Abstract
The morphological plasticity and community responses of algae competing with corals have not been assessed. We evaluated eight morphological characters of four species of stoloniferous clonal filamentous turf algae (FTA), including Lophosiphonia cristata (Lc) and Polysiphonia scopulorum var. villum (Psv) and the composition and number of turf algae (TA) in competition for space with the coral Orbicella spp. under experimental and non-manipulated conditions. All FTA exhibited morphological responses, such as increasing the formation of new ramets (except for Psv when competing with O. faveolata). Opposite responses in the space between erect axes were found when Psv competed with O. faveolata and when Lc competed with O. annularis. The characters modified by each FTA species and the number and composition of TA species growing next to coral tissue differed from that of the TA growing at ≥3 cm. The specific and community responses indicate that some species of TA can actively colonise coral tissue and that fundamental competitive interactions between the two types of organisms occur within the first millimetres of the coral−algal boundary. These findings suggest that the morphological plasticity, high number and functional redundancy of stoloniferous TA species favour their colonisation of coral tissue and resistance against coral invasion.
Similar content being viewed by others
Introduction
Coral reefs are being degraded around the world1 and those of the Caribbean are among the most affected2. Turf algae (TA) and macroalgae are significant assemblages on degraded reefs and the greater interaction between corals and algae under these conditions is part of a negative feedback mechanism that restricts reef recovery3; however, the direct involvement of algae in coral damage and coral mortality and the potential mechanisms used by algae to invade the coral tissue remain uncertain4. In general, it is assumed that corals must be injured, bleached, stressed, or dead before algae can colonise them5,6,7.
The relatively passive role that algae are assumed to play in colonising corals contrasts with their high morphological plasticity in biological interactions. Algal morphology is modified as a response to physical, chemical and biological factors8,9,10,11,12. It has also been suggested that algal phenotypic plasticity may be a key mechanism involved in the occupation of different ecological niches13 and in the invasion of species14, as observed in terrestrial clonal plants. For example, vascular clonal plants show morphological changes in their search for resources in spatially heterogeneous environments15,16 during fertilisation17, in response to disturbances (conferring a survival advantage)18 and in competitive interactions19. Fordyce20 concluded that ecological interactions mediated by phenotypic plasticity are common in nature and the results and the intensity of those interactions are mediated by the morphological responses of the interacting organisms.
TA constitute the most abundant algal assemblage on Caribbean reefs, frequently growing in contact with the tissue of Orbicella spp. (Orbicella = Montastraea annularis species complex)21, one of the most important reef-building coral genera in the Caribbean22. When these two organisms are in contact, TA stress, compete with and overgrow Orbicella spp.23,24. Typical or stoloniferous clonal algal species (attached to the substratum at several points through rhizoids from a prostrate axis, along which short and branched erect axes rise25,26) are commonly found within the TA assemblage.
The aim of this study was to determine the morphological plasticity and changes in the percentage of genets undergoing reproduction of four species of stoloniferous clonal filamentous turf algae (FTA): two under experimental conditions (reciprocal transplantation of healthy and dead coral cores) and three (including one of the first two) under non-manipulated conditions (growing in three dead coral zones close to live coral tissue). In this study, a genet is defined as a fragment consisting of a prostrate axis with several erect axes and a ramet as an erect axis that adopts the morphology of a prostrate axis with additional erect axes and rhizoids. The community of TA competing with Orbicella spp. was also evaluated for species composition, species number and percentage of species reproducing. Our results show that TA competing with the coral tissue of Orbicella spp. respond at both the species and community levels and these changes suggest that adaptive algal plasticity in morphology and community responses may impede coral defences against algal colonisation, that not all TA species are adapted to compete successfully for space with Orbicella spp. and that important coral−algal interactions occur within 0.5 cm of the coral−algal boundary.
Results
1) Turf algal species richness under non-manipulated and manipulated conditions
a) Non-manipulated conditions
The assemblages of TA growing in three zones in relation to O. annularis (the primary front, PF: the first 0.5 cm next to O. annularis tissue; the secondary front, SF: a 1-cm-wide band located 3–4 cm from coral tissue; and the rearguard zone, RE: ≥30 cm from the periphery of coral tissue) were composed of 96 taxa, with rhodophytes being the most abundant (55 taxa (57%)) than the other groups of autotrophs (18 Chlorophyta (19%), 10 Phaeophyceae (10%) and 13 Cyanobacteria (14%)) (see Supplementary Table S1).
b) Experimental conditions
The assemblages of TA growing on transplanted Orbicella faveolata cores were composed of 86 taxa, with rhodophytes being more abundant (51 taxa (59%)) than the other groups of autotrophs (15 Chlorophyta (18%), 14 Phaeophyceae (16%) and 6 Cyanobacteria (7%)) (see Supplementary Table S4).
2) Response of turf algal communities under non-manipulated and manipulated conditions.
a) Non-manipulated conditions:
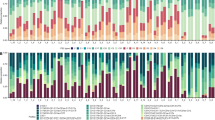
Composition of turf algal communities, number of species and percentage of species undergoing reproduction. The general composition of TA species in the PF differed from that in the SF and RE (pseudo-F2,27 = 1.98, p < 0.05). Five taxa (3 Rhodophyta, 1 Chlorophyta and 1 Cyanobacteria) contributed the most to the differences between zones (SIMPER): Anotrichium tenue was more frequent, whereas Taenioma nanum, Bryobesia johannae, and Lyngbya sordida were less frequent in the PF than in the SF and RE. Champia parvula var. prostrata was absent in samples from the PF but was a representative species of the SF and RE. Some species were found exclusively in one of the three zones: 6 in PF, 8 in SF and 19 in RE (see Supplementary Table S1).
