males to sex pheromone lures in Spodoptera exigua by over 100%46, making it possible that OBP99c is a gene involved with host and mating preference. A widespread sexual dimorphism in chemosensory genes expression, including OBPs, was documented in D. melanogaster, in response to different environmental, physiological and social conditions11. Indeed, responses to different odorants have been observed as sexually dimorphic47.
The results here obtained make Obp99c a good candidate for the application in techniques that use genetic manipulation like the Sterile Insect Technique (SIT), since its relative expression was almost exclusive to males. Likewise, the high expression of Obp56a on virgin females may be an important factor involved in their search for males and or mating sites. Some OBPs like Obp19d_2, Obp56a and Obp99c, may regulate parts of sexual behavior such as the search for hosts and sexual partners, therefore, an improvement on the knowledge about these proteins in agricultural pests like A. obliqua impacts not only the understanding of their biology, but may contribute to future research on the use and manipulation of OBPs for the development of new environmentally friendly pest control strategies. Furthermore, this knowledge may aid on the development of novel species-specific attractants and repellents, lures and traps for pest control programs.
Methods
Sampling
Flies were obtained from an A. obliqua population maintained in natural photoperiod at 28 °C ± 2.0 °C in the Population Genetics and Evolution laboratory, at the Federal University of São Carlos, Brazil. Different stages of development were used: 2nd and 3rd larval instars (LII and LIII) taken from mangoes offered to the population for oviposition, using mean standard length of each instar (3–5 mm for 2nd instar and around 8.6 mm for 3rd instar)48; early pupal stage (PI), taken around 24 hours after pupation, later pupal stage (PII), taken 10 days after pupation; sexually mature virgin males (MVM) and females (MVF), taken 10 days after adult emergence; and post-mating males (PMM) and females (PMF), taken 24 hours after mating. For each stage, three independent biological replicates were produced from pools of five different individuals.
RNA isolation and cDNA synthesis
Total RNA was extracted using the TRIzol/chloroform protocol49. RNA quality was visualized in an agarose gel electrophoresis for integrity, absorbance was measured in Nanodrop (Thermo ScientificTM) and then RNA was quantified using a Qubit® 2.0 Fluorometer (InvitrogenTM). The extracted RNA was treated with DNase I Amplification Grade (InvitrogenTM) prior to reverse transcription. One μg of treated RNA was converted into cDNA using iScriptTM cDNA Synthesis Kit (Bio-Rad). To perform the assays, all cDNA samples were diluted to 4 ng/μl. The samples were stored at −80 °C until used.
Candidate genes and primer design
For the validation of reference genes, eight constitutive genes were chosen, most of them already used as reference for qPCR experiments in insects24,31,32,33,50,51. In order to obtain DNA sequences for A. obliqua for these genes, D. melanogaster’s sequences from the following genes were selected from GenBank database: rpl18 (NM_139834.2), btub (EU980443.1), ef1a (GU339154.1), rps17 (NM_079278.2), gapdh (GU269901.1), syx (NM_168905.1), tpnC (EZ423429.1) and act (L12254.1). These sequences were used to BLAST against an A. fraterculus transcriptome (Rezende, V.B.; Congrains, C.; Lima, A.L.; Campanini, E.B.; Nakamura, A.M.; Oliveira, J.L.; Chahad-Ehlers, S.; Sobrinho-Jr., I.; de Brito, R.A.; unpublished data) that was the only transcriptome data available at that time in our lab. We did not consider this to be much of a problem, because A. fraterculus and A. obliqua are closely related species and these genes are evolutionarily very conservative. We performed a differential expression analysis between mature virgin male and post-mating male in the A. fraterculus transcriptome data set using the software edgeR following default specifications52 and identified three differentially expressed OBP genes (Obp19d_2, Obp56a and Obp99c) between these profiles. These genes were chosen following the premise that the difference in expression indicates that the gene may play an important role in reproduction. We used inferred amino acid sequences for each of these OBPs to perform a BLAST search on GenBank with the best close match options to C. capitata, which identified the sequences Obp19d_2 (XM_004524978.1), Obp56a (XM_004517843) and Obp99c (XM_004521129.1). We aligned these sequences to their orthologous copies obtained from the A. fraterculus transcriptome using MAFFT53 and designed primers to amplify conserved regions of each gene in A. obliqua. These PCR products were PEG purified and sent to a specialized company (MACROGEN INC., South Korea) to carry out the sequencing. Based on the Sanger sequences, qPCR primers were designed in regions with 100% of similarity between each A. obliqua sequence and A. fraterculus contig, using in flanking intron-exon junctions if possible and checked for duplex formation and secondary structures with the OligoAnalyzer tool (http://www.idtdna.com/analyzer/applications/oligoanalyzer/).