The average number of TA species was lower in the PF (20) than in the RE (30) (F2,27 = 11.35, p < 0.05). The percentages of species with reproductive structures were similar in all three zones (F2,27 = 0.05, p > 0.05).
b) Experimental conditions: Composition of turf algal communities and number of species
The TA composition differed between treatments (pseudo-F5,59 = 1.84, p < 0.05) and dates (pseudo-F1,59 = 5.61, p < 0.05), but no interaction between factors was found (pseudo-F5,59 = 0.85, p < 0.05); particularly, the composition of TA in contact with coral tissue (Treatment (T) 5) was different from all the other treatments (Fig. 1). According to SIMPER, three taxa (2 Rhodophyta and 1 Cyanobacteria) contributed the most to the differences between treatments: Centroceras clavulatum were more frequent, whereas Dichothrix penicillata and Polysiphonia havanensis were less frequent in T5 than in the other treatments.
The average number of TA species did not differ between the treatments (F5,70 = 1.73, p > 0.05) and dates (F1,70 = 0.48, p > 0.05) and no interaction between factors was found (F5,70 = 0.96, p > 0.05). Algal reproduction was virtually absent. Sexual and asexual reproductive structures were present in only 21 of 1504 specimens analysed (0.46% and 0.93%, respectively); in particular, reproductive structures were absent from the T5 thalli.
3) Response of clonal algae under non-manipulated and manipulated conditions
a) Non-manipulated conditions: Plasticity and reproduction of Herposiphonia bipinnata, Lophosiphonia cristata, and Polysiphonia scopulorum var. villum
The three FTA evaluated under non-manipulated conditions showed differences among the zones (see Supplementary Tables S2 and S3). In the PF, the genets exhibited a shorter SEA and HEA than those from the SF and RE, but a greater DPA, DR and FNR (Fig. 2, Table 1). The genets observed in both the PF and SF differed from those in the RE in presenting a greater length of the pericentral cells (LPC) of the prostrate axis and length of rhizoids (LR). SR was not affected by the zone in any species (see Supplementary Tables S2 and S3).
Genet of a ‘typical’ clonal alga, indicating the eight morphological characters evaluated in this study.
(a) Genet of the red alga Lophosiphonia cristata, indicating the distance (“spacer”) between erect axes (SEA), height of the erect axis (HEA) and the formation of new ramets (FNR); (b) an enlargement of (a) indicating the diameter of the prostrate axis (DPA), length of pericentral cells of the prostrate axis (LPC), distance (“spacer”) between rhizoids (SR), length of rhizoids (LR) and diameter of rhizoids (DR). Photo credit: H. Bahena-Basave.
The FNR in the three algal species was greater in the PF than in the SF and RE. In L. cristata, the FNR was greater in the PF than in SF. In both L. cristata and P. scopulorum var. villum, the LPC was greater in the PF and SF than in the RE. Regarding the other measurements (SEA, HEA, DPA, DR and LR), only one species (L. cristata or P. scopulorum var. villum) showed differences between the zones (see Supplementary Tables S2 and S3). Lophosiphonia cristata exhibited the most characters modified by the zones (SEA, DPA, LPC, DR, FNR), followed by H. bipinnata (HEA, LR, FNR) and Polysiphonia scopulorum var. villum (LPC, FNR).
The presence of reproductive structures was similar in all zones. When the algae were reproducing, the fragments of the three species showed only asexual tetrasporangia.
b) Experimental conditions: Plasticity and reproduction of Polysiphonia scopulorum var. villum and Parviphycus trinitatensis
The two clonal species evaluated under the experimental conditions displayed morphological differences between the treatments in direct contact with coral tissue (T1 and T5) and close to it (T6) in comparison with the other treatments (although the differences among treatments was not always well defined) and between dates (see Supplementary Tables S5 and S6). Thus, P. scopulorum var. villum exhibited a greater distance (“spacer”) between erect axes (SEA), whereas P. trinitatensis exhibited more formation of new ramets (FNR) when thalli were in contact with coral tissue than when they were surrounded by TA (Fig. 2, Table 1). In both species, the diameter of the prostrate axis (DPA) was greater when they were in contact with coral tissue than in contact with TA, whereas the height of erect axes (HEA) was similar between the treatments (see Supplementary Tables S5 and S6). In May, both species presented greater FNR, whereas P. scopulorum var. villum presented a greater SEA and DPA and P. trinitatensis presented a greater HEA and shorter distance (“spacer”) between rhizoids (SR) than in August (see Supplementary Tables S5 and S6). Four significant interactions were registered: for SR and FNR in P. scopulorum var. villum and for SEA and DPA in P. trinitatensis (see Supplementary Tables S5 and S6). Four characters were modified by P. scopulorum var. villum and two by P. trinitatensis between both the different treatments and dates (see Supplementary Tables S5 and S6).
Sexual and asexual reproductive structures developed in P. scopulorum var. villum in 2 of the 14 replicated cores in T3, but not in any of the P. trinitatensis plants in T1–T6.
Discussion
The four stoloniferous clonal algal species in competition for space with Orbicella spp. showed morphological changes under both experimental and non-manipulated conditions. These morphological responses have been observed in other clonal algal species when exposed to growth constraints imposed by water movements, substratum, depth, light and nutrient availability and invasion of new areas (e.g.,12,25,27,28). The morphological changes of the four clonal algae occurred when they were in contact with or close to the tissue of Orbicella spp., in comparison with the genets growing a few centimetres away from the coral−algal boundary. Similarly, morphological responses between the genets of neighbouring algae have been reported when shoots (modules) compete intraspecifically29,30. However, to our knowledge, this is the first report of morphological responses of clonal algae when they are in competition at the interspecific level.