qPCR
For qPCR assays, SsoFastTM Evagreen® Supermix (Bio-Rad) was used with final primer concentration of 0.3 μM in a total reaction of 10 μl. Reactions were performed in a CFX96 TouchTM Real-Time PCR Detection Systems (Bio-Rad) using the following protocol: 95° for 30 seconds followed by 40 cycles of dissociation at 95 °C for 10 seconds and annealing and extension at 60 °C for 1 min. For melt curve analysis we used a protocol with temperatures that varied from 65 °C to 95 °C with increments of 0.5 °C for 5 seconds and continuous fluorescent measurements. The efficiency of the reactions at high and low concentrations were assessed to ensure that primer efficiency would not vary significantly due to variations in cDNA concentration among samples. Therefore, efficiency of qPCR (E) and the correlation coefficients (R2) were calculated for each pair of primer using standard curves of correlation between cDNA concentration and Cq values. They were generated with a serial dilution (7x) of a pool with all cDNA samples with an initial concentration of 4 ng/μl, in triplicate. Moreover, to check for the uniform expression of reference genes across life stages, which is necessary for the gene stability calculation and to calculate the relative normalized expression for OBPs, assays were performed in triplicate, using each biological replicate at a concentration of 4 ng/μl. No-template control (NTC) was used in all qPCR experiments to confirm that there was no contamination with exogenous material.
Gene stability analyses of reference genes
The Cq average values calculated for the candidate reference genes in CFX ManagerTM software (Bio-Rad) were analyzed by three different descriptive statistic tools freely available online: NormFinder software (http://moma.dk/normfinder-software), BestKeeper software (http://www.gene-quantification.de/bestkeeper.html) and RefFinder web-based tool (http://www.leonxie.com/referencegene.php). NormFinder calculates not only the overall variation of the candidate normalization genes but also the variation between sample subgroups of the sample set54. The software BestKeeper estimates the correlation of expression levels of all candidates and the genes more stable are combined in an index. Three main indicators were used to determinate the best candidate: standard deviation, covariance and correlation analysis55. RefFinder ranks all candidates of reference genes based on the main algorithms currently used, including Normfinder and BestKeeper, as well as the geNorm algorithm56 and the Comparative ΔCt Method57. Thus, RefFinder allows the choice of the best candidates based on the final ranking results of different programs58,59,60,61,62.
Relative normalized expression calculation of OBPs
Like the reference genes, data analysis for OBPs was also performed using CFX Manager SoftwareTM (Bio-Rad). The values of relative normalized expression were calculated for each gene using the  method21, in contrast with the three validated reference genes expression. Since experiments were performed in different plates, inter-run calibrators were used and a common threshold was set. Data were statistically compared by ANOVA and Unpaired 2-tailed t test using Prism 5.01 software (GraphPad Software, San Diego, CA, USA).
method21, in contrast with the three validated reference genes expression. Since experiments were performed in different plates, inter-run calibrators were used and a common threshold was set. Data were statistically compared by ANOVA and Unpaired 2-tailed t test using Prism 5.01 software (GraphPad Software, San Diego, CA, USA).
Additional Information
How to cite this article: Nakamura, A.M. et al. Reference genes for accessing differential expression among developmental stages and analysis of differential expression of OBP genes in Anastrepha obliqua. Sci. Rep. 6, 17480; doi: 10.1038/srep17480 (2016).
References
Marsaro Júnior, A., Deus, E., Ronchi-Teles, R., Adaime, B. & Silva Júnior, R. Species of Anastrepha (diptera: Tephritidae) captured in a guava orchard (Psidium guajava l., myrtaceae) in Boa Vista, Roraima, Brazil. Brazilian Journal of Biology. 73, 879–886 (2013).
Bjelis, M., Ljubetic, V. & Novosel, N. Symposium on fruit flies of economic importance. Proceedings of the 7th International Symposium 7th, 255–259 (2007).
Heath, R. R., Epsky, N. D., Dueben, B. D., Guzman, A. & Andrade, L. E. Gamma radiation effect on production of four pheromonal components of male mediterranean fruit flies (Diptera: Tephritidae). Journal of Economic Entomology 87, 904–909 (1994).
Calcagno, G. E., Manso, F. & Vilardi, J. C. Comparison of mating performance of medfly (diptera, tephritidae Diptera, Tephritidae) genetic sexing and wild type strains: field cage and video recording experiments. Florida Entomologist 85, 41–50 (2002).