Five (SEA, HEA, DPA, DR and FNR) of the eight morphological characters evaluated under experimental and non-manipulated conditions were differentially modified among the four FTA species, depending on the coral species involved. For example, P. scopulorum var. villum increased SEA and DPA when competing with O. faveolata but increased FNR when competing with O. annularis; the two algal species competing with O. faveolata increased DPA, but none of the three species competing with O. annularis did; opposite responses were found when P. scopulorum var. villum, with longer SEA, competed with O. faveolata and when L. cristata, with shorter SEA, competed with O. annularis. This relative lack of a pattern in the morphological responses of algae in competition with O. annularis is similar to the morphological responses observed in clonal vascular plants when searching for resources, where the responses have been shown to differ among species, be absent or be the opposite of the expected responses17,19,31. The FNR exhibited the most regular morphological response, found in the four clonal red algae (but not P. scopulorum var. villum competing with O. faveolata), being greater in genets in contact with coral tissue than in those away from the coral−algal boundary. Similarly, it has been noted that the ramification of the stolon or rhizome of vascular plants (equivalent to the FNR here) is the most consistently modified character within the context of the plasticity of clonal growth forms19,32.
Increased ramification of the rhizomes of vascular plants maximises occupation and dispersion through the substratum33,34. In addition, a greater number of branches or ramets is a characteristic of plants presenting the phalanx growth form (with short spacers and dense and branched ramets) in comparison with plants presenting the guerrilla growth form (with longer spacers and less spread and branched ramets) and has been proposed as a strategy for excluding competitors and as a better adaptation for stressful conditions, in addition to being useful for resisting the invasion of other plants15,26,35. An increase in the FNR is also observed when algae occur in stressful environmental conditions, such as the charophyte Chara aspera exhibiting greater branching under high salinities36 and the brown alga Fucus radicans exhibiting an increased FNR (adventitious branches) under low salinities37. Additionally, the projection of prostrate axes to colonise corals (Fig. 3a), as can occur during the FNR, might indirectly initiate shading of coral tissue by TA23 at a microscale due to the formation of loose cushions (Fig. 3b). Based on the above information, the increase in the FNR in the four clonal algae would favour their colonisation of coral tissue as well as resistance to coral invasion and persistence under the stress caused by coral tissue but also a response of TA to the stress caused by corals. Corals might stress algae through their defence and attack mechanisms against competitors, such as the extrusion of mesenteric filaments accompanied by the discharge of nematocysts, swept by tentacles and polyps, growing over the competitor, mucus secretion and allelopathy5,38,39, as partially demonstrated by Nugues et al.40. In turn, some algae are allopathic to corals41. Therefore, both algae and corals exhibit active mechanisms for colonising each other as well as morphological and chemical mechanisms to protect themselves from such colonisation, although the responses between algae and corals depend on the species involved40.
Interactions between the coral Orbicella annularis and turf algae containing sediment.
(a) Filamentous turf algae projecting into coral tissue; note the sediment trapped by some algal axes and in the mat. (b) Loose cushions of dense turf algae (with deposited sediment) shading the coral tissue beneath. Photo credit: J. Espinoza-Avalos.
We suggest that relatively few algal species are well adapted to remain next to coral tissue and compete for space with Orbicella spp.; other algae show better performance in microenvironments away from contact with coral. This suggestion is based on the lower number of both total species and exclusive species registered in the PF compared to those found in the other zones under non-manipulated conditions and the differential composition of algal species in contact with or close to coral tissue vs. those not in contact with coral under both experimental and non-manipulated conditions. Nevertheless, the relatively low number of species in the PF represents a relatively high richness within a small band (0.5 cm) next to coral tissue, with many species presenting a clonal growth pattern. According to the clonal growth types differentiated by Collado-Vides25 and the thallus growth forms indicated through descriptions, diagrams and photographs by several authors (e.g.,42,43,44,45), we conservatively considered that 38 of the 53 species found in the PF under non-manipulated conditions and that 38 of the 65 species found in T1, T5 and T6 under experimental conditions were clonal algae. Of the clonal species registered under both conditions, 22 presented the same clonal growth type (stoloniferous) as the four algae in which we assessed morphological plasticity (see Supplementary Table S1). It is likely that some of those 22 stoloniferous clonal algae also present a diversity of responses in their morphological characters, including a greater FNR.
Turf algal assemblages with a relatively high richness, including groups of clonal algae with matching morphologies and phenotypic responses (showing functional redundancy, especially in the FNR), can promote the resilience of TA assemblages that compete with Orbicella, hindering the coral from re-occupying the space lost by the corals or gained by TA, due to complementarity in the competitive skills of clonal algae. Similarly, relatively diverse communities of vascular plants resist or reduce the successful invasion of exotic species46,47,48 and among seaweeds, the presence of different assemblages leads to resistance of invasion by the brown alga Sargassum muticum49. Additionally, sessile marine invertebrate communities with a richness of as low as four species can reduce the success of the invasion of space by competitors50. Naeem et al.51 observed a reduction in the success of invasive plants when using polycrops of up to 25 species, which is a low number in comparison with the species richness (ranging from 53 to 65) of the algal assemblages found in the vicinity of Orbicella tissue. Functional redundancy, or the capacity of a species to functionally compensate for the loss of another species with the same geometry and to occupy the same space or to be located at a short distance52,53,54, is important for maintaining resiliency52 and represents a form of biological insurance against the loss of any other redundant species50. For example, some plankton communities are resilient to disturbances due to the replacement of species that show similar performance in ecological functions55. Hence, the functional redundancy of clonal TA would allow them to be resilient when growing close to coral tissue, resisting potential attacks from the coral as a group, while the TA would simultaneously continue to exert their stressing effects on Orbicella spp.23,24.
In summary, algal assemblages responded at the species and community levels to competition for space with the coral Orbicella spp. and the responses of the algae in contact with or close to the coral tissue were significantly different from those growing a few centimetres away from the coral−algal boundary. The TA growing next to the tissue of Orbicella spp. displayed high responses in morphological characters of their genets as well as the same growth form (phalanx), relatively high species richness and species with functional redundancy. We suggest that all of these specific and community responses favour the colonisation of coral tissue by the algae, allowing resistance to invasion by the coral and persistence of algal assemblages under the stress caused by coral tissue, thus providing stability to algal assemblages competing with coral and hindering corals from re-occupying the space lost by the corals or gained by TA. The different composition of the algal assemblage of the PF, generally involving fewer total species and few exclusive species, suggests that species of TA are not homogeneously adapted to withstand potential attacks from coral and to compete successfully for space with Orbicella spp. Our results also suggest that fundamental active interactions between TA and Orbicella spp. occur over a distance of less than 1 cm between the two types of organisms and that a few centimetres away from that interface, the coral skeleton may simply serve as another hard substrate that can be colonised by additional species of algae that are not necessarily adapted to compete with corals. The high abundance of clonal algae bordering coral tissue gives them an additional competitive advantage because when ramets are lost through physical disturbances or herbivory, unaffected genets will rapidly re-occupy the space through vegetative propagation56. In addition, remnants or prostrate axes left behind after disturbances will regenerate erect axes8; for example, some TA species surrounding O. annularis regenerate erect axes from scarcely visible remnants of prostate axes left after the algae are manually scraped from the coral skeleton. To our knowledge, this is the first assessment of the morphological and community responses of TA in competition for space with corals. Our results suggest that these responses may favour the colonisation of coral tissue and the permanence of TA mats around the periphery of the coral. This small-scale study in the coral−TA interaction boundary revealed important, previously unnoticed information.
Methods
Study area
The study was carried out at Xcalak (18°15′41.6”N, 87°49′30”W) and Xahuayxol (18°30′11.9”N, 87°45′24.8”W), in the southern part of Quintana Roo, Mexican Caribbean (Fig. 4). Both sites are located in reef lagoons (approximately 1.5 and 2 m deep, respectively) near the breaker zone, within a Marine Protected Area (National Park “Arrecifes de Xcalak”). The reef lagoon at both study sites has a predominantly sandy bottom with seagrass beds of Thalassia testudinum and Syringodium filiforme, macroalgae, Alcyonacea corals and patches or aggregations of stony corals57. The Xcalak and Xahuayxol fringing reefs have similar average annual sea surface temperatures and salinities (27.80 °C and 27.81 ºC and 35.79 and 35.78, respectively see58), low coral cover (8.7% and 11.7%, respectively59) in fore reefs (~10 m) and high algal cover (41%60 and 58%61, respectively). Orbicella is the most abundant coral genus (50% of relative abundance), followed by Diploria (12%), Siderastrea (11%), Porites (9%), Agaricia (5%), Montastraea (4%), Colpophyllia (4%) and Acropora (3%)58,62. The coral species used for the experimental conditions (in Xcalak) and non-manipulated conditions (in Xahuayxol) were selected to better accomplish each objective of the study. At Xcalak, the morphological shape of the abundant coral Orbicella faveolata (frequently with relatively flat surfaces and large size, 2–3 m in diameter) facilitated the extraction of coral cores and to the reciprocal transplantations of live and dead (covered by TA) coral cores. At Xahuayxol, the dominant coral O. annularis, with individual lobules or ramets interacting with TA in a short perimeter of coral tissue (diameter ≥ 14 cm63), allowed us to collect TA from the whole periphery of ramets. The relative abundance of benthic algae at the Xcalak and Xahuayxol fore reefs was highest for turf algae (51% and 53%, respectively) in comparison to coralline algae and macroalgae (32% and 29% and 16% and 18%, respectively59). The assemblages of TA at Xahuayxol were approximately 8 mm in height and included abundant sediment with grains of less than 0.3 mm. The assemblages at both sites were mainly composed of Polysiphonia spp., Lophosiphonia cristata, Parviphycus trinitatensis, Herposiphonia spp., Centroceras clavulatum, Amphiroa fragilissima, Jania spp., Ceramium spp., Padina sp., Lyngbya spp. and Dichothrix spp., while Bryobesia johannae and Anotrichium tenue were common at Xahuayxol but not at Xcalak and Sphacelaria sp. was common at Xcalak but not at Xahuayxol24,64.
Sampling design and methods
Experimental conditions
Coral cores used as transplants and controls.
In order to evaluate the potential responses i) in morphological plasticity of the most abundant clonal filamentous algae (Polysiphonia scopulorum var. villum and Parviphycus trinitatensis) and ii) in the composition of turf algal (TA) communities towards the presence of the coral O. faveolata (e.g., competition for space), two different types of coral cores were reciprocally transplanted: i) cores covered with coral tissue (COCO) and ii) coral skeleton cores covered with TA (COTA). Both were transplanted to hosting coral colonies; controls were COTA left intact during the experiment (Fig. 5). The cores measured 5 cm in diameter (16.8 cm2) and approximately 2 cm in depth and were obtained with a pneumatic drill. The cores were cemented with marine epoxy in a hole previously made with the pneumatic drill in the coral colony hosting the transplants. Each core was identified with a steel rectangle (15 × 55 mm, marked with letters and numbers) nailed to a dead portion of the hosting colony. The top part of the implanted cores and the external surface of the hosting colony were accommodated at a similar level see23.