Schetelig, M. F., Milano, A., Saccone, G. & Handler, A. M. Male only progeny in Anastrepha suspensa by rnai-induced sex reversion of chromosomal females. Insect Biochemistry and Molecular Biology 42, 51–57 (2012).
Benton, R. On the origin of smell: odorant receptors in insects. Cell.Mol.Life Sci 63, 1579–1585 (2006).
Matsuo, T., Sugaya, S., Yasukawa, J., Aigaki, T. & Fuyama, Y. Odorant-binding proteins OBP57d and OBP57e affect taste perception and host-plant preference in Drosophila sechellia. PLoS Biol 5, e118 (2007).
Pelletier, J., Guidolin, A., Syed, Z., Cornel, A. J. & Leal, W. Knockdown of a mosquito odorant-binding protein involved in the sensitive detection of oviposition attractants. J Chem Ecol 36, 245–248 (2010).
Leal, W. S. Odorant reception in insects: roles of receptors, binding proteins and degrading enzymes. Annual Review of Entomology 58, 373–391 (2013).
Yi, X., Zhao, H., Wang, P., Hu, M. & Zhong, G. Bdor/orco is important for oviposition-deterring behavior induced by both the volatile and non-volatile repellents in Bactrocera dorsalis (Diptera: Tephritidae). Journal of Insect Physiology 65, 51–56 (2014).
Zhou, S., Stone, E. A., Mackay, T. F. C. & Anholt, R. R. H. Plasticity of the chemoreceptor repertoire in Drosophila melanogaster. PLoS Genet 5, e1000681 (2009).
Vogt, R. G. & Riddiford, L. M. Pheromone binding and inactivation by moth antennae. Nature 293, 161–163 (1981).
Zheng, W. et al. Identification and expression profile analysis of odorant binding proteins in the oriental fruit fly Bactrocera dorsalis. International Journal of Molecular Science 14, 14936–14949 (2103).
Siciliano, P. et al. Sniffing out chemosensory genes from the mediterranean fruit fly. Ceratitis capitata. PLoS ONE 9, e85523 (2014).
Bohbot, J. & Vogt, R. G. Antennal expressed genes of the yellow fever mosquito (Aedes aegypti l.); characterization of odorant-binding protein 10 and takeout. Insect Biochemistry and Molecular Biology 35, 961–979 (2005).
Leal, W. S. et al. Reverse and conventional chemical ecology approaches for the development of oviposition attractants for culex mosquitoes. PLoS ONE 3, e3045 (2008).
Lagarde, A. et al. The crystal structure of odorant binding protein 7 from Anopheles gambiae exhibits an outstanding adaptability of its binding site. Journal of Molecular Biology 414, 401–412 (2011).
Tsitsanou, K. E. et al. Anopheles gambiae odorant binding protein crystal complex with the synthetic repellent DEET: implications for structure-based design of novel mosquito repellents. Cell.Mol.Life Sci. 69, 283–297 (2012).
Bustin, S. A. et al. The miqe guidelines: Minimum information for publication of quantitative real-time pcr experiments. Clinical Chemistry 55, 611–622 (2009).
Li, S. et al. Multiple functions of an odorant-binding protein in the mosquito Aedes aegypti. Biochemical and Biophysical Research Communications 372, 464–468 (2008).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta ct) method. Method. Methods 25, 402–408 (2001).
Radonic, A. et al. Guideline to reference gene selection for quantitative real-time pcr. Biochemical and Biophysical Research Communications 313, 856–862 (2004).
Chen, D., Pan, X., Xiao, P., Farwell, M. A. & Zhang, B. Evaluation and identification of reliable reference genes for pharmacogenomics, toxicogenomics and small rna expression analysis. Journal of Cellular Physiology 226, 2469–2477 (2011).
Mamidala, P., Rajarapu, S. P., Jones, S. C. & Mittapalli, O. Identification and validation of reference genes for quantitative real-time polymerase chain reaction in Cimex lectularius. Journal of Medical Entomology 48, 947–951 (2011).
Shi, X. Q. et al. Validation of reference genes for expression analysis by quantitative real-time pcr in Leptinotarsa decemlineata (say). BMC Research Notes 6, 93 (2013).
Cook, P. E. et al. The use of transcriptional profiles to predict adult mosquito age under field conditions. Proceedings of the National Academy of Sciences 103, 18060–18065 (2006).
Hugo, L. E. et al. Field validation of a transcriptional assay for the prediction of age of uncaged Aedes aegypti mosquitoes in northern australia. PLoS Negl Trop Dis 4, e608 (2010).