Graphic representation of the six treatments carried out on the coral Orbicella faveolata under the experimental conditions.
The experimental design includes healthy (  ) and dead coral colonies (
) and dead coral colonies (  ), healthy (
), healthy ( ) and dead (
) and dead ( ) transplanted coral cores covered by turf algae (TA) and control cores (
) transplanted coral cores covered by turf algae (TA) and control cores ( ). Treatments: T1 = Algae to coral transplant; T2, T3 and T4 = control for T1; T5 = coral to algae transplant; and T6 = control for T5. COCO = cores covered with coral tissue; COTA = coral skeleton cores covered with TA. See text for details.
). Treatments: T1 = Algae to coral transplant; T2, T3 and T4 = control for T1; T5 = coral to algae transplant; and T6 = control for T5. COCO = cores covered with coral tissue; COTA = coral skeleton cores covered with TA. See text for details.
Algae to coral transplantation.
COTA growing on dead corals were transplanted into healthy O. faveolata colonies (treatment 1 (T1) in Fig. 5). Three controls were carried out for these transplants. The first consisted of COTA growing on dead corals transplanted into dead corals covered with algae (Fig. 5, T2), to test whether the biological parameters and species composition of TA were affected by the transplant manipulation. The second control consisted of TA growing on dead corals collected next to the cores used as the first control (Fig. 5, T3), to test whether algae covering dead coral colonies were affected by receiving the transplanted coral cores. The third control consisted of COTA obtained from non-manipulated TA growing on dead corals (Fig. 5, T4).
Coral to algae transplantation.
COCO were transplanted into dead coral colonies covered by TA (Fig. 5, T5). The controls for this treatment consisted of TA growing on dead coral collected next to the coral transplants (Fig. 5, T6). They were used to test whether TA growing on dead coral were affected by receiving the healthy COCO.
Before initiating the experiment, we placed 42 cores to be used in T1, T2 and T5 (n = 7 cores per treatment and date) inside the upper releasable part of two-part PVC tubes. The lower part of the tubes was cemented into a 50 × 42 × 10 cm concrete block. The cores in the tubes were left next to the experimental coral colonies for 3 weeks to allow regeneration of coral and algal tissue at their damaged edges. At the end of the regeneration time, 36 cores (n = 6 cores per treatment and date) were selected for the experiment, based on the best-cut skeleton cores and the presence of live coral or algae in all the periphery of cores.
The T1, T2 and T5 cores were placed under the experimental conditions in November 2003 (month 0) and cores from all treatments were extracted in May (month 6) and August 2004 (month 9). All the extracted cores were preserved in 4% formaldehyde in seawater.
Non-manipulated conditions
On 25 May 2010, TA that had overgrown O. annularis coral tissue ramets (the lobes of a coral colony) were collected at Xahuayxol from three zones at different distances from the O. annularis−algal boundary: from the first 0.5 cm next to O. annularis tissue (primary front, PF); from a 1-cm-wide band located 3–4 cm from the coral tissue (secondary front, SF); and from ≥30 cm away from the periphery of coral tissue (rearguard zone, RE). The TA area sampled was similar in all three zones. Ten samples per zone were collected with the aid of a knife and preserved in vials with 4% formaldehyde in seawater.
Experimental and non-manipulated conditions
Composition of turf algal communities, number of species and percentage of species undergoing reproduction
. The species composition and number of species were recorded after all the individuals/ramets of algae in each sample from both the experimental (cores of 16.8 cm2) and non-manipulated (samples of 4 cm2) conditions had been observed and identified. TA were identified, usually to species level, using common keys42,43,44,45 (with updated nomenclature from Algaebase65) and stereoscopic (magnifications: 0.67–4.5X) and compound (magnifications: 5X, 10X, 40X and 100X) Olympus microscopes. Diverse techniques were employed to identify the algal taxa depending on the specimen (e.g., decalcification, staining, transversal and longitudinal cuts, observations of cell shape and arrangement, cell size measurements (long and wide) and counting the number of pericentral cells and cell layers). Reproductive data were obtained only for the algae from the non-manipulated samples as follows: i) the percentage of species presenting sexual/asexual reproductive structures was obtained by examining all individuals/ramets in each sample; and ii) the presence of sexual/asexual reproductive structures in each of the species Herposiphonia bipinnata, Lophosiphonia cristata and Polysiphonia scopulorum var. villum was obtained by examining ramets in each sample. Overall, 113 slides of 84 algal taxa from the experimental conditions and 180 vials of 90 algal taxa from the non-manipulated conditions were deposited at the CIQR Herbarium at ECOSUR.
Plasticity and reproduction of Herposiphonia bipinnata, Lophosiphonia cristata, Polysiphonia scopulorum var. villum (Ceramiales) and Parviphycus trinitatensis (Gelidiales)
. Four clonal (i.e., algae with fragmented parts of the thallus, ramets, having the potential to reattach to the substratum and continue growing as independent organisms25) Rhodophyta species from two orders (Ceramiales and Gelidiales) growing in contact with the Orbicella spp. tissue were selected for the study, based on their high frequency in the pre-analysed samples and their same growth form (i.e., filamentous stoloniferous algae with prostrate axes from which erect axes emerge). Polysiphonia scopulorum var. villum and P. trinitatensis in contact with O. faveolata were selected to evaluate morphological characters under the experimental conditions. Similarly, Herposiphonia bipinnata, L. cristata, and P. scopulorum var. villum in contact with O. annularis were selected to evaluate the morphology responses and reproduction under the non-manipulated conditions.
In order to evaluate the potential effect of the coral−algal competition on the responses in morphological plasticity of clonal algae, eight morphological characters were evaluated (Fig. 2 and Table 1). Five morphological characters (a−c, e and h) were recorded for P. scopulorum var. villum and P. trinitatensis under the experimental conditions. The results from the experimental part of the study and the experience gained working with small clonal algae allowed us to evaluate eight morphological characters (a−h) for H. bipinnata, L. cristata, and P. scopulorum var. villum (Ceramiales) under the non-experimental conditions.
Under the experimental conditions, six fragments per species in each core (per treatment and date) were selected to evaluate the five morphological characters. A total of 12 average readings (6 samples per treatment and date) were obtained for P. scopulorum var. villum and P. trinitatensis for each morphological character. Under the non-manipulated conditions, five fragments per species from each sample per zone were selected to evaluate all morphological characters. A total of 24 average readings (8 samples per zone) were obtained for H. bipinnata and P. scopulorum var. villum and 30 average readings (10 samples per zone) were obtained for L. cristata for each morphological character. The first two species were absent in two of ten samples from two zones; therefore, the number of samples analysed was equalised to 8. Thirty samples (10 samples per zone) were examined to register the presence/absence of reproductive structures for each species.
Statistical analyses.
Shapiro-Wilk and Levene tests were used to determine normality and homogeneity of variances of the average values of the number of species, the percentage of species with reproductive structures in the TA samples and the morphological characters of the four clonal species. One- and two-way analyses of variance (ANOVA) were performed on the data obtained under the non-manipulated conditions (factor: zone; untransformed data) and experimental conditions (factors: treatment and extraction date; square root-transformed data), respectively. Scheffe and Games-Howell post hoc tests were subsequently applied to the data for the non-manipulated conditions (the last test, only for data without homoscedasticity); Student Newman-Keuls (SNK) post hoc tests were performed for the experimental conditions. The data (presence/absence of species) from the TA samples were analysed by one- and two-way permutational multivariate analysis of variance (PERMANOVA) to evaluate the difference in the specific composition between zones (non-manipulated conditions) and between treatments and dates (experimental conditions), respectively. Analyses were performed based on a Bray-Curtis matrix using Type III (partial) sums of squares and unrestricted permutation of raw data with 999 permutations. A non-metric multi-dimensional (nMDS) plot, using Bray-Curtis similarity, was performed to show the spatial distribution of algal composition in the treatments under experimental conditions. Subsequently, SIMPER analyses were performed to determine which turf species contributed to the differences observed in both non-manipulated and experimental conditions. To evaluate whether the presence/absence of reproductive structures in H. bipinnata, L. cristata and P. scopulorum var. villum depended on the zone of collection in the non-manipulated conditions, Pearson chi-square tests were applied.
Additional Information
How to cite this article: Cetz-Navarro, N. P. et al. Morphological and community changes of turf algae in competition with corals. Sci. Rep. 5, 12814; doi: 10.1038/srep12814 (2015).
References
De’ath, G., Fabricius, K. E., Sweatman, H. & Puotinen, M. The 27–year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl. Acad. Sci. USA 109, 17995–17999 (2012).
Gardner, T. A., Côté, I. M., Gill, J. A., Grant, A. & Watkinson, A. R. Long-term region-wide declines in Caribbean corals. Science 301, 958–960 (2003).
Mumby, P. J. & Steneck, R. S. The resilience of coral reefs and its implications for reef management, in Coral Reefs: An Ecosystem in Transition (eds. Dubinsky, Z. & Stambler, N. ) 509–519 (Springer, Netherlands 2011).
Wangpraseurt, D. et al. In situ oxygen dynamics in coral-algal interactions. PLoS ONE 7, e31192 (2012).
McCook, L. J., Jompa, J. & Diaz-Pulido, G. Competition between corals and algae on coral reefs: a review of evidence and mechanisms. Coral Reefs 19, 400–417 (2001).
Aronson, R. B. & Precht, W. F. Conservation, precaution and Caribbean reefs. Coral Reefs 25, 441–450 (2006).
Birrell, C. L., McCook, L. J., Willis, B. L. & Diaz-Pulido, G. A. Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. Oceanogr. Mar. Biol.: Annu. Rev. 46, 25–63 (2008).
Hay, M. E. The functional morphology of turf-forming seaweeds: persistence in stressful marine habitats. Ecology 62, 739–750 (1981).
Lewis, S. M., Norris, J. N. & Searles, R. B. The regulation of morphological plasticity in tropical reef algae by herbivory. Ecology 68, 636–641 (1987).
Yñiguez, A., McManus, J. & Collado-Vides, L. Capturing the dynamics in benthic structures: environmental effects on morphology in the macroalgal genera Halimeda and Dictyota. Mar. Ecol. Progr. Ser. 411, 17–32 (2010).
Charrier, B., Le Bail, A. & de Reviers, B. Plant Proteus: brown algal morphological plasticity and underlying developmental mechanisms. Trends Plant Sci. 17, 468–477 (2012).
Fernández-García, C., Cortés, J., Alvarado, J. J. & Nivia-Ruiz, J. Physical factors contributing to the benthic dominance of the alga Caulerpa sertularioides (Caulerpaceae, Chlorophyta) in the upwelling Bahía Culebra, north Pacific of Costa Rica. Rev. Biol. Trop. 60, 93–107 (2012).
Graham, M. H., Vasquez, J. A. & Buschmann, A. H. Global ecology of the giant kelp Macrocystis: From ecotypes to ecosystems. Oceanogr. Mar. Biol. 45, 39–88 (2007).