Shin, D., Jin, L., Lobo, N. F. & Severson, D. W. Transcript profiling of the meiotic drive phenotype in testis of Aedes aegypti using suppressive subtractive hybridization. Journal of Insect Physiology 57, 1220–1226 (2011).
Coggins, S. A., Estévez-Lao, T. Y. & Hillyer, J. F. Increased survivorship following bacterial infection by the mosquito Aedes aegypti as compared to Anopheles gambiae correlates with increased transcriptional induction of antimicrobial peptides. Developmental and Comparative Immunology 37, 390–401 (2012).
Cardoso, G. A., Matioli, C. C., Azeredo-Espin, A. M. L. & Torres, T. T. Selection and validation of reference genes for functional studies in the Calliphoridae family. Journal of Insect Science 14, 1–15 (2014).
Hornáková, D., Matoušková, P., Jirí, K., Valterová, I. & Pichová, I. Selection of reference genes for real-time polymerase chain reaction analysis in tissues from Bombus terrestris and Bombus lucorum of different ages. Analytical Biochemistry 397, 118–120 (2010).
Shen, G. M., Jiang, H. B., Wang, X. N. & Wang, J. J. Evaluation of endogenous references for gene expression profiling in different tissues of the oriental fruit fly Bactrocera dorsalis (Diptera: Tephritidae). BMC Molecular Biology 11, 76 (2010).
Ling, D. & Salvaterra, P. M. Robust RT-qPCR data normalization: validation and selection of internal reference genes during post-experimental data analysis. PLoS ONE 6, e17762 (2011).
Ponton, F., Chapuis, M. P., Pernice, M., Sword, G. A. & Simpson, S. J. Evaluation of potential reference genes for reverse transcription-qPCR studies of physiological responses in Drosophila melanogaster. Journal of Insect Physiology 57, 840–850 (2011).
Arya, A. L. & Weber, G. H. et al. Natural variation, functional pleiotropy and transcriptional contexts of odorant binding protein genes in Drosophila melanogaster. Genetics 186, 1475–1485 (2010).
Wolfner, M. F. Tokens of love: Functions and regulation of Drosophila male accessory gland products. Insect Biochemistry and Molecular Biology 27, 179–192 (1997).
Tram, U. & Wolfner, M. F. Seminal fluid regulation of female sexual attractiveness in Drosophila melanogaster. Proceedings of the National Academy of Sciences 95, 4051–4054 (1998).
McGraw, L. A., Gibson, G., Clark, A. G. & Wolfner, M. F. Genes regulated by mating, sperm, or seminal proteins in mated female Drosophila melanogaster. Current Biology 14, 1509–1514 (2004).
McKean, K. A. & Nunney, L. Sexual selection and immune function in Drosophila melanogaster. Evolution 62, 386–400 (2008).
DiBenedetto, A. J., Harada, H. A. & Wolfner, M. F. Structure, cell-specific expression and mating-induced regulation of a Drosophila melanogaster male accessory gland gene. Developmental Biology 139, 134–148 (1990).
Jang, E. B. Effects of mating and accessory gland injections on olfactory-mediated behavior in the female Mediterranean fruit fly, Ceratitis capitata Journal of Insect Physiology 41, 705–710 (1995).
Menuz, K., Larter, N. K., Park, J. & Carlson, J. R. An rna-seq screen of the Drosophila antenna identifies a transporter necessary for ammonia detection. PLOS Genetics 10, e1004810 (2014).
Wang, P., Lyman, R. F., Shabalina, S. A., Mackay, T. F. C. & Anholt, R. R. H. Association of polymorphisms in odorantbinding protein genes with variation in olfactory response to benzaldehyde in Drosophila. Genetics 177, 1655–1665 (2007).
Wang, P., Lyman, R. F., Mackay, T. F. C. & Anholt, R. R. H. Natural variation in odorant recognition among odorantbinding proteins in Drosophila melanogaster. Genetics 184, 759–767 (2010).
Rodríguez, A., Alquézar, B. & Peña, L. Fruit aromas in mature fleshy fruits as signals of readiness for predation and seed dispersal. New Phytologist 197, 36–48 (2013).
Deng, J. Y., Wei, H. Y., Huang, H. P. & Du, J. W. Enhancement of attraction to sex pheromones of Spodoptera exigua by volatile compounds produced by host plant. Journal of Chemical Ecology 30, 2037–2045 (2004).
Swarup, S., Williams, T. I. & Anholt, R. R. H. Functional dissection of odorant binding protein genes in Drosophila melanogaster. Genes, Brain and Behavior 10, 648–657 (2011).