Yun, H. Y. & Molis, M. Comparing the ability of a non-indigenous and a native seaweed to induce anti-herbivory defenses. Mar. Biol. 159, 1475–1484 (2012).
Lovett-Doust, L. Population dynamics and local specialization in a clonal perennial (Ranunculus repens). I. The dynamics of ramets in contrasting environments. J. Ecol. 69, 743–755 (1981).
López, F., Serrano, J. M. & Acosta, F. J. Parallels between the foraging strategies of ants and plants. Trends Ecol. Evol. 9, 150–153 (1994).
Dong, M., During, H. J. & Werger, M. J. A. Morphological responses to nutrient availability in four clonal herbs. Vegetatio 123, 183–192 (1996).
Fahrig, L., Coffin, D. P., Lauenroth, W. K. & Shugart, H. H. The advantage of long-distance clonal spreading in highly disturbed habitats. Evol. Ecol. 8, 172–187 (1994).
Sutherland, W. J. & Stillman, R. A. The foraging tactics of plants. Oikos 52, 239–244 (1988).
Fordyce, J. A. The evolutionary consequences of ecological interactions mediated through phenotypic plasticity. J. Exp. Biol. 209, 2377–2383 (2006).
Kramer, P. A. Synthesis of coral reef health indicators for the western Atlantic: Results of the AGRRA program (1997-2000). Atoll Res. Bull. 496, 1–57 (2003).
Edmunds, P. J. & Elahi, R. The demographics of a 15-year decline in cover of the Caribbean reef coral Montastraea annularis. Ecol. Monogr. 77, 3–18 (2007).
Quan-Young, L. I. & Espinoza-Avalos, J. Reduction of zooxanthellae density, chlorophyll a concentration and tissue thickness of the coral Montastraea faveolata (Scleractinia) when competing with mixed turf algae. Limnol. Oceanogr. 51, 1159–1166 (2006).
Cetz-Navarro, N. P., Espinoza-Avalos, J., Hernández-Arana, H. A. & Carricart-Ganivet, J. P. Biological responses of the coral Montastraea annularis to the removal of filamentous turf algae. PLoS ONE 8, e54810 (2013).
Collado-Vides, L. Morphological plasticity of Caulerpa prolifera (Caulerpales, Chlorophyta) in relation to growth form in a coral reef lagoon. Bot. Mar. 45, 123–129 (2002).
Santelices, B. A comparison of ecological responses among aclonal (unitary), clonal and coalescing macroalgae. J. Exp. Mar. Biol. Ecol. 300, 31–64 (2004).
Wright, J. T. Differences between native and invasive Caulerpa taxifolia: a link between asexual fragmentation and abundance in invasive populations. Mar. Biol. 147, 559–569 (2005).
Demes, K. W. & Graham, M. H. Abiotic regulation of investment in sexual versus vegetative reproduction in the clonal kelp Laminaria sinclairii (Laminariales, Phaeophyceae). J. Phycol. 47, 463–470 (2011).
Viejo, R. M. & Åberg, P. Effects of density on the vital rates of a modular seaweed. Mar. Ecol. Progr. Ser. 221, 105–115 (2001).
Arenas, F., Viejo, R. M. & Fernández, C. Density-dependent regulation in an invasive seaweed: responses at plant and modular levels. J. Ecol. 90, 820–829 (2002).
Tworkoski, T. J., Benassi, T. E. & Takeda, F. The effect of nitrogen on stolon and ramet growth in four genotypes of Fragaria chiloensis L. Sci. Hortic-Amsterdam 88, 97–106 (2001).
De Kroon, H. & Hutchings, M. J. Morphological plasticity in clonal plants: the foraging concept reconsidered. J. Ecol. 83, 143–152 (1995).
Marbà, N. & Duarte, C. M. Rhizome elongation and seagrass clonal growth. Mar. Ecol. Progr. Ser. 174, 269–280 (1998).
Turner, S. J. Growth and productivity of intertidal Zostera capricorni in New Zealand estuaries. New Zeal. J. Mar. Fresh. 41, 77–90 (2007).
Humphrey, L. D. & Pyke, D. A. Demographic and growth responses of a guerrilla and a phalanx perennial grass in competitive mixtures. J. Ecol. 86, 854–865 (1998).
Blindow, I. & Schütte, M. Elongation and mat formation of Chara aspera under different light and salinity conditions. Hydrobiologia 584, 69–76 (2007).
Pereyra, R. T., Bergström, L., Kautsky, L. & Johannesson, K. Rapid speciation in a newly opened postglacial marine environment, the Baltic Sea. BMC Evol. Biol. 9, 70 (2009).
Hughes, R. N. Evolutionary ecology of colonial reef-organisms, with particular reference to corals. Biol. J. Linn. Soc. 20, 39–58 (1983).
Lang, J. C. & Chornesky, E. A. Competition between scleractinian reef corals. A review of mechanisms and effects, in Ecosystems of the world. Coral reefs (ed. Dubinsky, Z. ) 209–252 (Elsevier, Amsterdam,1990).
Nugues, M. M., Delvoye, L. & Bak, R. P. M. Coral defense against macroalgae: differential effects of mesenterial filaments on the green alga Halimeda opuntia. Mar. Ecol. Progr. Ser. 278, 103–114 (2004).
Bonaldo, R. M. & Hay, M. E. Seaweed-coral interactions: Variance in seaweed allelopathy, coral susceptibility and potential effects on coral resilience. PLoS ONE 9, e85786 (2014).
Taylor, W. R. Marine algae of the eastern tropical and subtropical coast of the Americas. (Univ. Michigan Press, Ann Arbor, 1960).
Littler, D. S. & Littler, M. M. Caribbean reef plants. An identification guide to the reef plants of the Caribbean, Bahamas, Florida and Gulf of Mexico. (OffShore Graphics, Inc. Washington, 2000).
Komárek, J., Kling, H. & Komárková, J. Filamentous Cyanobacteria, in Freshwater algae of North America (eds. Wehr, J. D. & Sheath, R. G. ) 117–196 (Elsevier Science, USA, 2003).
Dawes, C. J. & Mathieson, A. C. The seaweeds of Florida. (Univ. Press of Florida, USA, 2008).
Tilman, D. Community invasibility, recruitment limitation and grassland biodiversity. Ecology 78, 81–92 (1997).
Dukes, J. S. Biodiversity and invasibility in grassland microcosms. Oecologia 126, 563–568 (2001).
Fargione, J. E. & Tilman, D. Diversity decreases invasion via both sampling and complementarity effects. Ecol. Lett. 8, 604–611 (2005).
Britton-Simmons, K. H. Functional group diversity, resource preemption and the genesis of invasion resistance in a community of marine algae. Oikos 113, 395–401 (2006).
Stachowicz, J. J., Whitlatch, R. B. & Osman, R. W. Species diversity and invasion resistance in a marine ecosystem. Science 286, 1577–1579 (1999).
Naeem, S. et al. Plant diversity increases resistance to invasion in the absence of covarying extrinsic factors. Oikos 91, 97–108 (2000).
Walker, B., Kinzig, A. & Langridge, J. Plant attribute diversity, resilience and ecosystem function: The nature and significance of dominant and minor species. Ecosystems 2, 95–113 (1999).
Loreau, M. Biodiversity and ecosystem functioning: recent theoretical advances. Oikos 91, 3–17 (2000).
Nyström, M. Redundancy and response diversity of functional groups: implications for the resilience of coral reefs. Ambio 35, 30–35 (2006).
Fischer, J. M., Frost, T. M. & Ives, A. R. Compensatory dynamics in zooplankton community responses to acidification: Measurement and mechanisms. Ecol. Appl. 11, 1060–1072 (2001).
Airoldi, L. Roles of disturbance, sediment stress and substratum retention on spatial dominance in algal turf. Ecology 79, 2759–2770 (1998).
CONANP. Programa de manejo Parque Nacional Arrecifes de Xcalak. (Comisión Nacional de Áreas Naturales Protegidas, México, DF, 2004).
Chávez-Hidalgo, A. Conectividad de los arrecifes coralinos del Golfo de México y Caribe Mexicano. MSc thesis, Instituto Politécnico Nacional (2009).
Ruiz-Zárate, M. A., Hernández-Landa, R. C., González-Salas, C., Núñez-Lara, E. & Arias-González, J. E. Condition of coral reef ecosystems in central-southern Quintana Roo, Mexico (Part I: Stony corals and algae). Atoll Res. Bull. 496, 318–337 (2003).
García-Salgado et al. Línea base del estado del Sistema Arrecifal Mesoamericano. Programa de monitoreo sinóptico 2004 y 2005. Documento Técnico del SAM (18) (2006).
Bastida-Zavala, J. R., Beltrán-Torres, A. U., Gutiérrez-Aguirre, M. A. & Fuente-Betancourt, G. Rapid assessment of reef patches in Majagual, Quintana Roo, Mexico. Rev. Biol. Trop. 48, 137–143 (2000).
Steneck, R. S. & Lang, J. C. Rapid assessment of México’s Yucatán reef in 1997 and 1999: pre- and post-1998 mass bleaching and hurricane Mitch (stony corals, algae and fishes). Atoll. Res. Bull. 496, 294–317 (2003).
Cetz-Navarro, N. P., Carpizo-Ituarte, E. J., Espinoza-Avalos, J. & Chee-Barragán, G. The effect of filamentous turf algal removal on the development of gametes of the coral Orbicella annularis. PLoS ONE 10, e0117936 (2015).
Quan-Young, L. I. Respuestas de parámetros biológicos a la competencia por espacio entre macroalgas y el coral Montastraea faveolata (Scleractinia). PhD dissertation, ECOSUR (2007).
Guiry, M. D. & Guiry, G. M. AlgaeBase. World-wide electronic publication. Available at: http://www.algaebase.org. (Accessed: 21st June 2014).
Acknowledgements
We thank the staff of the National Park “Arrecifes de Xcalak” for providing work facilities; R. Herrera-Pavón and J.A. Batún-Catzín for field assistance; H. Bahena-Basave for the photograph (Fig. 2); H. Weissenberger for his advising in the mapping; J. Moreno-Caraveo for registering the algal specimens in the CIQR Herbarium at ECOSUR; A. Sentíes for their valuable comments on the manuscript; H. Hernández-Arana for suggesting improvements to the experimental sampling design and for advising with statistical analyses; and A. Grant and C. Harris for editorial assistance. We are grateful to the PADI Foundation (register number: 64, 2009; NPCN) and ECOSUR (federal funds; JEA) for funding this study. NPCN and LIQY were supported by CONACYT with PhD scholarships 32985 and 162716, respectively. SAGARPA, Mexico, provided the collecting permit (DGOPA.10745.121009.3629) to accomplish this study.
Author information
Authors and Affiliations
Contributions
N.P.C.-N., L.I.Q.-Y. and J.E.-A. conceived the study, accomplished the fieldwork, analysed the data and wrote the manuscript. N.P.C.-N. and L.I.Q.-Y. identified and measured algal specimens.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Cetz-Navarro, N., Quan-Young, L. & Espinoza-Avalos, J. Morphological and community changes of turf algae in competition with corals. Sci Rep 5, 12814 (2015). https://doi.org/10.1038/srep12814
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep12814
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.