DaCruz, I. B. M., Nascimento, J. C., Taufer, M. & Oliveira, A. K. in. Moscas-das-frutas de importância econômica no Brasil: conhecimento básico e aplicado. Holos Editora 6, 55–66.
Chomczynski, P. & Mackey, K. Modification of the tri reagent procedure for isolation of RNA from polysaccharide- and proteoglycan-rich sources. Biotechniques 19, 942–945 (1995).
Bagnall, N. & Kotze, A. Evaluation of reference genes for real-time PCR quantification of gene expression in the Australian sheep blowfly. Lucilia cuprina. Medical and Veterinary Entomology 24, 176–181 (2010).
Majerowicz, D. et al. Looking for reference genes for real- time quantitative PCR experiments in Rhodnius prolixus (hemiptera: Reduviidae). Insect Molecular Biology 20, 713–722 (2011).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. EdgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 25, 139–140 (2010).
Katoh, K. & Standley, D. M. Mafft multiple sequence alignment software version 7: improvements in performance and usability. molecular biology and evolution. Molecular Biology and Evolution 30, 772–780 (2013).
Andersen, C. L., Jensen, J. L. & Orntoft, T. F. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Research 64, 5245–5250 (2004).
Pfaffl, M. W., Tichopad, A., Prgomet, C. & Neuvians, T. P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: Bestkeeper – excel-based tool using pair-wise correlations. Biotechnology 26, 509–515 (2004).
Vandesompele, J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology 3, research0034–research0034.0011 (2002).
Silver, N., Best, S., Jiang, J. & Thein, S. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Molecular Biology 7, 33 (2006).
Ovesná, J. et al. Validation of the b-amy1 transcription profiling assay and selection of reference genes suited for a RT-qPCR assay in developing barley caryopsis. PLoS ONE 7, e41886 (2012).
Chao, W. S., Dogramaci, M., Foley, M. E., Horvath, D. P. & Anderson, J. V. Selection and validation of endogenous reference genes for qRT-PCR analysis in leafy spurge (Euphorbia esula). PLoS ONE 7, e42839 (2012).
Jacob, F. et al. Careful selection of reference genes is required for reliable performance of RT-qPCR in human normal and cancer cell lines. PLoS ONE 8, e59180 (2013).
Cheng, D., Zhang, Z., He, X. & Liang, G. Validation of reference genes in Solenopsis invicta in different developmental stages, castes and tissues. PLoS ONE 8, e57718 (2013).
Wang, Y. et al. Identification of endogenous reference genes for RT-qPCR analysis of plasma micrornas levels in rats with acetaminophen-induced hepatotoxicity. Journal of Applied Toxicology 33, 1330–1336 (2013).
Acknowledgements
This project was supported by generous grants and fellowships from the Brazilian agencies CNPq (Conselho Nacional de Pesquisa e Desenvolvimento Científico), FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo), particularly FAPESP grant 2010/20455-4 and CAPES (Coordenacão de Aperfeiçoamento de Pessoal de Nível Superior), especially CAPES PVE 056/2013.
Author information
Authors and Affiliations
Contributions
A.M.N., S.C.E., A.L.A.L., C.H.T., I.S.J., F.R.T. and R.A.B. planned the experiments. A.M.N., S.C.E., C.H.T. and F.R.T. performed the experiments. A.M.N., S.C.E., F.R.T. and R.A.B. analysed the results and prepared the manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Nakamura, A., Chahad-Ehlers, S., Lima, A. et al. Reference genes for accessing differential expression among developmental stages and analysis of differential expression of OBP genes in Anastrepha obliqua. Sci Rep 6, 17480 (2016). https://doi.org/10.1038/srep17480
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep17480
This article is cited by
-
Selection of species specific panel of reference genes in peripheral blood mononuclear cells of native livestock species adapted to trans-Himalayan region of Leh-Ladakh
Scientific Reports (2022)
-
Transcriptome analysis of Anastrepha fraterculus sp. 1 males, females, and embryos: insights into development, courtship, and reproduction
BMC Genetics (2020)
-
mRNA extraction of Xanthomonas fragariae in strawberry and validation of reference genes for the RT-qPCR for study of bacterial gene expression
Molecular Biology Reports (2019)
-
Identification of Reference Genes for Normalizing Quantitative Real-Time PCR in Urechis unicinctus
Journal of Ocean University of China (2018)
-
Odorant-binding proteins expression patterns in recently diverged species of Anastrepha fruit flies
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.